Abstract
The kinetics of the martensitic transformation in Fe–0·80C has been determined from dilatometry data and shows no significant variation when the cooling rate is changed by two orders of magnitude. All kinetic data can be adequately simulated by the Koistinen and Marburger (KM) equation using a specific start temperature TKM and rate parameter αm. This finding supports the suggestion that the transformation is athermal, and moreover, the absence of a time dependence strongly indicates that autocatalytic nucleation does not contribute to the transformation kinetics in plain carbon steels on measurable time scales. Furthermore, dilatometry experiments with different austenitising conditions were conducted to examine the effect of the prior austenite grain size on the overall kinetics of martensite formation. The present results indicate that the progress of martensite formation beyond a fraction f = 0·15 is independent of the prior austenitising treatment. It is therefore concluded that austenite–austenite grain boundaries have no significant effect on the overall nucleation and growth of athermal martensite, which is consistent with a model proposed by Ansell and co-workers.
Introduction
Although much research has been performed on martensite formation in steels,Citation1–Citation6 the exact mechanisms by which martensite nucleates and grows are not yet understood. Experimental investigations and their interpretation have proven to be complicated since the growth of martensite is very fast, and therefore each nucleation event directly leads to the formation of a typical volume of the new phase. Regarding the transformation kinetics, three different types of behaviour have been identified. These kinetic modes are athermal, isothermal, and burst kinetics. Although some common features of the three kinetic modes are known, a unifying kinetic theory does not yet exist.Citation7–Citation9 Moreover, there seems to be no general consensus regarding some fundamental aspects of the transformation.Citation10,Citation11
Athermal martensite is usually observed in low-alloy carbon steels.Citation8 As argued by Entwisle,Citation7 the apparent independence of martensitic athermal transformation on time might be explained by extrapolating the typical isothermal transformation kinetics observed at low temperatures in Fe–Ni–Mn alloys to the higher temperatures which are comparable to the Ms temperature of alloys exhibiting athermal kinetics. Such a translation would lead to transformation times of less than 1 ms and thus not resolvable with conventional experimental capabilities.Citation7
Isothermal martensite is typically formed in high-Ni iron based alloys at sub-zero temperatures.Citation9 The isothermal transformation can be preceded by the formation of a volume fraction of athermal or burst martensite formed during cooling to the holding temperature.Citation12 In some alloys with Mn addition a truly isothermal transformation has been reported.Citation7
In the formation of burst martensite, which is probably the least-understood transformation mode, the transformation occurs abruptly at a certain temperature during cooling of the austenite.Citation9,Citation13,Citation14 The fraction formed at the burst temperature can vary from a few percentage to more than 50 vol.-%, and this burst transformation can take place within a millisecond. This chain reaction of the formation of plates is usually regarded as an extreme form of autocatalysis.Citation9
In the comprehensive review by RaghavanCitation9 it is argued that the present understanding of the martensite kinetics is largely confined to the isothermal mode, with a limited extension of the concepts to the athermal and burst modes. In the case of athermal martensite, the nucleation is often believed to take place at structural imperfections in the parent phase and these defects are stimulated to grow into martensite crystals at different degrees of undercooling below Ms.Citation8 However, the nature of the defect sites at which heterogeneous nucleation occurs is still not well understood. In addition to nucleation at pre-existing defects, the overall transformation rate can, in principle, be enhanced by so called autocatalytic nucleation: the stimulus of nucleation by previously formed martensite crystals. In addition the mechanism of this type of nucleation is not fully understood.
Based on the considerations discussed in Ref. 15 it seems plausible to assume that the freshly formed martensite strengthens the surrounding austenite during its formation, and that this mechanical stabilisation impedes the plastic accommodation of the subsequent transformation. When a martensite lath or plate is formed, dislocations are generated in the surrounding austenite by plastic deformation due to the volumetric and shear strains.Citation9 On the one hand, it is sometimes assumed that the dislocations produced in the austenite during transformation can assist the subsequent nucleation of martensite, which is known as autocatalytic nucleation.Citation16–Citation19 However, the dislocations induced in the austenite due to prior transformation can also retard the subsequent transformation to martensite, which is known as mechanical stabilisation.Citation4,Citation20–Citation23 The dislocation debris interferes with the movement of the glissile interface that constitutes the growth. When the strain build-up in the remaining austenite accompanying the transformation exceeds a critical value, the further transformation at a certain temperature below Ms is suppressed.Citation22 In this sense mechanical stabilisation can play an essential role in the athermal character of the martensitic transformation in low-alloyed carbon steels.Citation15
When the athermal martensitic transformation is interrupted at a temperature below Ms by stopping the cooling, the remaining austenite is often observed to decompose; however, there is not yet consensus on the exact transformation mechanism and the classification of this isothermal transformation product in low-alloyed steels. It has frequently been argued that isothermal martensite forms below Ms.Citation24,Citation25 In contrast, in another investigationCitation26 it was shown that below Ms the remaining austenite continues to decompose isothermally with a certain rate, which is in agreement with the kinetics of bainite formation above Ms. Based on this it was concluded that isothermal bainite can be formed below Ms, which was supported by SEM images showing a mixed martensite–bainite microstructure.Citation26 A renewed literature survey was conducted by the present authors which shows that the isothermal decomposition to bainite below Ms was already suggested by Davenport and Bain in 1930,Citation27 although no experimental evidence was shown. Some years later Howard and CohenCitation1 investigated the transformation kinetics and microstructures formed both above and below Ms in plain carbon steels with approximately 1 wt-%. Based on their data, TTT diagrams were constructed displaying isothermal kinetics below and above Ms and they concluded that ‘the bainite formation below Ms takes place at a rate more or less predictable from the normal shape of the bainite curves extending down from above Ms’, which is fully consistent with interpretation of the results for Fe–0·66C discussed elsewhere.Citation26 Howard and Cohen found no evidence for isothermal martensite formation in steels with 0·75–1·35 wt-%C at temperatures below Ms.Citation1
In the first part of the present work the possible time dependence of the martensitic transformation will be investigated by applying different cooling rates to Fe–0·80C samples. In the analysis of the results special attention will be paid to the influence of possible instrumental effects like thermal gradients across the sample length. Furthermore, the influence of autotempering on dilatometry results is discussed. In the second part the kinetics of the transformation are determined from the dilatometry data and compared with simulations using the Koistinen Marburger (KM) equation.Citation28
In the final part of this investigation it is attempted to get more insight into the role of the austenite grain size and grain boundaries in the nucleation and growth of martensite. Although it is known that microstructural features such as the grain size can cause variations in the Ms temperature,Citation29–Citation35 little systematic work has been conducted to quantify the effect of the grain size on the progress of the transformation below Ms. In the present paper, dilatometry measurements are analysed to quantify the effect of the austenite grain size on both the Ms temperature and the kinetics of the transformation below Ms.
Experimental
Steel Fe–0·80C used in this study has a chemical composition that is close to the eutectoid composition as shown in . The dilatation of the samples as a function of temperature and time was measured using a Bähr 805A/D dilatometer.Citation36 Cylindrical dilatometric samples were machined of 10 mm in length and 5 mm in diameter. The temperature was controlled using a thermocouple spot-welded onto the middle of the sample. In order to get an estimate of the thermal gradient in the axial direction of the sample a second thermocouple was welded approximately 4 mm from the centre of the sample. In of Ref. 36, a schematic drawing of the sample in the dilatometer is presented, which shows the position of the two thermocouples. Results of repeated heat treatments with different samples sometimes showed differences in the temperature at which the onset of expansion during cooling appeared (the martensite-start temperature), and these differences seemed to correlate with changes in the thermal gradient along the sample length. Despite these differences in Ms the characteristics of the dilatometry curves below Ms were very similar. In contrast to these irreproducible variations in Ms, repeated heat treatments with the same sample did not show any anomalous results. It is noted that Tsuzaki et al. observed similar differences when different samples were used.Citation37 Analysis showed that these systematic errors are probably due to inaccuracies in the spot-welding of the thermocouples on the sample, and the exact position of the thermocouple wires relative to the nozzles of the He quench gas spray system. In order to avoid these errors and to ensure an unambiguous analysis, all experiments were conducted with the same sample.
Table 1. Chemical composition of steel Fe–0·80C/wt-%
In the experiments with varying cooling rates, the sample was heated with 10°C s−1 to a temperature of 900°C, and austenitised for 2 min at a pressure of less than 5×10−5 mbar. Subsequently, the sample was cooled using He gas to 500°C with an average cooling rate between 800 and 500°C of approximately 75°C s−1. Below 500°C the cooling rate was decreased gradually to approximately 15°C s−1 at 400°C with the aim to minimise the temperature differences in the sample before the transformation to martensite starts. The dilatation signal as a function of temperature indicated that no ferrite or bainite formation occurred at the cooling rates employed.
To investigate the possible effect of the cooling rate on the transformation kinetics, the sample was cooled from approximately 400°C to room temperature with five different cooling rates in the range of 0·2 to 12°C s−1 (see ). For cooling rates higher than 2°C s−1, He quench gas was applied; the lowest cooling rates required induction heating. A number of experiments were conducted with natural cooling subsequent to the initial fast cool. This gave highly reproducible results, not only for the experiments with the same sample but also in the case that different samples were used. Therefore, the natural cooling conditions are considered to give results that are least susceptible to inaccuracies due to temperatures differences in the sample. Natural cooling in an He atmosphere results in a cooling rate of approximately 2°C s−1 close to Ms and decreases gradually to 0·3°C s−1 just above room temperature.
Table 2. Experimental details and results of experiments with varying cooling rates
Experiments with different austenitising conditions were conducted to examine the effect of the prior austenite grain size on the kinetics of martensite formation in Fe–0·80C. The five austenitising treatments discussed in detail in this paper are listed in . After austenitising in the range 800–1050°C, the samples were cooled with the natural cooling conditions explained above.
Table 3. Experimental details and results of experiments with varying austenitising treatments*
Results
Influence of cooling rate on dilatometry measurements
The dilatation curves measured as a function of temperature for the five investigated cooling rates are shown in . It is seen that the three dilatometry curves for cooling with He quench gas, the cooling rates 2, 5 and 12°C s−1, are mutually not very different, but they are distinctly different from the two dilatation signals measured for the slowest cooling rates (0·2°C s−1 and natural cooling). This difference between the experiments with and without He quench gas can be attributed to the thermal gradients in the sample, which are depicted in the same figure. The right-hand y axis of shows the temperature difference ΔT measured between the centre and the edge of the sample. It is seen that ΔT is less than 3°C near Ms for the experiments without active cooling. In contrast, ΔT is quite large for the experiments using He quench gas: ΔT is approximately 18°C near Ms, and increases somewhat for the higher cooling rates (see ). It should be noted that the maximum thermal gradient in the sample may even be somewhat larger than measured with the two thermocouples. For the measurements with active cooling it is also seen that just below Ms the thermal gradient decreases more strongly: the ΔT curve exhibits a small dip. This behaviour may be due to the release of latent heat accompanying the martensitic transformation. The regions near the edge of the sample with the lowest temperature will transform first, and the local heat release increases the temperature, which reduces ΔT. Based on above observations it is concluded that the dilatometry curves measured during active cooling of samples do not always give a very accurate measure of the kinetics of the transformation. The Ms temperature, as determined from the change in slope of these dilatometry curves,Citation38 is about 15°C higher than the Ms temperature evaluated from the more accurate measurements performed without the use of He quench gas (see ).
1. a measured dilatation as a function of temperature due to martensite formation during cooling of Fe–0·80 samples with different rates. Right-hand y-axis shows measured temperature difference between two thermocouples (ΔT) and b dilatometry data plotted against temperature corrected for thermal gradients
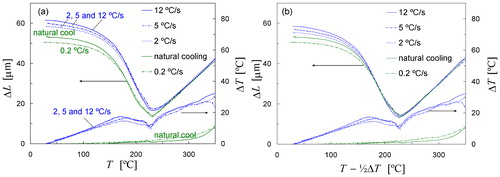
The average sample temperature of quenched samples is lower than the temperature recorded with the control thermocouple in the middle of the sample. An estimate of the average sample temperature is needed to correct for the influence of thermal gradients on the dilatometry curves. In the present work it is assumed that the average sample temperature can be approximated by T–½ΔT. shows the dilatometry data plotted against this corrected temperature. Owing to this correction all five dilatometry curves are very similar in the temperature range 160–240°C. Although the correction for thermal gradients indicates that the cooling rate has no influence on the kinetics of martensite formation at the beginning of transformation (see ), it is seen that in the final stage of transformation (below 160°C) the dilatation becomes significantly smaller with decreasing cooling rate. This difference in kinetics is thought to be related to autotempering of martensite: the tempering of the as formed martensite during subsequent slow cooling. Tempering of martensite, and therefore also autotempering, is accompanied with a volume decrease.Citation39 Moreover, this thermally activated process is stronger for the lower cooling rates. In fact, there are two simultaneous effects on the measured dilatation due to autotempering. At the relatively low temperatures at which the martensite is formed, the so called first stage of tempering is probably dominant,Citation40 which means partitioning of carbon to dislocations or the remaining austenite. In addition, carbide formation takes place, and both processes involved in tempering result in a negative change in lengthCitation39 and can thus explain the smaller dilatation for the lower cooling rates. The magnitude of the dilatation due to tempering will be discussed later in this section.
Furthermore, at very low temperatures the carbide formation may become very sluggish, and consequently the austenite is stabilised when carbon partitions from the supersaturated martensite to the austenite. Such chemical stabilisation of the austenite implies that the transformation shifts to lower temperatures. The resultant decrease in the transformation kinetics also results in a smaller dilatation signal. In relation to this, it is noted that in the literature the stabilisation of austenite due to a lower cooling rate or interrupted cooling has sometimes been called thermal stabilisation of austenite.Citation41
In order to further test this explanation of the differences in dilatation in terms of the degree of autotempering taking place during cooling, both isothermal aging and heating experiments were conducted to obtain an estimate of the dilatation owing to the tempering of martensite. To investigate tempering under isothermal conditions, a sample of Fe–0·80C was first fully transformed to martensite by quenching in liquid nitrogen, and subsequently heated with 20°C s−1 to 180°C in the dilatometer and isothermally annealed at that temperature for 5 min. The result shown in indicates that the sample length decreases approximately 2–2·5 μm in some tens of seconds. This estimate of the magnitude of the length decreases owing to tempering is in fair agreement with the differences observed between dilatometry curves for natural cooling and cooling with 0·2°C s−1. The second contribution of autotempering to the dilatation, the stabilisation of the remaining austenite owing to autotempering during cooling, cannot be simulated with an additional experiment, but may also have contributed to the difference between the two experiments without active cooling.
2. a dilatation due to tempering of fully martensitic sample at 180°C as a function of time: length decrease of about 2–2·5 μm in some tens of seconds and b dilatation as a function of temperature showing tempering characteristics during heating of samples which were made martensitic with different cooling rates as shown in
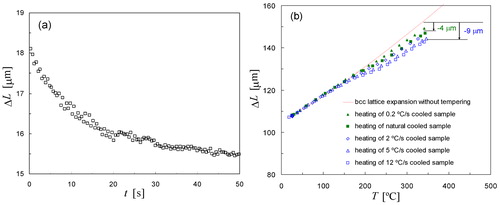
As discussed above, repeated heat treatments with the same sample were conducted because this proved to yield the most reproducible results. Thus all samples, for which the dilatometry data for different cooling rates are shown in , were heated with 10°C s−1 in a subsequent experiment. shows that the degree of tempering of samples depends on the cooling rate in the previous experiment. As a reference, the solid line (red) shows the thermal expansion of samples in the absence of tempering. Samples cooled with He quench gas (open blue symbols) show a stronger tempering behaviour during heating than the samples that were cooled slowly (solid green symbols). The susceptibility to tempering of a martensitic sample during heating depends on the degree of autotempering that occurred during cooling. Thus the relatively small tempering during heating of slowly cooled samples indicates that the martensitic microstructure was already autotempered to a certain degree due to the slow cooling.
Kinetics of martensite formation
By applying different cooling rates it has been shown that the progress of martensite formation has no time-dependence, at least not on experimentally accessible time scales, which confirms the athermal character of the transformation. It seems to be widely accepted that the extent of transformation as a function of temperature can be well described by the Koistinen and Marburger equationCitation8,Citation15,Citation28(1) in which αm is a rate parameter, and TKM is the theoretical martensite-start temperature, which is typically somewhat lower than the conventional (experimental) Ms determined as the onset of expansion during cooling in a dilatometry experiment. As discussed earlier, the experiments for continuous natural cooling are most accurate and therefore the measured dilatation during natural cooling after austenitising at 900°C (see ) is used for a further quantitative analysis using the KM equation. These data are shown by the open diamonds in . The sample cooled to room temperature was investigated by X-ray diffraction (XRD) to confirm the presence of retained austenite in the microstructure, and quantitative analysis of the XRD pattern yielded a volume fraction austenite of 0·10±0·02, which is comparable to results of previous investigations on steels with a similar carbon content.Citation28,Citation42
3. a measured dilatation as a function of temperature due to martensite formation during natural cooling (open squares). Solid lines represent thermal expansion of both austenite (fcc) and martensite (bcc); open circles describe dilatation of fully martensitic sample during heating and b experimental volume fraction of martensite as a function of temperature derived from Fig. 3a (open squares) can be described by KM equation (solid line) using TKM = 218°C, αm = 0·0122 K−1. Volume fraction of retained austenite at room temperature is approximately 0·10
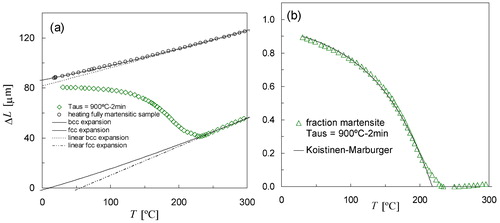
The martensite transformation kinetics has been evaluated from dilatometry data with the Lever rule (the linear law of mixtures) applied to the data using the non-linear equations for the thermal contraction of bcc and fcc lattices (solid lines) derived in Ref. 43. The non-linear thermal expansion of bcc phaseCitation43 is found to agree well with the dilatation data measured during heating of a Fe–0·80C sample that was made fully martensitic (see open circles in ). This sample was produced by rapid cooling to room temperature, followed by quenching in liquid nitrogen. For comparison, to demonstrate the importance of using the non-linear expressions of thermal expansion, the dashed lines in represent linear approximations for thermal expansion, which are seen to have a less adequate agreement with the experimental data over the whole temperature range. The fraction retained austenite of 10 vol.-% at room temperature, which was derived from XRD measurements, specifies the relation between the dilatation and the bcc phase fraction at room temperature. This means that for the present analysis a length change ΔLα−γRT = 86 μm (see ) is caused by 100% austenite-to-martensite transformation. shows that the thermal expansion of 100% bcc phase (martensite) is significantly larger than the slope of the dilatometry curve just above room temperature, which indicates that the transformation is not finished at room temperature.
The volume fraction of martensite as a function of temperature as represented by the open triangles in shows the best agreement with the KM equation using TKM = 218°C, αm = 0·0122 K−1. The rate parameter found for Fe–0·80C is higher than the well known value of 0·011 K−1 obtained by Koistinen and Marburger for plain carbon steels with 1 to 1·1 wt-% carbon.Citation28 The differences in the values of αm for different steels are thought to be related to their chemical composition,Citation44 in particular the carbon content.Citation45 The rate parameter αm = 0·0122 K−1 derived from the dilatometry data is comparable to the value αm = 0·0129 K−1 predicted using the equation proposed in.Citation45
shows the volume fraction of martensite as a function of temperature corresponding to the dilatation signal shown in . It is seen that the volume fraction calculated with the KM equation (solid line) is in very good agreement with the experimental result except for some deviations near the start of the transformation. The Ms temperature as derived from the change in slope is approximately 230°C, which is 12°C higher than the value of TKM = 218°C determined from the best fit simulated with the KM equation. It is noted that this gradual beginning of the transformation can only partly be explained by temperature differences in the sample. In principle, thermal gradients in the sample lead to localised transformations as the sample approaches the martensite-start temperature, and this may effectively cause an apparent gradual beginning of the transformation. However, for the experiment with natural cooling the thermal gradient measured over the sample length is very small, approximately 2°C near the Ms temperature. A deviation of 12°C in the martensite start temperature can only be caused by temperature gradients of at least 12°C, whereas the experiments show that the gradients are not more than 2°C. This means that thermal gradients in the sample cannot fully explain the gradual beginning of transformation, which is different from the modelled kinetics. In the following section it will be demonstrated that the temperature range where the gradual start of the transformation occurs is dependent on the austenitising treatment.
Effect of prior austenite grain size on martensitic transformation
shows the dilatation curves measured during cooling of five samples that were given different austenitising treatments in order to change the prior austenite grain size. The estimated grain sizes of the samples are listed in . shows that distinct differences are observed in the early stage of transformation. The inset of shows that the Ms temperature derived from the change in slope increases from Ms = 221°C for Taus = 800°C to Ms = 238°C for Taus = 1050°C (see ). It is noted that each experimental result is to some extent subject to thermal gradients, but the measured temperature difference ΔT between the centre and the edge of the sample is always smaller than 3°C in the temperature range of transformation. Furthermore, ΔT is approximately the same for all measurements. The observation that Ms depends on the austenite grain size is consistent with results reported previously for various steels.Citation29–Citation35 However, a systematic study of the effect of the austenite grain size on the kinetics of martensite formation has not been reported in the literature. Only a qualitative description of this gradual beginning of transformation in relation to the austenitising treatment has been given by Sastri and West.Citation29
4. Change in length as a function of temperature due to martensite formation in Fe–0·80C during natural cooling of samples which were given different austenitising treatments. Inset shows magnification of dilatometry curves close to Ms temperature
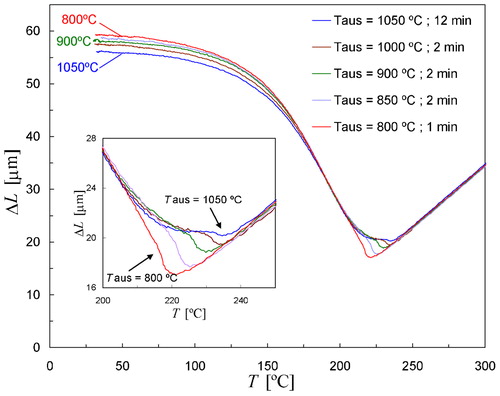
The small differences in the dilatometry curves of at the final stage of transformation are possibly due to different degrees of transformation plasticity for different austenite grain sizes. Analysis of the sample length after multiple thermal cycles on a single sample frequently showed that the sample becomes about 2 μm shorter in the case Taus = 800°C, and approximately 8 μm shorter when Taus = 1050°C. The final microstructures of four samples with different prior austenite grain sizes were investigated by XRD, and a similar volume fraction of retained austenite was found in each sample as shown in .
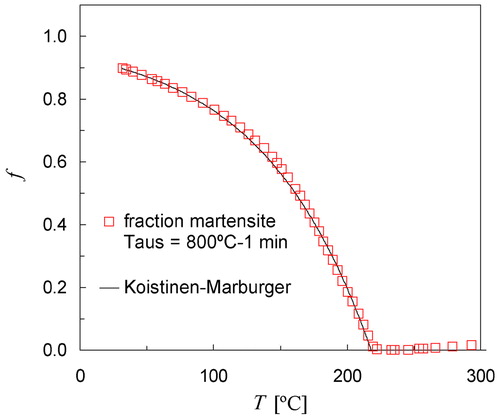
The fraction of martensite as a function of temperature was determined from the five dilatation curves shown in using the Lever rule as demonstrated earlier for Taus = 900°C (see ). The fraction curve for the smallest austenite grain size (Taus = 800°C) is shown in . The solid line through the data represents best fit to the KM equation calculated with TKM = 218°C, αm = 0·0122 K−1. The Ms temperature as derived from the change in slope is approximately 221°C, which is only 3°C higher than TKM = 218°C. It is noted that this deviation can be fully explained by temperature differences in the sample. It is therefore concluded that for Taus = 800°C the gradual beginning of the transformation is not significant, whereas for Taus = 850°C and higher a distinct deviation is found (see ).
5. Volume fraction of martensite as a function of temperature derived from measured dilatation data shown in for Taus = 800°C (open squares). Solid line represents progress of transformation calculated with KM equation using TKM = 218°C, αm = 0·0122 K−1
The overall transformation kinetics for the other three austenitising temperatures can also be well described using KM equation by taking TKM = 218°C, and αm = 0·0122 K−1 (see ). These model parameters lead to the best agreement in the temperature range from room temperature to Ms, although some discrepancies are observed close to Ms depending on Taus. shows all five experimental fraction curves corresponding to the inset of , and the best simulation for the transformation kinetics. As demonstrated in , for Taus = 800°C the measured fraction curve agrees very well with the calculated kinetics at each stage of the transformation. With increasing austenite grain size the agreement with the KM model becomes poorer for the early stages of transformation (see ), and the maximum deviations are observed for Taus = 1050°C. Austenitising at Taus = 800°C for 1 min resulted in Dγ≈12 μm (). It was, however, not possible to investigate the martensite kinetics for even smaller grain sizes because for very small grain sizes it becomes impossible to prevent bainite formation during the fastest cooling. The kinetics of martensite formation was also investigated for grain sizes somewhat smaller and larger than the largest in , Dγ = 130 μm. It was found that the dilatometry curve measured after austenitising at Taus = 1050°C for 5 min (Dγ≈100 μm) or 18 min (Dγ≈150 μm) did not differ significantly from the result for taus = 12 min shown in although the austenite grain size was different.
6. Martensite fraction as a function of temperature corresponding measured dilatation signals shown in inset of . Black line represents transformation kinetics calculated with KM equation using TKM = 218°C, and αm = 0·0122 K−1
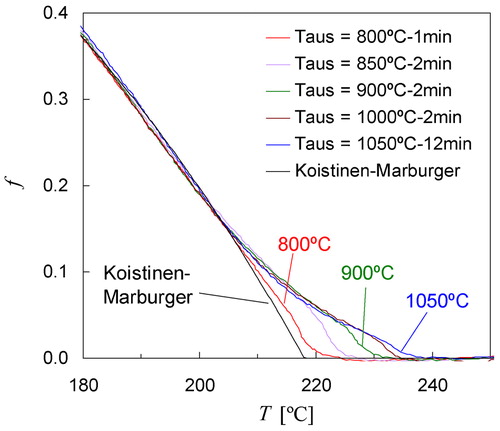
Based on abovementioned observations it is concluded that the effect of the austenite grain size on the start temperature is large when the grain size is small and diminishes as the grain size increases. This effect on Ms is consistent with the observations reported elsewhere.Citation29,Citation30,Citation32,Citation35 In addition to the effect on Ms, it is seen in and that the initial transformation rate between Ms and TKM decreases when the austenite grain size increases. Similar observations have been reported by Sastri and West,Citation29 and indicate that not only Ms, but also the early stages of the martensitic transformation just below Ms are dependent on the initial austenite grain structure. The fraction curves in also indicate that the progress of martensite formation beyond f = 0·15 (i.e. below 205°C) is nearly independent of the prior austenitising treatment. It can therefore be concluded that the major part (85%) of the overall kinetics is not significantly influenced by the prior austenite grain size.
Discussion
Autocatalytic nucleation
By applying different cooling rates it has been shown in the foregoing that the martensite formation has no time-dependence, at least not on experimentally accessible time-scales (). This may imply that the nucleation process takes place without thermal activation, i.e. the activation energy is zero in the temperature range of transformation.Citation9 This is called truly athermal behaviour.Citation9 However, the transformation kinetics may also be too fast for experimental observation when the activation energy is very small. In this case the transformation would be thermally activated, and therefore this kind of transformation is sometimes called anisothermal.Citation12 For most steels a distinction cannot be made because of experimental limitations. Nevertheless, the present results for Fe–0·80C confirm the athermal character of the transformation as it is frequently defined in literature, meaning that it is practically impossible to measure time dependence, and thus the volume fraction of martensite appears to vary only with temperature below Ms.
Since martensite nucleation and growth events in plain carbon steels take place too rapidly at any given temperature to be measurable as a function of time, it is not immediately evident that autocatalysis takes place in athermal martensitic transformations. However, it cannot be ruled out that autocatalysis plays a role on very small time scales. Possibly martensitic laths, which appear to be grouped into a single packet in the final microstructure, are formed simultaneously in a very fast transformation process/event in which autocatalytic nucleation does play an important role.
In contrast to the present findings, a significant cooling rate dependence of the kinetics was reported by Tsuzaki et al. for lath martensite formation in a Fe–15Ni alloy.Citation37 For the lowest cooling rate they observed steps in the transformation curveCitation37 in the temperature range 300–400°C, which are quite similar to fraction curves observed for burst martensite formation,Citation8,Citation14 and they concluded that the transformation has an isothermal character in addition to the athermal one. Similar transformation behaviour has been reported for alloys with higher Ni contents transforming below room temperature.Citation12 The observation of Tsuzaki et al. that the progress of transformation depends on the cooling rate,Citation37 and thus has a time dependence, strongly indicates that autocatalysis took place in the steel studied by Tsuzaki et al. For example, kinetics of isothermal martensite formation exhibits a typical S-shaped transformation curve during isothermal holding, which can be best understood and described under the assumption of an autocatalytic contribution to the overall nucleation kinetics. The autocatalytic nucleation is accounted for in displacive models for isothermal martensite and bainite by an additional autocatalytic term in the total nucleation rate.Citation7,Citation18,Citation46
The mechanism of autocatalytic nucleation is usually assumed to be associated with the dislocations produced in the austenite during transformation, which are considered to assist the subsequent nucleation of martensite.Citation16,Citation47 In this respect, autocatalysis can be regarded as very similar to strain induced transformation with the strain generated by the transformation itself. However, the dislocations induced in the austenite due to prior transformation can also retard the subsequent transformation to martensite,Citation30 which is known as mechanical stabilisation.Citation23 The dislocation debris interferes with the movement of the glissile interface that constitutes the growth. If the strain in remaining austenite accompanying the transformation exceeds a critical value,Citation22 then mechanical stabilisation suppresses further transformation. This mechanism is assumed to control the kinetics of athermal martensite formation in low-alloy carbon steels.Citation15
Kinetics of athermal martensite
shows the kinetics of martensite formation as a function of temperature below Ms. For steels in general, the driving force at Ms is approximately 1700 J mol−1 of which about 600 J mol−1 (Refs. 23 and 48) is considered to be stored in the surrounding austenite as strain energy after the formation of martensite crystals. The athermal kinetics observed for low-alloy carbon steels (e.g. the present results in ) can be well explained with the martensite model proposed in Ref. 15 in which it is assumed that the shape deformation accompanying the formation of a certain volume of martensite increases the strength of the remaining austenite and stops the transformation. This model explaining the athermal nature of the martensitic transformation is consistent with the ‘growth-resistance of austenite’ hypothesis proposed by Edmondson and Ko.Citation49 Since the resistance of the remaining austenite against plastic deformation increases due to the formation of a number of plates or laths, an increase in driving force is required for the transformation to recommence. Based on these assumptions the well known KM equation was derived,Citation15 and the rate parameter αm was linked to the strain energy stored in austenite. On the basis of fitting experimental fraction curves to the KM equation it was found that the rate parameter αm decreases with increasing carbon content.Citation44,Citation45 This empirical finding is possibly related to dislocation strengthening of austenite which can be expected to increase for higher carbon steels.
Austenite grain size dependence of martensite kinetics
Since the first detailed investigation on the influence of the prior austenite grain size on martensite formation,Citation29 different explanations were proposed for the depression of Ms with austenite grain refinement as shown in for Fe–0·80C. At present the most plausible explanation seems to be the mechanism based on Hall–Petch strengthening of the austeniteCitation50 proposed by Ansell and his co-workers.Citation33–Citation35 This explanation has been supported by other investigators,Citation30 and is adopted in the present work. Ansell argued that the Ms temperature is not directly affected by the austenite grain size, but controlled by the austenite yield strength.Citation34 Breinan and Ansell used a high temperature tensile apparatus and showed that an increasing austenite yield strength due to grain refinement correlated with decreasing Ms temperatures.Citation33 The increasing resistance of austenite to plastic deformation makes it more difficult to accommodate the shape change accompanying the martensitic transformation.
The mechanism based on Hall–Petch strengtheningCitation50 proposed by Ansell et al. assumes that the grain interior is locally soft compared to the grain boundary regions.Citation35 The local strength of the austenite determines the resistance against plastic deformation and thus the strain energy involved in the formation of plates or laths of martensite. It is well known that the austenite to martensite transformation occurs when the change in the chemical free energy accompanying the transformation is larger than the energy necessary to overcome the resistance to volume deformation, strain energy and the creation of new surfaces/interfaces. This means that when the resistance of austenite to plastic deformation is locally reduced, the transformation can occur at a higher temperature. Thus with increasing austenite grain size the non-chemical free energy opposing the transformation decreases, which leads to a higher Ms.Citation35
Based on the micrographs seen in Fig. 10 of Ref. 15 and further consideration of the locally soft austenite grain interior assumed in the Ansell model, it is postulated that the initial formation of martensite occurs preferably in the grain interior. The first-formed martensite plates effectively divide the prior austenite grainCitation3,Citation9,Citation51 and strengthen the surrounding austenite, and the subsequent formation of martensite plates in the same geometrically partitioned austenite grain require a larger driving force. In the model for athermal martensiteCitation15 the local austenite strength determines the transformation temperature of a certain region. The transformation of such a region to martensite leads to an increase in the strength of the surrounding austenite and therefore each austenite region has a specific decomposition temperature. Thus in this model the nucleation at certain defects or grain boundaries is not considered to be the rate-controlling step in the transformation, but the resistance of the austenitic parent phase to the growth of the nuclei.
This transformation induced stabilisation mechanism,Citation15 which controls the kinetics of martensite formation, can also aid to understand the evolution of martensite formation in large grained structures compared to fine grained structures. shows that the effect of the austenite grain size on both Ms and the initial rate of transformation is such that the degree of transformation is similar for all measurements at approximately 205°C, which corresponds to a volume fraction martensite f = 0·15. This indicates that the stabilisation in a large grained structure evolves in such a way that at f = 0·15 the resistance of the remaining austenite against plastic deformation becomes equal to that for the fine grained structure. The subsequent decomposition of austenite can be described accurately by the KM equation. Apparently, the first-formed martensite plates (or laths) in a large grained structure have a relatively strong stabilisation effect on the remaining austenite. Possibly this is due to the fact that these martensite plates have a relatively large aspect ratio. It is well known that the strain energy term increases with increasing aspect ratio,Citation18 and this may explain that the first-formed martensite plates have a stronger stabilising effect.
For isothermal martensite a similar study has been undertaken by Raghaven and Entwisle using isothermal resistivity measurements on a Fe–Ni–Mn alloy with grain diameters in the range 10 to 100 μm.Citation6 They found that the incubation time increases with decreasing austenite grain size, which was in agreement with their model calculations. Based on this it was concluded that grain boundaries and grain corners are not preferred nucleation sites,Citation6 which is in agreement with the conclusion drawn from the present study of athermal martensite formation. This standpoint seems to be widely accepted; only Kajiwara argued that certain types of austenite grain boundaries provide potential nucleation sites for martensite formation.Citation30
Conclusions
It is demonstrated that the martensite transformation kinetics is not dependent on the cooling rate, which means that the transformation can be regarded as athermal for the experimentally accessible cooling conditions in the range of 0·2–12°C s−1. The finding that the progress of transformation is independent of the cooling trajectory below Ms and thus exhibits no time dependence strongly indicates that autocatalytic nucleation does not play a vital role in the overall transformation kinetics in plain carbon steels. The methodology of cooling has been found to have a significant influence on temperature gradients within a dilatometry sample, which in turn can lead to experimental deviations in the acquired dilatometry data. Furthermore, the influence of autotempering of the as formed martensite has been discussed. It is concluded that experiments with natural cooling conditions in the temperature range of transformation are most reliable, and the kinetic data thus obtained can be accurately simulated by the KM equation using a specific (alloy dependent) start temperature TKM and rate parameter αm. Dilatometry experiments with different austenitising conditions were conducted to examine the effect of the prior austenite grain size on the overall kinetics of martensite formation. In contrast to the known sizable effect on Ms, the present results indicate that the kinetics of martensite formation beyond f = 0·15 is independent of the prior austenitising treatment. It is therefore concluded that austenite–austenite grain boundaries have no effect on the overall nucleation and growth of athermal martensite.
Notes
This paper is part of a special issue on Adventures in the Physical Metallurgy of Steels
References
- Howard RT and Cohen M: Trans. AIME, 1948, 176, 384–397.
- Averbach BL and Cohen M: Trans. AMS, 1949, 41, 1024–1036.
- Fisher JC, Hollomon JH and Turnbull D: Trans. AIME, 1949, 185, 691–700.
- Machlin ES and Cohen M: Trans. AIME, 1951, 191, 267–274.
- Machlin ES and Cohen M: Trans. AIME, 1952, 194, 489–499.
- Raghavan V and Entwisle AR: Special Report No. 93, 30, The Iron and Steel Institute, London, UK, 1965.
- Entwisle AR: Metall. Trans., 1971, 2, 2395–2407.
- Magee CL: ‘The nucleation of martensite’, in ‘Phase transformations’, 115–144; 1970, Metals Park, OH, ASM.
- Raghavan V: in ‘Martensite’, (ed. Olson G B and Owen W S), 202–225; 1992, Metals Park, OH, ASM.
- Zhao XQ and Liu BX: Scr. Mater., 1998, 38, 1137–1142.
- Otsuka K, Ren X and Takeda T: Scr. Mater., 2001, 38, 145–152.
- Lin M, Olson GB and Cohen M: Metall. Mater. Trans. A, 1992, 23A, 2987–2999.
- Bhadeshia HKDH: J. Mater. Sci., 1982, 15, 383–386.
- Brook R and Entwisle AR: J. Iron Steel Inst., 1965, 203, 905–912.
- van Bohemen SMC and Sietsma J: Metall. Mater. Trans. A, 2009, 40A, 1059–1068.
- Olson GB and Cohen M: Metall. Trans. A, 1975, 6A, 791–795.
- Olson GB and Cohen M: Metall. Mater. Trans. A, 1976, 7A, 1897–1923.
- Bhadeshia HKDH: ‘Bainite in steels’; 2001, London, The Institute of Materials.
- Hsu TY: Mater. Sci. Eng. A, 2006, A438–A440, 64–68.
- Strife JR, Carr MJ and Ansell GS: Metall. Trans. A, 1977, 8A, 1471–1483.
- Bhadeshia HKDH: Mater. Sci. Eng. A, 1999, A275, 58–66.
- Chatterjee S, Wang HS, Yang JR, and Bhadeshia HKDH: Mater. Sci. Technol., 2006, 22, 641–644.
- Chatterjee S and Bhadeshia HKDH: Mater. Sci. Technol., 2007, 23, 1101–1104.
- Yakubtsov IA and Purdy GR: Metall. Mater. Trans. A, 2012, 43A, 437–446.
- Kim D, Lee S and de Cooman BC: Metall. Mater. Trans. A, 2012, 43A, 4967–4983.
- van Bohemen SMC, Santofimia MJ and Sietsma J: Scr. Mater., 2008, 58, 488–491.
- Davenport ES and Bain EC: Trans. AIME, 1930, 90, 117–131.
- Koistinen DP and Marburger RE: Acta Metall., 1959, 7, 59–60.
- Sastri AS and West DRF: J. Iron Steel Inst., 1965, 203, 138–149.
- Kajiwara S: Metall. Trans. A, 1986, 17A, 1693–1702.
- Hayzelden C and Cantor B: Acta Metall., 1986, 34, 233–242.
- Umemoto M and Owen WS: Metall. Trans., 1974, 5, 2041–2046.
- Breinan EM and Ansell GS: Metall. Trans., 1970, 1, 1513–1519.
- Nichol TJ: Metall. Trans. A, 1977, 8, 1877–1883.
- Brofman PJ and Ansell GS: Metall. Trans. A, 1983, 14, 1929–1931.
- Kop TA, Sietsma J and van der Zwaag S: J. Mater. Sci., 2001, 36, 519–526.
- Tsuzaki K, Maki T and Tamura I: Scri. Met., 1987, 21, 1693–1698.
- Yang HS and Bhadeshia H: Mater. Sci. Technol., 2007, 23, 556–560.
- Mittemeijer EJ, Cheng L, Vanderschaaf PJ, Brakman CM and Korevaar BM: Metall. Trans. A, 1988, 19A, 925–932.
- Waterschoot T, Verbeken K and de Cooman BC: ISIJ Int., 2006, 46, 138–146.
- Morgan ER and Ko T: Acta Metall., 1953, 1, 36–48.
- Marder AR and Krauss G: Trans. ASM, 1967, 60, 651–660.
- van Bohemen SMC: Scr. Mater., 2013, 69, 315–318.
- van Bohemen SMC and Sietsma J: Mater. Sci. Technol., 2009, 25, 1009–1012.
- van Bohemen SMC: Mater. Sci. Technol., 2012, 28, 487–495.
- van Bohemen SMC: Philos. Mag., 2012, 93, 388–408.
- Suezawa M and Cook HE: Acta Metall., 1980, 28, 423–432.
- Christian JW: in ICOMAT ’79, (ed. Olson G B and Cohen M), 145–154; 1979, Boston, MA, MIT.
- Edmondson B and Ko T: Acta Metall., 1954, 2, 235–241.
- Hirth JP: Metall. Trans., 1972, 3, 3047–3067.
- Ghosh G and Olson GB: J. Phys. IV, 2003, 112, 139–142.
