Abstract
Rigid endoscopic resection using a thick sheath (ViewSite) may be a viable method for the resection or biopsy of selected deep-seated brain tumors, such as thalamic gliomas and malignant lymphomas. Neuroendoscopic biopsy is one technique used for the histological verification of suspected brain tumors. There are a number of advantages to using this technique. For example, under direct vision, it is possible to carefully observe the tumor surface and avoid vessels during tumor resections. It is also possible to collect enough specimens for a pathological diagnosis. Eighteen consecutive patients safely underwent rigid endoscopic resection or biopsy using a thick sheath (ViewSite). The two-handed endoscopic technique that utilized a mounted rigid endoscope proved very useful and safe, since it enabled easy stanching of hemorrhages. The aim of this study was to demonstrate how to use a thick sheath for deep-seated or intraventricular tumors. The rigid endoscopic approach with a thick sheath provides an alternative medial approach with improved visualization and a wider working space.
Introduction
Brain retraction is necessary for approaching deep-seated intracranial tumors. Greenberg introduced the first self-retaining retractor for neurosurgery in 1981.Citation1 Strong retraction may cause significant brain and vascular damage.Citation2,Citation3 Some reports have demonstrated that tubular retractors can help minimize retraction injury in adultCitation3–Citation5 and pediatric patients.Citation6 While the use of long retractors is necessary for deep lesions in the brain, they significantly reduce visualization. The rigid endoscopic technique using a thick tubular sheath provides an alternative medial approach that improves visualization and increases the working space. Furthermore, the two-handed technique is particularly useful for stanching hemorrhages. Although previous studies have described the procedures for this surgery, our study includes more cases, and for the first time, provides a detailed, step-by-step description of the techniques and demonstrates the amount of tissue that can be obtained when using this method.
Materials and Methods
A navigation system (BrainLab, Feldkirchen, Germany) was used to identify the entry and target points. The following instruments were used in this study: HOPKINS 2·7 mm endoscope (Karl Storz, Tuttlingen, Germany); ViewSite sheath part# TC171107 (width: 17 mm, height: 11 mm, length: 7 cm; Vycor Medical, OH, USA); sucker for a 2·0 or 2·5 mm endoscope (Fujita Medical Instrument Co., Tokyo, Japan); and a UniARM endoscope holder (Mitaka Kohki Co., Tokyo, Japan).
Surgical procedures using a thick sheath (ViewSite)
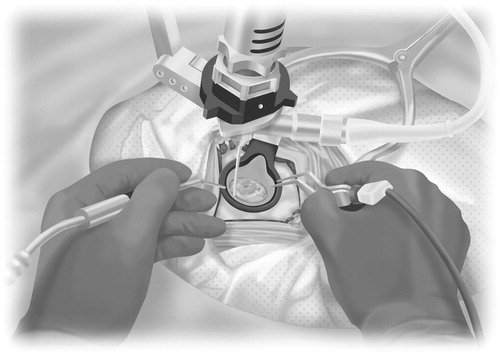
Straight incisions were performed following a small craniotomy with a single burr hole. The dural and pial entry points were coagulated with bipolar electrocautery forceps, and the dura mater was opened widely and sharply. A corticotomy was performed on the non-eloquent cortex, and crossing a sulcus was avoided. A sheath was inserted with the help of a navigation guide to the preoperatively set target (). A ventricular penetration needle was used to penetrate the ventricular wall since this is difficult to perform with the thick sheath. The sheath was then gently inserted under the guidance of the endoscope, because otherwise, the ventricular wall may have been pushed, or the ventricular shape may have been changed, or the sheath may have reached a location that we did not intend to reach. Suctioning of the ventricular wall, around the hole penetrated by the needle, was performed to create the surgical space. Double-handed manipulation during the operation was made possible using an endoscope holder ( and ). Dissection of the tumor from the normal brain tissue was easily conducted using this technique, similar to how it is performed under a microscope (). In the case of transcortical white matter tumor resection, the superficial part of the tumor (arrow) with some surrounding white matter (arrowheads) was visible through the transparent sheath (). Brain contusion in the trajectory was minimal. The diameter was approximately 15 mm or less immediately following sheath removal. After the operation, the trajectory continued to decrease.
Figure 1. (A), Photograph showing the procedures for inserting the clear plastic sheath (ViewSite) into the brain using a navigation system (BrainLab). (B) The bimanual (bipolar forceps and suction) technique as visualized through the endoscope into the sheath. (C) Intraoperative photographs showing the procedures for dissecting the tumor from the surrounding normal brain tissue using the bimanual technique. These procedures are similar to those used when performing the operation under a microscope. (D) The superficial part of the tumor (arrow) with some surrounding white matter (arrowheads) is visible through the transparent sheath. Preoperative [contrast-enhanced T1-weighted axial, (E)] magnetic resonance (MR) images revealing a right thalamic low-grade glioma prior to endoscopic resection. Subtotal resection was achieved. (F) Postoperative computed tomography axial images obtained in a patient who underwent subtotal endoscopic resection of a thalamic low-grade astrocytoma; the patient was neurologically unchanged after surgery.
![Figure 1. (A), Photograph showing the procedures for inserting the clear plastic sheath (ViewSite) into the brain using a navigation system (BrainLab). (B) The bimanual (bipolar forceps and suction) technique as visualized through the endoscope into the sheath. (C) Intraoperative photographs showing the procedures for dissecting the tumor from the surrounding normal brain tissue using the bimanual technique. These procedures are similar to those used when performing the operation under a microscope. (D) The superficial part of the tumor (arrow) with some surrounding white matter (arrowheads) is visible through the transparent sheath. Preoperative [contrast-enhanced T1-weighted axial, (E)] magnetic resonance (MR) images revealing a right thalamic low-grade glioma prior to endoscopic resection. Subtotal resection was achieved. (F) Postoperative computed tomography axial images obtained in a patient who underwent subtotal endoscopic resection of a thalamic low-grade astrocytoma; the patient was neurologically unchanged after surgery.](/cms/asset/0722a156-31f9-4db0-8468-f6cef7003679/yner_a_11746983_f0001_b.jpg)
Figure 2. Schematic diagram showing the procedures for using the bipolar forceps and suction bimanually with a fixed rigid endoscope. The bimanual (bipolar forceps and suction) technique as visualized through the endoscope into the sheath.
The resected tumor volume was assessed by subtracting the residual tumor volume from the preoperative volume on gadolinium-enhanced T1-weighted magnetic resonance (MR) images pre- and postoperatively. Postoperative MR images were acquired within 3 days after surgery.
Results
Our 18 cases are summarized in . We were able to collect sufficient specimens for pathological diagnosis in all 18 patients. None of the patients had any complications after surgery.
Table 1. Patient characteristics
An illustrative case
A 62-year-old male patient presented with a narrowing right-sided visual field and left-sided facial dysesthesia. Magnetic resonance (MR) imaging revealed a right thalamic mass with no enhancement (). Transventricular surgical resection through the right posterior horn of the lateral ventricle was performed. Subtotal removal could be performed without any postoperative deterioration of the patient’s neurological deficits (). The pathological diagnosis was diffuse astrocytoma. The MIB-1 index was 20%. Radiochemotherapy was initiated.
Discussion
Previous studies have reported novel techniques for the pure endoscopic removal of brain tumors.Citation7–Citation10 Harris et al. demonstrated frame-based stereotactic techniques for intraventricular tumors.Citation7 Considering the small diameter of endoscopes and the high number of available instruments for endoscopic surgery, it is possible to use this technique for almost complete resection of tumors, even thalamic lesions. The conduit (sheath) creates a space that allows for bimanual dissection, a concept that was initially developed and modified for the stereotactic tubular retraction system.Citation11–Citation13 Microsurgical techniques with a similar translucent tubular retractor were reported for deep-seated or ventricular brain tumors.Citation14 Some authors showed usefulness of the same retractor for deep-seated tumors in the brain;Citation5,Citation6 neuroendoscopic techniques with a similar tubular retractor for third ventricular tumorsCitation15,Citation16 and intraparenchymal tumorsCitation17 were also reported. Recently, another report demonstrated the use of the same retractor.Citation18 Collectively, these reports emphasize the usefulness of the translucent tubular retractor for ventricular and deep-seated brain tumors. While the procedures for this surgery have been published previously,Citation15–Citation18 our study provides data from more cases and offers a more detailed, step-by-step description of the techniques than previous studies. We also show, for the first time, how much tissue can be obtained when using this method.
There are a number of advantages to using this technique. For example, under direct vision, it is possible to carefully observe the tumor surface and avoid vessels during tumor resections. It is also possible to collect enough specimens for a pathological diagnosis. Ten of the 18 cases described in this article only underwent biopsies. Their diagnoses were malignant lymphoma, germinoma, or demyelinating tissues. With these diagnoses, additional resections should not be performed because removing additional tissue does not induce a better outcome. Although a stereotactic or neuronavigational needle biopsy might have been sufficient and less invasive in these cases, at least one study has suggested that stereotactic needle biopsy specimens may not provide a sufficiently accurate diagnosis to reliably guide the treatment of brain neoplasms.Citation19 In general, the accuracy rate of the stereotactic needle biopsy is 76–90%.Citation19–Citation22 The additional tumor tissue that can be removed safely with a tubular retractor for deep-seated brain tumors such as malignant lymphoma may not provide a better prognosis for longer life, but can provide a more accurate diagnosis, which could offer information about whether an additional tumor resection should be performed, for example, in malignant gliomas. The major limitation of using stereotactic techniques is related to the lack of intraoperative visualization and direct monitoring of the procedures, as well as to changes in the intracranial coordinates following cerebrospinal fluid loss during the management of intraventricular lesions.
Using the inserted route, we could observe some hemorrhages directly, and thus manipulate them while gradually pulling out the sheath. However, there are some disadvantages to using this technique. For one, the brain contusion on the trajectory to the tumor is larger in comparison to that using stereotactic biopsy. That said, we never experienced permanent or serious adverse effects when a particular trajectory was carefully planned. Hemorrhage control during endoscopic neurosurgery is critical due to the lack of suitable instruments for coagulation. Bleeding may cause significant visual deterioration during an endoscopic procedure. Moreover, it was slightly more challenging to manipulate some instruments in sheaths using endoscopic two-dimensional monitoring compared to using three-dimensional microscopic procedures; however, the learning curve could be satisfactorily overcome.
We emphasize that rigid endoscopic resection is a very useful and safe approach for removing a target specimen. Rigid endoscopes, in comparison to flexible endoscopes, make bimanual procedures possible, which in turn makes it easier to control bleeding during operations (). Therefore, we believe that this technique is a safer, more reliable, and less invasive method for the treatment of deep-seated brain tumors.
Conclusions
Rigid endoscopic resection may be a viable method for the resection of certain deep-seated brain tumors, such as thalamic gliomas and malignant lymphomas. Further experience with this technique will help to determine its applicability and safety.
Disclaimer Statements
Contributors All authors are aware of and have consented to the submission. Each author has participated sufficiently in this article to take public responsibility for the content relevant to their contributions as following: analyzing the data and writing the article by Dr Akiyama as corresponding author, collecting the data by Dr Horita, Komatus, Suzuki, and Otaki, analyzing the data by Dr Wanibuchi and Mikami, and revising the article by Dr Mikuni.
Funding None.
Conflicts of interest None of the authors have any conflict of interest concerning the financial, material, or methods used in this study.
Ethics approval This study was approved by the Sapporo Medical University Institutional Review Board (IRB # 25-103).
References
- Greenberg IM. Self-retaining retractor and handrest system for neurosurgery. Neurosurgery. 1981;8(2):205–8.
- Rosenørn J, Diemer NH. Reduction of regional cerebral blood flow during brain retraction pressure in the rat. J Neurosurg. 1982;56(6):826–9.
- Zhong J, Dujovny M, Perlin AR, Perez-Arjona E, Park HK, Diaz FG. Brain retraction injury. Neurol Res. 2003;25(8):831–8.
- Greenfield JP, Cobb WS, Tsouris AJ, Schwartz TH. Stereotactic minimally invasive tubular retractor system for deep brain lesions. Neurosurgery. 2008;63(4 Suppl 2):334–9.
- Raza SM, Recinos PF, Avendano J, Adams H, Jallo GI, Quinones-Hinojosa A. Minimally invasive trans-portal resection of deep intracranial lesions. Minim Invasive Neurosurg. 2011;54(1):5–11.
- Recinos PF, Raza SM, Jallo GI, Recinos VR. Use of a minimally invasive tubular retraction system for deep-seated tumors in pediatric patients. J Neurosurg Pediatr. 2011;7(5):516–21.
- Harris AE, Hadjipanayis CG, Lunsford LD, Lunsford AK, Kassam AB. Microsurgical removal of intraventricular lesions using endoscopic visualization and stereotactic guidance. Neurosurgery. 2005;56(1 Suppl):125–32.
- Kassam AB, Engh JA, Mintz AH, Prevedello DM. Completely endoscopic resection of intraparenchymal brain tumors. J Neurosurg. 2009;110(1):116–23.
- Moshel YA, Link MJ, Kelly PJ. Stereotactic volumetric resection of thalamic pilocytic astrocytomas. Neurosurgery. 2007;61(1):66–75.
- Sood S, Hoeprich M, Ham SD. Pure endoscopic removal of pineal region tumors. Childs Nerv Syst. 2011;27(9):1489–92.
- Kelly PJ, Goerss SJ, Kall BA. The stereotaxic retractor in computer-assisted stereotaxic microsurgery. J Neurosurg. 1988;69(2):301–6.
- Otsuki T, Jokura H, Yoshimoto T. Stereotactic guiding tube for open-system endoscopy: a new approach for the stereotactic endoscopic resection of intra-axial brain tumors. Neurosurgery. 1990;27(2):326–30.
- Russell SM, Kelly PJ. Volumetric stereotaxy and the supratentorial occipitosubtemporal approach in the resection of posterior hippocampus and parahippocampal gyrus lesions. Neurosurgery. 2002;50(5):978–88.
- Cohen-Gadol AA. Minitubular transcortical microsurgical approach for gross total resection of third ventricular colloid cysts: technique and assessment. World Neurosurg. 2013;79(1):e7–10.
- Jho HD, Alfieri A. Endoscopic removal of third ventricular tumors: a technical note. Minim Invasive Neurosurg. 2002;45(2):114–9.
- Engh JA, Lunsford LD, Amin DV, Ochalski PG, Fernandez-Miranda J, Prevedello DM, et al. Stereotactically guided endoscopic port surgery for intraventricular tumor and colloid cyst resection. Neurosurgery. 2010;67(3 Suppl Operative): ons198–204; discussion ons204–5.
- Akai T, Shiraga S, Sasagawa Y, Okamoto K, Tachibana O, Lizuka H. Intra-parenchymal tumor biopsy using neuroendoscopy with navigation. Minim Invasive Neurosurg. 2008;51(2):83–6.
- Kishida Y, Sato T, Oda K, Ichikawa M, Sakuma J, Saito K. Pure endoscopic resection of deep intracranial tumors using the ViewSite. No Shinkei Geka. 2014;42(4):311–25.
- Jackson RJ, Fuller GN, Abi-Said D, Lang FF, Gokaslan ZL, Shi WM, et al. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro Oncol. 2001;3(3):193–200.
- Grunert P, Ungersböck K, Bohl J, Kitz K, Hopf N. Results of 200 intracranial stereotactic biopsies. Neurosurg Rev. 1994;17(1):59–66.
- Woodworth G, McGirt MJ, Samdani A, Garonzik I, Olivi A, Weingart JD. Accuracy of frameless and frame-based image-guided stereotactic brain biopsy in the diagnosis of glioma: comparison of biopsy and open resection specimen. Neurol Res. 2005;27(4):358–62.
- Owen CM, Linskey ME. Frame-based stereotaxy in a frameless era: current capabilities, relative role, and the positive- and negative predictive values of blood through the needle. J Neurooncol. 2009;93(1):139–49
