Abstract
Objectives: To evaluate the predictive significance of F-18 FDG PET/CT quantization parameters for progression-free survival (PFS) in patients with diffuse large B cell lymphoma (DLBCL) before chemotherapy.
Methods: We conducted a retrospective study involving 60 patients with DLBCL between January 2010 and August 2014 who had undergone F-18 FDG PET/CT scan prior to treatment. Maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), total lesion glycolysis (TLG), and number of enlarged lymph nodes (>2 cm) were measured. The primary outcome measure was PFS. Spearman rank correlation analysis, univariate and multivariate Cox regression models, receiver operating characteristic (ROC) analysis, and Kaplan–Meir survival curves were used.
Results: Spearman analysis determined that the MTV and TLG values were positively related to Ann Arbor stage, National Comprehensive Cancer Network International Prognostic Index (NCCN-IPI) score, and lactate dehydrogenase (LDH) level. The number of enlarged lymph nodes was positively related only to LDH level. The SUVmax value and clinical characteristics were not related. Univariate Cox regression determined that the MTV and TLG values, number of enlarged lymph nodes, and NCCN-IPI score were predictive factors. Multivariate Cox regression determined that the MTV and TLG values and number of enlarged lymph nodes predicted PFS independently of the NCCN-IPI score. The SUVmax value was not predictive of PFS. According to the cut-off determined from ROC analysis, lower MTV and TLG values were highly predictive of favorable PFS.
Conclusions: In contrast to SUVmax, the MTV and TLG may be significant prognostic markers for PFS in DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common of all lymphomas, accounting for 30–35% of non-Hodgkin lymphoma casesCitation1,Citation2; it is characterized as a heterogeneous disease with variable patient survival. Accurate assessment of prognosis and early identification of high-risk patients before conventional therapy are of great value in clinical treatment decisions. The International Prognostic Index (IPI) and the revised IPI have been acknowledged as highly predictive and are useful prognostic tools pre-immunochemotherapy.Citation3,Citation4 However, due to differences in biological behavior, the IPI system is not suitable for all lymphomas and was recently proven inconsistent for stratifying patients with intermediate IPI scores.Citation4,Citation5 Recently, it was proposed that the National Comprehensive Cancer Network IPI (NCCN-IPI) allows better risk stratification of patients, but is insufficient for accurately identifying patients with immunochemotherapy-refractory disease.Citation6 Over the past decade, F-18 FDG PET/CT has been widely used for initial staging, follow-up, and treatment response evaluation in lymphoma.Citation7,Citation8 The maximum standardized uptake value (SUVmax) acquired using PET is commonly used in clinical practice as a criterion for malignancy; high SUVmax is commonly considered a poor prognostic factor.Citation9 With the development of software programs, recent studies have found that metabolic tumor volume (MTV) and total lesion glycolysis (TLG) may be reliable prognostic markers in human solid tumors.Citation10,Citation11 Although PET/CT for assessing lymphoma is widely implemented in daily clinical practice, the quantitative parameters of FDG PET/CT have not been comparatively investigated as prognostic factors in untreated DLBCL. A few recent studies evaluated the prognostic values of baseline FDG PET/CT quantitative metrics in DLBCL; their results were inconclusive and contradictory.Citation12–Citation17 The reason why studies reached different conclusions may be explained by the differences in treatment protocols, distributions of risk groups according to IPI scoring system, the inclusion and exclusion criteria of patients, the optimal cut-off values for survival prediction and the Cox proportional hazard regression methods.Citation18 Therefore, more studies are needed to evaluate the prognostic values of these FDG PET/CT measurements.
In this study, we investigated the value of the PET/CT quantization parameters SUVmax, MTV, TLG, and number of enlarged lymph nodes for predicting PFS in DLBCL before first-line chemotherapy.
Patients and methods
Patients
One hundred and five patients with histologically proven lymphoma underwent PET/CT scan prior to beginning first-line chemotherapy from January 2010 to August 2014. Among them, 60 patients met the inclusion criteria: histologically confirmed DLBCL; aged ≥18 years; treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) as first-line chemotherapy. The following individuals were excluded: patients with a lymphoma subtype other than DLBCL on diagnostic biopsy, transformed lymphoma, or previously treated/relapsed lymphoma, women who were lactating or pregnant, and those with HIV, heart failure with an ejection fraction of <40%, severe psychiatric diseases, or hepatic or renal dysfunction. Clinical parameters and hematological data (Ann Arbor stage, NCCN-IPI score, pathological type, LDH level, Ki-67 index, bone marrow smear, subsequent treatment planned) were determined from the medical records. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. All patients provided written informed consent to participate in the study.
PET/CT technique and evaluation
A PET/CT scan was performed at baseline prior to the commencement of chemotherapy. All patients were fasted for at least 6 hours before the intravenous injection of 0.1 mCi/kg F-18 FDG. All patients had glucose levels between 90 and 160 mg/dL at the time of injection. Sixty minutes after the tracer injection, PET and CT images were obtained using a commercial PET/CT scanner (Siemens Biograph 64; Siemens Healthcare, Germany). Sequential overlapping CT scans were acquired to cover the neck, chest, abdomen, and pelvis. Subsequently, PET scanning was performed, acquiring 3 minutes per bed position and five to six beds per patient, depending on patient height. CT images were acquired at 120 mA, 120 kV, and 5-mm axial slice thickness. CT and PET images were displayed both independently and in fusion mode in transaxial, coronal, and sagittal planes. The PET reconstruction parameters were as following: Recon method: TrueX; iterations: 2; subsets: 14; filter: all-pass; FWHM (mm): 0. These parameters are compliant with EARL (The European Association of Nuclear Medicine Research Ltd)/NEDPAS (The Netherlands protocol for standardization of FDG whole body PET studies in multi-center trials) recommendations.
The data were processed using a HERMES Workstation (HERMES Medical Solutions, Sweden). The SUVmax, MTV (mL), and TLG (g) were evaluated using TUMOR FINDER software application. First, the mean liver SUV (LiverSUVmean) was measured by placing a 14-mL cubic volume of interest in the liver. For each patient, all lesions were segmented using an automated 3D region-growing algorithm with a defined minimum SUV threshold [LiverSUVmean + SD × 2.00]. After removing physiological activity above the measured threshold, this computation defined the MTV. The atelectasis area size (three maximum diameters) was also measured. The SUVmax and SUVmean were derived from the MTV, and the TLG was then calculated as [TLG = SUVmean × MTV].
Follow-up assessment
The follow-up procedures included physical examination; hematological examination (red blood cell count, differential white blood cell count, platelet count); laboratory tests, including LDH level and bone marrow biopsy; neck, oxter, and groin echography; and chest, abdominal, and pelvic CT scan. Follow-up data were recorded at scheduled or unscheduled visits as well as by phone interview. The primary endpoint was PFS. An event was defined as registration to progression (documentation of a new lesion or enlargement of a previous existing lesion), relapse, or death from any cause. All patients were followed until disease progression or for a maximum 40 months.
Statistical analysis
Continuous variables were expressed as means ± SD. The correlation between the F-18 FDG PET/CT quantization parameters and patient clinical characteristics was assessed using Spearman rank correlation analysis. A P-value <0.05 was considered statistically significant. Estimates of the predictive effect for PFS were expressed as hazard ratios (HRs) in univariate and multivariate Cox regression analyses with a 95% confidence interval (CI). Receiver operating characteristic (ROC) analysis was performed to determine optimal cut-off values for MTV, TLG, and number of enlarged lymph nodes for predicting PFS (event vs. no event). Progression-free survival curves were constructed using the Kaplan–Meier method and were compared with the log-rank test. Differences between the results of comparative tests were considered significant if the 2-sided P-value was <0.05. All statistical analyses were performed using STATA version 12.0 (StataCorp, College Station, TX, USA).
Results
Patient characteristics
In this analysis, we included 60 patients with DLBCL who had undergone baseline PET/CT examination prior to chemotherapy. The clinical features of the patients are summarized in Table . The median age was 60.5 years, and 56% were male. Ann Arbor stage, NCCN-IPI score, DLBCL subtype by Hans's algorithm (GCB vs. non-GCB), and the number of patients with extranodal site or bone marrow involvement are also shown in Table . Almost 80% of patients were considered to have intermediate-risk DLBCL (NCCN-IPI score 2–5). The median LDH was 431.8 U/L. Tissue specimens were collected from 52 nodal locations, six gastric sites, and two bone marrows. The median Ki-67 index was 57%. All patients received R-CHOP with a median 4.6 cycles as first-line therapy after PET/CT examination.
Table 1 Patient characteristics
Correlation between PET/CT quantization parameters and clinical features
The median SUVmax was 35.26 (range, 6.1–292.5), median MTV was 1285 mL (range, 35–9113 mL), and median TLG was 7094 g (range, 276–37361 g). The correlation between the F-18 FDG PET/CT quantization parameters (SUVmax, MTV, TLG, number of enlarged lymph nodes), the F-18 FDG PET/CT quantization parameters and clinical characteristics (age, Ann Arbor stage, NCCN-IPI score, LDH level, Ki-67 index) were assessed using Spearman rank correlation analysis (Table ). Spearman analysis showed that the F-18 FDG PET/CT quantization parameters were highly correlated with each other, except that the SUVmax and the number of enlarged lymph nodes were not relevant (P = 0.285). The SUVmax and clinical features were not related. The MTV and TLG values were positively related to Ann Arbor stage, NCCN-IPI score, and LDH level, with rs of 0.334, 0.386, 0.557; and 0.361, 0.445, 0.564, respectively. The number of enlarged lymph nodes was positively related only to LDH level (P = 0.003). Based on the above analysis, higher MTV and TLG appear to indicate worse disease stage and prognosis.
Table 2 Spearman rank correlation analysis of the relation between F-18 FDG PET/CT quantization parameters and patient clinical characteristics
Predictive value for event occurrence
At a median follow-up of 17 months (range, 2–40 months), 20 of 60 patients reached the endpoint. Among them, 17 experienced progression (28%) and three died (5%). Univariate analysis for PFS in the Cox regression model that included the SUVmax, MTV, TLG, number of enlarged lymph nodes, NCCN-IPI score, LDH level, and Ki-67 index was performed (Table ). The variables significantly associated with PFS were MTV (HR = 1.030, 95%CI 1.017–1.044, P = 0.000), TLG (HR = 1.078, 95%CI 1.042–1.116, P = 0.000), number of enlarged lymph nodes (HR = 1.108, 95%CI 1.031–1.191, P = 0.005), and NCCN-IPI score (HR = 1.577, 95%CI 1.141–2.181, P = 0.006).
Table 3 Univariate Cox proportional hazard regression analysis
In GCB or non-GCB subgroup, MTV (P = 0.017, 0.002, respectively) and TLG (P = 0.008, 0.001, respectively) parameters were still considered as prognostic indicators for PFS. On multivariate Cox analysis adjusted by NCCN-IPI score, the MTV (HR = 1.028, 95%CI 1.014–1.043, P = 0.001), TLG (HR = 1.071, 95%CI 1.032–1.112, P = 0.001), and number of enlarged lymph nodes (HR = 1.089, 95%CI 1.014–1.169, P = 0.018) remained predictive factors of PFS independently of NCCN-IPI score (Table ).
Table 4 Multivariate Cox proportional hazard regression analysis adjusted by NCCN-IPI
ROC analysis
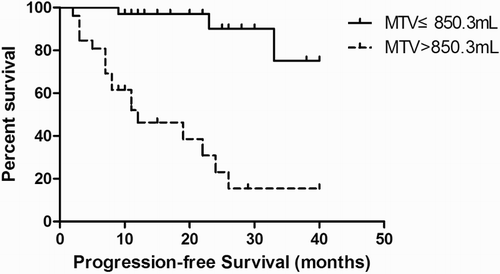
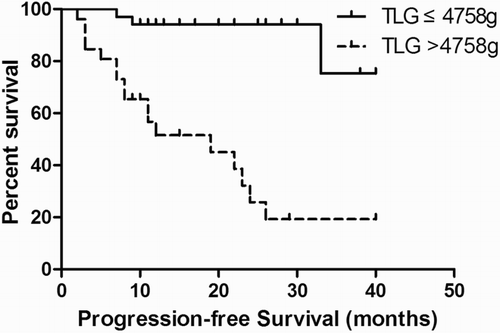
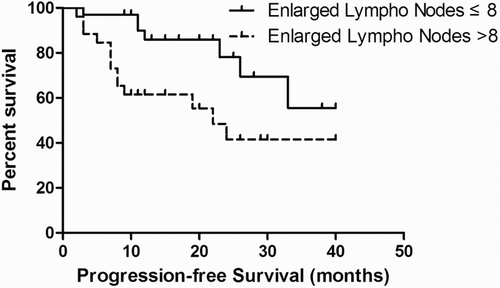
ROC curve analysis was performed to determine the accuracy and optimal cut-off values of the PET/CT quantization parameters in predicting PFS. The Area Under the Curve (AUC) for MTV and TLG was 0.900 and 0.896, respectively; the MTV and TLG cut-off values were 850.3 mL and 4758 g, respectively. The AUC for number of enlarged lymph nodes was 0.678, with a cut-off value of 8.
Kaplan–Meier survival analysis
Kaplan–Meier survival analysis was performed according to the cut-off of the ROC curves. A significant difference in PFS was observed between patients with MTV above and below the 850.3 mL cut-off (log-rank test, P = 0.000) (Fig. ). A significant difference in PFS was also observed between patients with TLG above and below the 4758 g cut-off (log-rank test, P = 0.000) (Fig. ). Similarly, better survival was associated with fewer enlarged lymph nodes (log-rank test, P = 0.014) (Fig. ).
Discussion
Recently, the potential prognostic value of quantitative FDG PET/CT parameters in pretreatment evaluation of DLBCL was investigated.Citation12–Citation17 Among the PET parameters, SUVmax is the most commonly used semi-quantitative index of F-18 FDG uptake, reflecting the tumor glucose metabolism of the most aggressive cell component, and previous studies have suggested an association between SUVmax and tumor aggressiveness.Citation19,Citation20 However, SUVmax has limited prognostic value because it lacks information regarding the tumor burden.Citation21 Moon et al. also reported that SUVmax may not reflect tumor prognosis in its entirety, as it may vary with the partial volume effect, body composition, uptake period, and plasma glucose level, or mixed effects.Citation22 A few recent studies investigated the relationship between SUVmax and prognosis in DLBCL; the findings were discordant, alternately concluding that the SUVmax was valuable or worthless. For example, a retrospective study involving 76 patients reported that SUVmax was significantly related to survival outcome in patients with primary extranodal DLBCL, while high SUVmax (cut-off value 11.0) was a poor prognostic factor of PFS and overall survival (OS).Citation16 Chihara et al. showed that SUVmax was associated with lower PFS and OS independently of IPI score.Citation17 However, some recent studies drew the opposite conclusion. Multivariate Cox regression analysis in a retrospective study involving 140 patients with DLBCL showed that SUVmax was not predictive of PFS.Citation15 A retrospective study involving 20 patients also showed that SUVmax did not predict survival, although the number of patients in that study was too low to draw conclusions.Citation14 Univariate Cox regression analysis in another retrospective study indicated that low SUVmax was associated with shorter event-free survival (EFS), and high SUVmax was considered a favorable predictor of EFS.Citation23 Our findings suggest that SUVmax is not related to clinical features such as age, Ann Arbor stage, NCCN-IPI score, LDH level, or Ki-67 index. When using SUVmax to evaluate PFS in DLBCL, we found that the pretreatment SUVmax did not significantly correlate with prognosis. Some researchers believe that the SUVmax has relatively large variability,Citation24 which may lead to great uncertainty regarding the outcome, and the SUVmax only represents the highest level of glucose metabolism in tumor tissue. Whether the glucose metabolism truly represents the tumor biological characteristics in terms of therapeutic sensitivity or vulnerability to metastasis has not been established.Citation25
Following the development of software programs, MTV or TLG may provide additional valuable information for evaluating tumor reaction to treatment and for assessing the patient prognostic value. MTV is a measure of the viable tumor fraction, and may better estimate tumor burden than anatomical imaging. TLG, calculated as a product of MTV and mean SUV within the volume, represents the metabolic burden of disease that depends on both tumor volume and glucose utilization rate. Thus far, MTV and TLG have been evaluated as prognostic factors for survival or treatment response in various solid tumorsCitation21,Citation26; however, the outcomes of five recent studies that focused on DLBCL were inconsistent.Citation12–Citation15,Citation23 One retrospective study indicated that high TLG values were independently predictive of reduced PFS and OS in DLBCL, whereas Ann Arbor stage and IPI score did not predict survival.Citation15 Multivariate Cox regression analysis in another retrospective study showed that MTV was the only independent predictor of both PFS and OS; TLG did not predict PFS and was less predictive of OS than MTV.Citation13 In a retrospective study by Esfahani et al., univariate Cox regression analysis showed that only baseline TLG was associated with PFS.Citation14 Two other studies drew the opposite conclusion: both MTV and TLG were not predictive of survival outcomes in DLBCL. Following univariate analysis, Gallicchio et al. reported that MTV and TLG were not predictive of EFS.Citation23 Adams et al. showed that MTV and TLG did not provide any DLBCL prognostic information beyond what could already be derived from the NCCN-IPI, and the NCCN-IPI remained the most important prognostic tool in DLBCL.Citation12 Our results imply that MTV and TLG are positively related to lymphoma staging and are highly associated with NCCN-IPI score and LDH level. Multivariate analysis adjusted by NCCN-IPI score indicated that rather than SUVmax, MTV, and TLG are PFS prognostic factors independent of NCCN-IPI score. Our data also indicate a strong link between the number of enlarged lymph nodes and LDH levels. Elevation of serum LDH in DLBCL is believed to reflect cellular turnover and tumor burden. Therefore, we speculated that the number of enlarged lymph nodes (>2 cm) may reflect body tumor burden to some degree but does not fully represent other clinical features, such as Ann Arbor stage, age, and physical status. Our study also demonstrated the protective significance of fewer enlarged lymph nodes, whereas lower LDH and Ki-67 staining did not. Based on our results, we speculate that MTV and TLG, rather than SUVmax, may truly represent tumor biological characteristics and reflect the actual tumor burden, and their values were consistent with clinical stage and current NCCN-IPI score. This implies that tumor volume and overall metabolic activity of the volume, or metabolic tumor burden, may be more important than the highest metabolic activity within the tumor for predicting treatment outcomes in DLBCL.
The optimal cut-off values of the PET/CT quantization parameters varied greatly in the recent studies, with the cut-off values ranging from 220 to 550 mL for MTVCitation12–Citation14,Citation27 and ranging from 415.5 to 4576 g for TLG.Citation12–Citation15 The optimal cut-off values of MTV and TLG values in our study were similar to the ones reported by Sasanelli et al.Citation13 We speculate that several factors may cause the difference of optimal cut-off values, including the different distributions of risk groups in patients, the different definitions of the minimum SUV threshold and whether to apply ROC curve analysis instead of using the median values as the cut-off values.
Both the IPI and revised IPI are highly predictive for patients at risk of early relapse/progression, and constitute valuable stratifying tools. However, they have been proven insufficient for accurate discrimination of patients within the gray area of the intermediate score.Citation4,Citation5,Citation28 The NCCN-IPI was proposed recently and proved more accurate for identifying low- or high-risk patients despite their older age, and more patients with increased LDH.Citation29 This novel index places more importance on age and high LDH levels. In our study, univariate Cox regression analysis showed that the NCCN-IPI score was a significant predictor of PFS but was less predictive of PFS than MTV and TLG. Our results also showed significant correlations between MTV and NCCN-IPI score and between TLG and NCCN-IPI score. Therefore, we used multivariate analysis to evaluate the prognostic values adjusted by NCCN-IPI score, and concluded that MTV, TLG, and number of enlarged lymph nodes were significant PFS predictors independent of NCCN-IPI score.
Due to the small number of included patients in the present study, large-scale prospective studies are needed to confirm the prognostic value of the F-18 FDG PET/CT quantization parameters. Moreover, there is a lack of actual standardized criteria for determining MTV and TLG values, and the optimal cut-off values have not been explicated. Future studies should also focus on these issues.
Conclusions
Our preliminary study shows that the MTV and TLG are positively related to lymphoma stage, NCCN-IPI score, and LDH level. The SUVmax and clinical characteristics are not related. Rather than SUVmax, the MTV, TLG, and number of enlarged lymph nodes are important predictors for PFS in DLBCL independent of NCCN-IPI score. Quantitative assessment by MTV and TLG via F-18 FDG PET/CT may be helpful for managing patients with DLBCL prior to treatment.
Disclaimer statements
Contributors MX: conception and design of the study, protocol development, analysis and interpretation of data, drafting the article; YX and WH: conception and design of the study, protocol development, modifying the article; SC, HZ, and WZ: operation of the equipment (PET–CT), screening patients, collection of data, analysis and interpretation of data.
Funding None.
Conflicts of interest The authors report no conflicts of interest with this study.
Ethics approval The study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
References
- Flowers CR, Sinha R, Vose JM. Improving outcomes for patients with diffuse large B-cell lymphoma. CA Cancer J Clin. 2010;60:393–408.
- Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematol Am Soc Hematol Educ Program. 2011;2011:498–505. doi: 10.1182/asheducation-2011.1.498
- Shipp MA, Harrington DP, Anderson JR, Armitage JO, Bonadonna G, Brittinger G, et al. A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–94. doi: 10.1056/NEJM199309303291402
- Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 2007;109:1857–61. doi: 10.1182/blood-2006-08-038257
- Martelli M, Ferreri AJ, Agostinelli C, Di Rocco A, Pfreundschuh M, Pileri SA. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. 2013;87:146–71. doi: 10.1016/j.critrevonc.2012.12.009
- Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014;123:837–42. doi: 10.1182/blood-2013-09-524108
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403
- Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–8. doi: 10.1200/JCO.2006.08.2305
- Zhu SH, Zhang Y, Yu YH, Fu Z, Kong L, Han DL, et al. FDG PET-CT in non-small cell lung cancer: relationship between primary tumor FDG uptake and extensional or metastatic potential. Asian Pac J Cancer Prev. 2013;14:2925–9. doi: 10.7314/APJCP.2013.14.5.2925
- Arslan N, Tuncel M, Kuzhan O, Alagoz E, Budakoglu B, Ozet A, et al. Evaluation of outcome prediction and disease extension by quantitative 2-deoxy-2-[18F] fluoro-d-glucose with positron emission tomography in patients with small cell lung cancer. Ann Nucl Med. 2011;25:406–13. doi: 10.1007/s12149-011-0478-y
- Paidpally V, Chirindel A, Chung CH, Richmon J, Koch W, Quon H, et al. FDG volumetric parameters and survival outcomes after definitive chemoradiotherapy in patients with recurrent head and neck squamous cell carcinoma. AJR Am J Roentgenol. 2014;203:W139–45. doi: 10.2214/AJR.13.11654
- Adams HJ, de Klerk JM, Fijnheer R, Heggelman BG, Dubois SV, Nievelstein RA, et al. Prognostic superiority of the National Comprehensive Cancer Network International Prognostic Index over pretreatment whole-body volumetric-metabolic FDG-PET/CT metrics in diffuse large B-cell lymphoma. Eur J Haematol. 2015;94:532–9. doi: 10.1111/ejh.12467
- Sasanelli M, Meignan M, Haioun C, Berriolo-Riedinger A, Casasnovas RO, Biggi A, et al. Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2014;41:2017–22. doi: 10.1007/s00259-014-2822-7
- Esfahani SA, Heidari P, Halpern EF, Hochberg EP, Palmer EL, Mahmood U. Baseline total lesion glycolysis measured with (18)F-FDG PET/CT as a predictor of progression-free survival in diffuse large B-cell lymphoma: a pilot study. Am J Nucl Med Mol Imaging 2013;3:272–81.
- Kim TM, Paeng JC, Chun IK, Keam B, Jeon YK, Lee SH, et al. Total lesion glycolysis in positron emission tomography is a better predictor of outcome than the International Prognostic Index for patients with diffuse large B cell lymphoma. Cancer. 2013;119:1195–202. doi: 10.1002/cncr.27855
- Oh MY, Oh SB, Seoung HG, Kim JH, Kim SM, Kim TK, et al. Clinical significance of standardized uptake value and maximum tumor diameter in patients with primary extranodal diffuse large B cell lymphoma. Korean J Hematol. 2012;47:207–12. doi: 10.5045/kjh.2012.47.3.207
- Chihara D, Oki Y, Onoda H, Taji H, Yamamoto K, Tamaki T, et al. High maximum standard uptake value (SUVmax) on PET scan is associated with shorter survival in patients with diffuse large B cell lymphoma. Int J Hematol. 2011;93:502–8. doi: 10.1007/s12185-011-0822-y
- Xie M, Wu K, Liu Y, Jiang Q, Xie Y. Predictive value of F-18 FDG PET/CT quantization parameters in diffuse large B cell lymphoma: a meta-analysis with 702 participants. Med Oncol. 2015;32:446. doi: 10.1007/s12032-014-0446-1
- Schoder H, Noy A, Gonen M, Weng L, Green D, Erdi YE, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:4643–51. doi: 10.1200/JCO.2005.12.072
- Hutchings M, Loft A, Hansen M, Ralfkiaer E, Specht L. Different histopathological subtypes of Hodgkin lymphoma show significantly different levels of FDG uptake. Hematol Oncol. 2006;24:146–50. doi: 10.1002/hon.782
- Hyun SH, Ahn HK, Kim H, Ahn MJ, Park K, Ahn YC, et al. Volume-based assessment by (18)F-FDG PET/CT predicts survival in patients with stage III non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 2014;41:50–8. doi: 10.1007/s00259-013-2530-8
- Moon SH, Choi JY, Lee HJ, Son YI, Baek CH, Ahn YC, et al. Prognostic value of 18F-FDG PET/CT in patients with squamous cell carcinoma of the tonsil: comparisons of volume-based metabolic parameters. Head Neck 2013;35:15–22. doi: 10.1002/hed.22904
- Gallicchio R, Mansueto G, Simeon V, Nardelli A, Guariglia R, Capacchione D, et al. F-18 FDG PET/CT quantization parameters as predictors of outcome in patients with diffuse large B-cell lymphoma. Eur J Haematol. 2014;92:382–9. doi: 10.1111/ejh.12268
- Weber WA, Ziegler SI, Thodtmann R, Hanauske AR, Schwaiger M. Reproducibility of metabolic measurements in malignant tumors using FDG PET. J Nucl Med. 1999;40:1771–7.
- Li YM, Lin Q, Zhao L, Wang LC, Sun L, Dai MM, et al. Pre-treatment metabolic tumor volume and total lesion glycolysis are useful prognostic factors for esophageal squamous cell cancer patients. Asian Pac J Cancer Prev. 2014;15:1369–73. doi: 10.7314/APJCP.2014.15.3.1369
- Park GC, Kim JS, Roh JL, Choi SH, Nam SY, Kim SY. Prognostic value of metabolic tumor volume measured by 18F-FDG PET/CT in advanced-stage squamous cell carcinoma of the larynx and hypopharynx. Ann Oncol. 2013;24:208–14. doi: 10.1093/annonc/mds247
- Song MK, Chung JS, Shin HJ, Lee SE, Lee HS, Lee GW, et al. Clinical significance of metabolic tumor volume by PET/CT in stage II and III of diffuse large B cell lymphoma without extranodal site involvement. Ann Hematol. 2012;91:697–703. doi: 10.1007/s00277-011-1357-2
- Shipp MA. Prognostic factors in aggressive non-Hodgkin's lymphoma: who has ‘high-risk’ disease? Blood 1994;83:1165–73.
- Melchardt T, Troppan K, Weiss L, Hufnagl C, Neureiter D, Trankenschuh W, et al. A modified scoring of the NCCN-IPI is more accurate in the elderly and is improved by albumin and beta-microglobulin. Br J Haematol. 2015;168:239–45. doi: 10.1111/bjh.13116