Abstract
Objectives: The occurrence of cardiac iron deposition is one of the late effect of iron over load which causes cardiovascular disease (CVD) in patients who are affected by beta-thalassemia major. Evaluation of some cardiovascular risk factors plays a crucial role in prediction and prevention of CVD.
Subjects and methods: This study consisted of 70 young adult subjects with beta-thalassemia major (beta-TM) (aged <30 years) and 71 age- and sex-matched healthy subjects as control group in the range of 20–30 years. Hematological and biochemical laboratory parameters including apolipoprotein (Apo)A1 and ApoB, oxidative stress biomarker pro-oxidant–antioxidant balance (PAB), homocysteine, serum high-sensitivity C-reactive protein (hs-CRP), and lipid profile were evaluated.
Results: ApoA1, ApoB, lipid profiles, and homocysteine were significantly decreased in patients group (P < 0.001); however, very low-density lipoprotein and also mean corpuscular hemoglobin concentration (P > 0.05) were different. Some elements included ferritin (P < 0.001), PAB (P < 0.001), and ApoB/apoA1 ratio (P < 0.05) statistically increased in patients, whereas hs-CRP (P > 0.05) was not significantly different in study groups. Exception of high-density lipoprotein (P > 0.05), other lipid profiles, and apoB had a negative meaningful correlation with PAB (P < 0.05). Likewise, apoA1, apoB, apoB/A1 ratio with apoB and homocysteine showed a strong correlation (P < 0.05). We did not find a slight correlation between apoB/A1 ratio in the company of oxidative stress marker PAB (r = −0.366; P = 0.086). We found a statistical correlation between apoB/A1 and homocysteine (P < 0.05).
Discussion: Higher level of some risk factors like PAB values, apoB/A1 ratio concentration, and lipid profiles is able to involve in the prognostic pathological consequences in patients with beta-thalassemia major. Even so, they contribute toward the gradual development of CVD.
Introduction
Beta-thalassemia is a hereditary hematological disorder (β+/β° thalassemia) caused by nearly 300 mutations of beta-globin gene which lead to impaired production of beta-globin chains. Thalassemia syndromes are the most important hemolytic problems in some countries like Greece, Italy, the Gulf countries, India, and Iran.Citation1,Citation2 Patients who carry both defective beta-globin gene or beta-thalassemia major (beta-TM) usually suffer from severe anemia that necessitates regular blood transfusion along with iron overload and ineffective erythropoiesis which result in impaired oxygen delivery and also produce oxidative stress to tissue dysfunctions namely heart.Citation3,Citation4 Cardiac involvement which springs from ineffective erythropoiesis, excessive iron deposition, and free radical formation in the myocardium of beta-TM patients,Citation5 eventually cause irreversible damage in patients.Citation6 It is confirmed that patients who are affected by beta-thalassemia have an high incidence of morbidity and mortality due to cardiovascular disease (CVD); however, underlying mechanisms of CVD are not completely clear.Citation5,Citation7 Inspite of being multiple conventional risk factors, a complete explanation of CVD in beta-TM has not been yet offered. Even so, dramatic increase in some apolipoproteins is one of the strongest predictive risk factors for the future of CVD.Citation8–Citation10 Apolipoproteins are significant structural and functional proteins in lipoprotein particles which transport lipids. Apolipoprotein B (apoB), a single molecule protein, contains low-density lipoprotein cholesterol (LDL-C) and also very low-density lipoprotein cholesterol (VLDL-C) which is responsible for transporting cholesterol to tissues; moreover, it plays a crucial role in inflammation.Citation11,Citation12 Likewise, Apolipoprotein A1 (apoA1), the major protein component in high-density lipoprotein (HDL), performs its anti-atherogenic properties by transporting excess cholesterol to the liver.Citation8,Citation13,Citation14 It has been suggested that apoA1, in addition to having anti-atherogenic effects, has antioxidant,Citation15 as well as, anti-inflammatory properties by means of inhibiting the cytokines production from macrophages.Citation16,Citation17 Furthermore, elevated homocysteine (Hcy) concentration, which is an amino acid,Citation18 widely accepted as a major risk factor for CVDs,Citation19,Citation20 has taken its own place among other risk factors such as cholesterol, oxidative stress, and inflammatory. Having said that, some confirmed evidence indicates that hyperhomocysteinemia is an important independent risk factor of CVD; despite the fact that, the related mechanism is yet unclear.Citation21,Citation22
The aim of this study mainly was to evaluate the prospective importance of serum pro-oxidant–antioxidant balance (PAB) level as a marker of oxidative stress, Hcy, apoB, apoA1, and apoB/apoA1 ratio in young adult patients who suffer from beta-TM to predict the risk of CVD. At the end, the role of inflammation in this context was also evaluated.
Materials and methods
Study population
In both patients and control groups, approximately equal number of young adults less than 30 years old at the time of survey were entered in this case–control study. The patients who diagnosed as beta-thalassemia major were registered in the Thalassemia and Hemoglobinopathy Research Center, Iran in 2013. All patients groups were under regular transfusion and they received packed cell every month. They received iron chelator together with regular transfusion like Desferal or Deferiprone, simultaneously. Based on complete blood count (CBC) and hemoglobin, electrophoresis had been participated for scrutiny on CVD risk factors. At the same, control group with proven healthy history by complete clinical and laboratory examination was recruited from our center, as well. Participants who are taking vitamin supplements, had a history of smoking, clinical evidence of heart disease, and arterial hypertension were refused to take part in survey. Furthermore, individuals who had diabetes mellitus and other endocrine disorders, or those taking pharmacological agents which may affect the cardiovascular system were excluded prior to entry into the study. All subjects were informed about the study protocol and written consent was obtained from each participant in the study which had been approved by Tehran University of Medical Science Ethics Committee.
Blood sample collection
Sampling in both control and patients groups for checking lipid profiles has been performed in the morning after 12 hours fasting. At first, 5 ml peripheral blood was taken in aseptic conditions from each subject and was collected in plain and EDTA glass tube and CBC by automatic hematology analyzer as well (Sysmex KX21; Sysmex, Kobe, Japan). Meanwhile, serum was separated by centrifugation at 2500 rpm for 15 minutes at room temperature and divided into several aliquots and was kept at −70°C until it was analyzed.
Laboratory measurements and clinical definitions
Measurement of hematological and biochemical markers
By the way of vast comprehensive hematological tests, participants were examined for the count of red blood cells (RBC), hemoglobin (Hb), hemotocrit (Hct), mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration (MCHC), and ferritin. Biochemical cardiac risk factors consist of lipid profile comprising total cholesterol (TC) and triglyceride (TG) assessed by enzymatic colorimetric methods; even so, LDL-C was estimated by the Friedewald equation. Evaluation of HDL-C was carried out by means of enzymatic method of elimination.
Assessment of serum oxidative stress
The PAB was measured in serum samples of both subjects as described by Alamdari et al.Citation23 In summary, this method was based on measurement of the balance between oxidants and antioxidants simultaneously by using chromogen TMB throughout the assessment, TMB can be either oxidized to a color cation by oxidants or reduced to a colorless compound by antioxidants which finally provides a redox index. In order to provide standard solutions, various proportions (0–100%) of 250 μM hydrogen peroxide as an oxidant substance were mixed with 3 mM uric acid (in 10 mM NaOH), as antioxidants. The absorbance of samples was measured with an enzyme-linked immunosorbent assay reader at 450 nm and the values of PAB are expressed in arbitrary (HK) unit. Afterward, the value of unknown samples was calculated using the standard curve.
Measurement of inflammatory biomarker hs-CRP
High-sensitivity C-reactive protein was measured in serum samples by a polyethylene glycol-enhanced immunoturbidimetry method with an Alcyon® analyzer (ABBOTT, Chicago, IL, USA).
Evaluation of apolipoproteins and homocysteine in serum
Levels of apoB, apoA1, and also, cardiac risk factor Hcy in serum accomplished by means of the turbidimetric method using an automatic analyzer (ABBOTT, Chicago, IL, USA) based on a competitive immunoassay.
Statistical methods
All data were expressed as mean ± standard deviation (SD) or frequency as per the parameter based on testimonial. Normally distributed parametric variables between groups were performed using Student's t-test, Mann–Whitney U test, and Spearman's correlation univariate correlation analysis conducted for relationship between all parameters. Multiple linear regression analysis was performed to determine the level of association between apoB, apoA1 vs. the independent variables (ferritin, hs-CRP, hemocysteine and PAB). Data were analyzed using the SPSS for Windows software (version 18 software package SPSS Inc., Chicago, IL, USA). A P-value less than 0.05 was accepted statistically significant.
Results
Participants’ characteristics
This study consists of two groups: 70 young adult beta-TM subjects (34 males and 36 females) between 20 and 30 years as a patients group and 71 age- and sex-matched healthy subjects as control group (33 males and 38 females) with the mean age of 25.9 years and without any abnormality or disease at the time of study. Demographic information and acquired hematologic results were summarized in Table . With the exception of age, gender, and MCHC index, other acquired hematological indices in cases were significantly different from normal subjects (P < 0.001). Furthermore, the hematological tests, results of Hb, Hct, and RBC count in normal control group were within reference range; whereas thalassemic patients had shown statistically lower than normal subjects (P < 0.001).
Table 1 Demographic and hematological characteristics of the study subjects
Cardiovascular risk parameters
Collective data were also analyzed separately to assess the cardiovascular risk parameters. As described in Table , there are noticeably elevated clinical characteristics of apoB/apoA1 ratio (P < 0.05), ferritin, and PAB oxidative stress marker in beta-thalassemia major subjects compared to normal healthy group (P < 0.001); whereas, we could not find any significant difference between participants about VLDL and hs-CRP (P > 0.05).
Table 2 Cardiovascular risk parameters in patients and controls
Components cardiovascular risk factors in patients
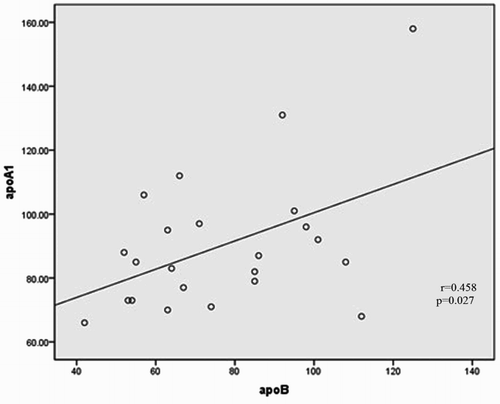
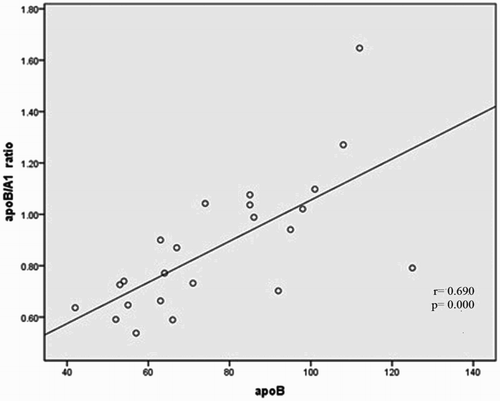
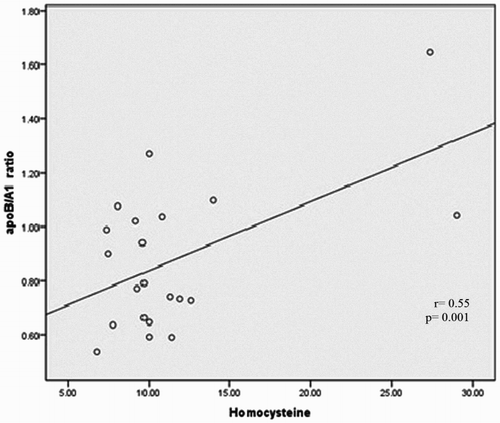
Spearman's correlation univariate analysis was conducted to evaluate the association between risk factors apoA1, apoB, apoB/A1 ratio, and homocysteine vs. hs-CRP, ferritin, and PAB. As shown in Table , statistical analysis shows the meaningful correlation between apoB with apoA1 levels (Fig. ) (P = 0.027) and apoB/A1 ratio (Fig. ) (P = 0.000), as well as, weak reverse correlation with PAB variable (r = −0.432; P = 0.039). Correlations between apolipoproteins, oxidative stress marker, ferritin, homocysteine, and inflammatory variable with using the Spearman rank correlation test show that no significant difference was observed between these parameters with hs-CRP in cases individuals (P > 0.05). There was also a tendency elevation significance between apoB/A1 ratio with ferritin (r = 0.359; P = 0.093), whereas it was negative between apoB/A1 ratio and PAB (r = −0.366; P = 0.086). Moreover, Serum Hemocysteine was positively correlated with apoB/A1 ratio (P = 0.001) (Fig. , Table ).
Table 3 Correlations between cardiovascular risk factors in patients
Association between lipid profiles with other lab finding risk factors
Table represents information about the relationship between lipid profiles and other biochemical risk factors. It was remarkable to consider a negative significant correlation between PAB with dangerous variable objects TG (r = −0.331, P = 0.045), VLDL (r = −0.321, P = 0.043), and TC (r = −0.502, P = 0.002) which were observed, as well as it was shown between HDL and Hyc (r = −0.372, P = 0.035). Serum HDL was completely correlated with apoA1 (P < 0.001) and apoB (P = 0.004); moreover, among TG (P = 0.032) and VLDL (P = 0.040) with homocysteine, we found a straight correlation, like exactly what have seen between TC with apoB (P < 0.001) and apoB/A1 ratio (P = 0.004). Ultimately, there was not any meaningful association between serum TC and apoA1 (P = 0.084).
Table 4 Correlations between cardiovascular risk factors with lipid profile in patients
Multivariate analysis
Multiple linear regression analysis was performed to explore the predictors of apoA1, apoB, and apoB/A1ratio. As we specified in Table , only homocysteine was found to have a significant independent association when apoB/A1 ratio was treated as a dependent variable (r = 0.023, P = 0.018).
Table 5 Multiple linear regression analysis of apoA1, apoB, and apoB/A1 ratio (dependent variables) vs. independent variables (ferritin, homocysteine, PAB, and hs-CRP)
Discussion
Thalassemias are the most common single gene hemoglobin disorders caused by some mutations which can reduce the synthesis of alpha- or beta-globin chains. More than 90 million people are affected by this inherited defect all around the world.Citation2,Citation24 This global problem in Iran and other countries as well includes a vast number of beta-thalassemia major patientsCitation25 who have significantly decreased in synthesis of beta-globin chain by incidence of monogenic disorder. In beta-TM patients, heart disease and cardiac insufficiency, due to cardiac iron overload, are the major causes of their mortality.Citation26,Citation27 Traditional risk factors for CVD, such as diabetes mellitus, dyslipidemia, hypertension, smoking, and low physical activity, have been used to assess the risk of CVD,Citation28,Citation29 but these characteristics cannot completely predict cardiovascular risk. Therefore, advent new cardiac risk factors apoB, apoA1, hs-CRP, oxidative stress, and homocystein for CVD have been recently added to the previous established risk factors.Citation18 Elevated concentrations of ApoB, an increased apoB/apoA1 ratio or diminution in HDL-C and apoA1 levels are able to increase the risk of CVD.Citation11 The metabolism of apolipoproteins is closely associated with the development of atherosclerosis and among them, most of the studies intensively focused on apoB, a protein constituent of the atherogenic lipoproteins VLDL, IDL, and LDL; furthermore, apoA1, the structural protein component of HDL.Citation30 Thus, both the apoB level and the apoB/apoA1 ratio are supposed to be useful predictors of cardiovascular events.Citation11,Citation31 Similar to previous studies,Citation2,Citation32–Citation34 our findings indicate that in young adult beta-thalassemia patients the levels of TC, LDL- cholesterol, and HDL-cholesterol were significantly lower than normal controls. In present study, as described in Table and in accordance with Al-Quobaili and Abou Asali,Citation35 beta-thalassemia major patients had significantly lower apolipoproteins B, A1, and lipid profile compared with control individuals (P < 0.05); on the other hand, our obtained serum TG levels in patients group were lower than in comparison with controls, so it made a contrast with Bordbar et al.,Citation34 Al-Quobaili and Abou Asali,Citation35 and MadaniCitation32 studies who found higher TG serum level in patients vs. controls. This result is confusing but can be partially explained by the low fat intake in our young study population than children. Moreover, the reduction was significant in apolipoproteins B and A1 in patients compared to control subjects (P < 0.001). Maioli et al.Citation36 reported that serum lipids TC, HDL-C, and apoA1 and apoB levels in 20 patients with beta-thalassemia major were significantly lower than 20 control subjects and it was discordance with our report. Alongside, he described that TGs did not differ in both groups. It seems that liver damage plays an important role in determining the altered some of lipid pattern in beta-thalassemia major. However, other factors may also contribute to cause such lipid changes. Refer to the previous studies, apoA1 has been reported to have strong anti-inflammatory properties.Citation8,Citation37 Our achieved result agrees with this result which it found inflammatory marker hs-CRP does not have any association between groups in this study. Likewise, concomitance with Hyka et al.Citation16 who showed that HDL-associated apoA1 was decreased in acute inflammation and suggested that it may possibly induce concomitantly production of TNF-alpha and a chronic inflammation,Citation17 besides, in our study, apoA1 decreased in patients and above-mentioned result was confirmed. Several studies demonstrated that apoB level represents the total atherogenic particles, similar to Xu et al.Citation11 our subjects of beta-TM population, the baseline of both apoA and apoB levels was lower than healthy population. In the other side, apoB/apoA1 ratio reflects the balance between pro-atherogenic IDL, VLDL, LDL particles, and anti-atherogenic HDL particles.Citation15 Despite the fact that, the apoB/apoA1 ratio is a statistically significant predictor, it is emphasizing the importance of apolipoproteins rather than the lipid levels for the predictive of CVD in beta-TM patients (Table ). In our study, the result of the Mann–Whitney U test analysis demonstrated that apoB/A1 ratio level was significantly higher in patients (P < 0.005). Our analysis underscores that the most pronounced cardiovascular risk factors apoB, apoB/A1 ratio, TC, and Hcy contained a strong correlation in thalassemic patients to fall sick heart disease.
We also evaluate hs-CRP to determine the important inflammation with the aim may exist in case subjects. Numerous studies reported that inflammation plays an essential role during all phases of atherosclerosis which leads to severe clinical events. High-sensitivity C-reactive protein is an easy measured and reliable biomarker of inflammatory status. Some clinical trials have indicated that hs-CRP is an independent predictor for future cardiovascular events in populations.Citation38,Citation39 Similar results have also been found in patients with stable coronary artery diseases.Citation40 Thus, to the best of our knowledge, it is valuable to identify the trigger of vascular inflammation to decrease the incidence of cardiovascular events. Interestingly, the levels of C-reactive biomarker were less increased in patients in comparison to control but were not statistically significant with healthy subjects in response to elevated of ferritin, PAB and apoB/apoA1 ratio (Table ). Likewise, we found no statistical relationship between the inflammatory marker hs-CRP and any of the biochemical risk parameters. However, neither HDL-C nor ApoA1 was an independent predictor of hs-CRP. The reason of this result may be that the anti-inflammatory effect of HDL was influenced by evaluated in apoB/A1 ratio or liver damage. Furthermore, result demonstrates that hs-CRP level did not correlate with Hyc, apoB, apoB/A1 ratio, and lipid profile (P = 0.087). Therefore, the concentration of these parameters might not reflect the severity of inflammation. In several studies,Citation2,Citation25,Citation41 increased oxidative stress has been reported which is able to cause oxidative damage in thalassemia and it is related to generation of free radicals by an excess of denature alpha or beta-globin chains, intracellular iron overload and low concentration of normal Hb. Because of this fact that iron-related cardiac disease remains the most common cause of death in patients with thalassemia yet.Citation42 Specifically, similar to Ghayour study,Citation43 our results indicate that the levels of PAB in patients presenting with beta-thalassemia major were significantly higher when compared with healthy control subjects. High levels of PAB in thalassemic patients are significantly reverse correlated with TG, VLDL, TC, and apolipoprotein B (Table ). The reason for this phenomenon seems that all through the under conditions of iron overload, increased free radical production, peroxidative damage to tissues especially liver and depletion of endogenous antioxidants may be expected.
Another factor, homocysteine is an precious predictive biomarker for CVD.Citation28,Citation44 In present study, the levels of homocysteine in beta-TM patients were significantly lower than normal subjects (P < 0.001) (Table ). With Spearman's correlation univariate analysis, we found a positive correlation between Hcy with TG, VLDL, and apoB/A1 ratio (P < 0.05) and negative relation with HDL (r = −0.372 P = 0.035). Several studies demonstrated a relationship between Hcy and risk of obesity, diabetes, and dyslipidemia,Citation45 although Veerkamp et al.Citation46 reported that no association was between Hcy concentration and plasma lipid levels. Fascinatingly, only a significant linear correlation was found between level of homocysteine and level of apoB/A1 ratio in patients which is able to be a possible risk factor for CVD (Table ) (P = 0.018). However, no such linear correlation was clarified in Table . Data concerning the level of Hcy in beta-thalassaemia are limited. Like to our result, Ozdem et al.,Citation47 demonstrated a significant reduction of Hcy concentration in thalassaemic patients. In contrast with these mentioned studies, Sherief et al.,Citation48 reported that Hcy significantly elevated in patients compared to healthy individuals. Although the reason for this discrepancy remains unclear, differences in number of subjects and study designs may play a role.
Clearly, finding indicate that (A) levels of oxidative stress indicator PAB, ferritin, and serum apoB/A1 ratio what the most cardiovascular risk factors is that predictive CVD were significantly increment in beta-thalassaemia patients compared to control and other lab findings were statistically lower in patients. (B) Homocysteine that is main CVD risk factor just have a positive correlation with lipid risk factors TG and VLDL. (C) Measurement of PAB and hs-CRP may be useful marker for oxidative stress and inflammation and helpful diagnostic factor to prevent injury and its development to liver and other tissues. At the end, we investigated there are significant relationship between two major risk factors apoB/A1 ratio and Hcy and these factors are usefulness for predicting CVD in subjects with beta-thalassemia major.
Disclaimer statements
Contributors They have contributed to the research.
Funding None.
Conflicts of interest None.
Ethics approval Ethical approval was obtained from all the participants.
Acknowledgments
The researchers would like to thank sincerely Mrs Hajar Nasiri from Hematology–Oncology and Stem Cell Transplantation Research Center, Tehran University of Medical Science, Tehran, Iran, for her valuable cooperation and English editing in this study.
References
- Finotti A, Gambari R. Recent trends for novel options in experimental biological therapy of beta-thalassemia. Expert Opin Biol Ther. 2014;14(10):1443–54. doi: 10.1517/14712598.2014.927434
- Ehteram H, Bavarsad MS, Mokhtari M, Saki N, Soleimani M, Parizadeh SM, et al. Prooxidant–antioxidant balance and hs-CRP in patients with beta-thalassemia major. Clin Lab. 2014;60(2):207–15.
- Bakr A, Al-Tonbary Y, Osman G, El-Ashry R. Renal complications of beta-thalassemia major in children. Am J Blood Res. 2014;4(1):1–6.
- Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331(9):567–73. doi: 10.1056/NEJM199409013310902
- Shahramian I, Razzaghian M, Ramazani AA, Ahmadi GA, Noori NM, Rezaee AR. The correlation between troponin and ferritin serum levels in the patients with major beta-thalassemia. Int Cardiovasc Res J. 2013;7(2):51–5.
- Atefi A, Binesh F, Hashemi A, Atefi A, Aminorroaya M. Seroprovalence of herpes simplex1, 2 IgG antibodies in patients with beta thalassemia in a major tertiary care hospital located in Yazd, Iran. Iran J Ped Hematol Oncol. 2014;4(2):64–7.
- Wood JC. Impact of iron assessment by MRI. Hematology Am Soc Hematol Educ Program 2011:443–50. doi:10.1182/asheducation-2011.1.443.
- Ohman M, Ohman ML, Wallberg-Jonsson S. The apoB/apoA1 ratio predicts future cardiovascular events in patients with rheumatoid arthritis. Scand J Rheumatol. 2014;43(4):259–64. doi: 10.3109/03009742.2013.877158
- Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren WM, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Atherosclerosis 2012;223(1):1–68. doi: 10.1016/j.atherosclerosis.2012.05.007
- Ingelsson E, Schaefer EJ, Contois JH, McNamara JR, Sullivan L, Keyes MJ, et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA 2007;298(7):776–85. doi: 10.1001/jama.298.7.776
- Xu W, Li R, Zhang S, Gong L, Wang Z, Ren W, et al. The relationship between high-sensitivity C-reactive protein and ApoB, ApoB/ApoA1 ratio in general population of China. Endocrine 2012;42(1):132–8. doi: 10.1007/s12020-012-9599-x
- Faraj M, Messier L, Bastard JP, Tardif A, Godbout A, Prud'homme D, et al. Apolipoprotein B: a predictor of inflammatory status in postmenopausal overweight and obese women. Diabetologia 2006;49(7):1637–46. doi: 10.1007/s00125-006-0259-7
- Zannis VI, Chroni A, Krieger M. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med (Berl.) 2006;84(4):276–94. doi: 10.1007/s00109-005-0030-4
- Savas Erdeve S, Simsek E, Dallar Y, Biyikli Z. Utility of ApoB/ApoA1 ratio for the prediction of cardiovascular risk in children with metabolic syndrome. Indian J Pediatr. 2010;77(11):1261–5. doi: 10.1007/s12098-010-0217-8
- Lu M, Lu Q, Zhang Y, Tian G. Apob/apoA1 is an effective predictor of coronary heart disease risk in overweight and obesity. J Biomed Res. 2011;25(4):266–73.
- Hyka N, Dayer JM, Modoux C, Kohno T, Edwards CK, 3rd, Roux-Lombard P, et al. Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood 2001;97(8):2381–9. doi: 10.1182/blood.V97.8.2381
- Burger D, Dayer JM. High-density lipoprotein-associated apolipoprotein A-I: the missing link between infection and chronic inflammation? Autoimmun Rev. 2002;1(1–2):111–7. doi: 10.1016/S1568-9972(01)00018-0
- Marti-Carvajal AJ, Sola I, Lathyris D, Karakitsiou DE, Simancas-Racines D. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev.1:CD006612.
- Ajabnoor MA, MN AL-A, Banjar Z, Rafee AA, Sheweita SA. Homocysteine level and other biochemical parameters in cardiovascular disease patients with diabetes mellitus. Med Sci Monit. 2003;9(12):CR523–7.
- Graham IM, Daly LE, Refsum HM, Robinson K, Brattstrom LE, Ueland PM, et al. Plasma homocysteine as a risk factor for vascular disease. The European concerted action project. JAMA 1997;277(22):1775–81. doi: 10.1001/jama.1997.03540460039030
- El Oudi M, Aouni Z, Mazigh C, Machghoul S. Total homocysteine levels and cardiovascular risk factors in healthy Tunisians. East Mediterr Health J. 2011;17(12):937–42.
- Lawrence de Koning AB, Werstuck GH, Zhou J, Austin RC. Hyperhomocysteinemia and its role in the development of atherosclerosis. Clin Biochem. 2003;36(6):431–41. doi: 10.1016/S0009-9120(03)00062-6
- Alamdari DH, Paletas K, Pegiou T, Sarigianni M, Befani C, Koliakos G. A novel assay for the evaluation of the prooxidant–antioxidant balance, before and after antioxidant vitamin administration in type II diabetes patients. Clin Biochem. 2007;40(3–4):248–54. doi: 10.1016/j.clinbiochem.2006.10.017
- Colah R, Gorakshakar A, Nadkarni A. Global burden, distribution and prevention of beta-thalassemias and hemoglobin E disorders. Expert Rev Hematol. 2010;3(1):103–17. doi: 10.1586/ehm.09.74
- Cighetti G, Duca L, Bortone L, Sala S, Nava I, Fiorelli G, et al. Oxidative status and malondialdehyde in beta-thalassaemia patients. Eur J Clin Invest. 2002;32(Suppl 1):55–60. doi: 10.1046/j.1365-2362.2002.0320s1055.x
- Bayar N, Kurtoglu E, Arslan S, et al. Assessment of the relationship between fragmented QRS and cardiac iron overload in patients with beta-thalassemia major. Anadolu Kardiyol Derg. April 2.
- Kremastinos DT, Tsetsos GA, Tsiapras DP, Karavolias GK, Ladis VA, Kattamis CA. Heart failure in beta thalassemia: a 5-year follow-up study. Am J Med. 2001;111(5):349–54. doi: 10.1016/S0002-9343(01)00879-8
- Kang JY, Park IK, Lee JY, Sung SH, Chang YK, Park YK, et al. Use of serum homocysteine to predict cardiovascular disease in Korean men with or without metabolic syndrome. J Korean Med Sci. 2012;27(5):500–5. doi: 10.3346/jkms.2012.27.5.500
- Hamer M, Stamatakis E. Physical activity and risk of cardiovascular disease events: inflammatory and metabolic mechanisms. Med Sci Sports Exerc. 2009;41(6):1206–11. doi: 10.1249/MSS.0b013e3181971247
- Marcovina SM, Crea F, Davignon J, Kaski JC, Koenig W, Landmesser U, et al. Biochemical and bioimaging markers for risk assessment and diagnosis in major cardiovascular diseases: a road to integration of complementary diagnostic tools. J Intern Med. 2007;261(3):214–34. doi: 10.1111/j.1365-2796.2006.01734.x
- Sierra-Johnson J, Fisher RM, Romero-Corral A, Somers VK, Lopez-Jimenez F, Ohrvik J, et al. Concentration of apolipoprotein B is comparable with the apolipoprotein B/apolipoprotein A-I ratio and better than routine clinical lipid measurements in predicting coronary heart disease mortality: findings from a multi-ethnic US population. Eur Heart J. 2009;30(6):710–7. doi: 10.1093/eurheartj/ehn347
- Madani H, Rahimi Z, Manavi-Shad M, Mozafari H, Akramipour R, Vaisi-Raygani A, et al. Plasma lipids and lipoproteins in children and young adults with major beta-thalassemia from western Iran: influence of genotype. Mol Biol Rep. 2011;38(4):2573–8. doi: 10.1007/s11033-010-0397-3
- Papanastasiou DA, Siorokou T, Haliotis FA. Beta-Thalassaemia and factors affecting the metabolism of lipids and lipoproteins. Haematologia (Budap.) 1996;27(3):143–53.
- Bordbar M, Haghpanah S, Afrasiabi A, Dehbozorgian J, Karimi M. Genotype–phenotype correlation related to lipid profile in beta-thalassemia major and intermedia in southern Iran. J Clin Lipidol. 2012;6(2):108–13. doi: 10.1016/j.jacl.2011.12.005
- Al-Quobaili FA, Abou Asali IE. Serum levels of lipids and lipoproteins in Syrian patients with beta-thalassemia major. Saudi Med J. 2004;25(7):871–5.
- Maioli M, Cuccuru GB, Pranzetti P, Pacifico A, Cherchi GM. Plasma lipids and lipoproteins pattern in beta-thalassemia major. Acta Haematol. 1984;71(2):106–10. doi: 10.1159/000206566
- Rye KA, Barter PJ. Antiinflammatory actions of HDL: a new insight. Arterioscler Thromb Vasc Biol. 2008;28(11):1890–1. doi: 10.1161/ATVBAHA.108.173575
- Bassuk SS, Rifai N, Ridker PM. High-sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol. 2004;29(8):439–93.
- Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–65. doi: 10.1056/NEJMoa021993
- Haverkate F, Thompson SG, Pyke SD, Gallimore JR, Pepys MB. Production of C-reactive protein and risk of coronary events in stable and unstable angina. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet 1997;349(9050):462–6. doi: 10.1016/S0140-6736(96)07591-5
- Walter PB, Fung EB, Killilea DW, Jiang Q, Hudes M, Madden J, et al. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. Br J Haematol. 2006;135(2):254–63. doi: 10.1111/j.1365-2141.2006.06277.x
- Walter PB, Macklin EA, Porter J, Evans P, Kwiatkowski JL, Neufeld EJ, et al. Inflammation and oxidant-stress in beta-thalassemia patients treated with iron chelators deferasirox (ICL670) or deferoxamine: an ancillary study of the Novartis CICL670A0107 trial. Haematologica 2008;93(6):817–25. doi: 10.3324/haematol.11755
- Ghahremanlu E, Banihashem A, Saber H, Tavallaie S, Mirhosseini N, Ghayour-Mobarhan M, et al. Increased serum heat shock protein 27 antibody titers and prooxidant–antioxidant balance in patients with beta-thalassemia major. Acta Haematol. 2013;129(1):1–9. doi: 10.1159/000339502
- Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA 1995;274(13):1049–57. doi: 10.1001/jama.1995.03530130055028
- Real JT, Martinez-Hervas S, Garcia-Garcia AB, Chaves FJ, Civera M, Ascaso JF, et al. Association of C677 T polymorphism in MTHFR gene, high homocysteine and low HDL cholesterol plasma values in heterozygous familial hypercholesterolemia. J Atheroscler Thromb. 2009;16(6):815–20. doi: 10.5551/jat.2196
- Veerkamp MJ, de Graaf J, den Heijer M, Blom HJ, Stalenhoef AF. Plasma homocysteine in subjects with familial combined hyperlipidemia. Atherosclerosis 2003;166(1):111–7. doi: 10.1016/S0021-9150(02)00312-X
- Ozdem S, Kupesiz A, Yesilipek A. Plasma homocysteine levels in patients with beta-thalassaemia major. Scand J Clin Lab Invest. 2008;68(2):134–9. doi: 10.1080/00365510701516343
- Sherief LM, Abd El-Salam SM, Kamal NM, El Safy O, Almalky MA, Azab SF, et al. Nutritional biomarkers in children and adolescents with beta-thalassemia-major: an Egyptian center experience. Biomed Res Int. 2014:261761, doi:10.1155/2014/261761. Epub 2014 Apr 8.