Abstract
The field of high entropy alloys has exploded in its first 10 years. Vast opportunities for new compositions and microstructures are offered by this idea, but current efforts have become surprisingly focused on a narrow set of systems and on the search for single phase solid solution alloys. This perspective outlines the challenge to re-engage the full range of compositional and microstructural complexity in the search for new structural metals. We draw on extensive experience in the development of exquisite multiphase microstructures and define new high throughput experiments and computations that must be integrated to respond to this challenge. Broadening the scope is expected to provide dramatic new opportunities in the field of complex concentrated alloys.
Introduction
High entropy alloys have five or more elements with atomic concentrations between 5 and 35%.Citation1 Increasing configurational entropy ΔSconf is a key motivation for high entropy alloys (HEAs), with the intent of stabilising solid solution phases over competing intermetallic compounds.Citation1–Citation3 The rationale often given to support this strategy is that intermetallic phases can embrittle structural alloys, while solid solution alloys with simple crystal structures can have good strength and may retain ductility. This motivation has led to a strong emphasis on the search for single phase alloys and on development of simple, phenomenological rules to guide their selection.
Another major benefit is that HEAs provide a vast number of new alloys.Citation4 Conventional alloy development starts with a base element and then modifies properties by adding relatively minor amounts of other elements. As many as a dozen alloy elements can be added, but the base element still almost always accounts for >50% of the alloy. With this approach, each element represents an alloy base, so that a palette of a dozen elements (for example) gives only 12 alloy bases (Al base, Ti base, etc.). By contrast, an HEA base alloy has significant concentrations of five or more elements, so that the same palette of 12 elements gives 3302 different HEA bases. Each HEA base can be modified by adjusting proportions of the base elements around the equimolar composition, and by relatively minor additions of other elements as is carried out for conventional alloys, so that the number of unexplored compositions is truly astronomical. This suggests a strong potential for new discoveries in both scientific knowledge and practical usefulness. Supporting this expectation, HEAs have already been produced with a wide range of properties and microstructures, including single phase, multiphase, nanocrystalline and amorphous.Citation5,Citation6
In spite of these advantages, current HEAs cover a surprisingly narrow range of systems that are typically based on the transition metals Cr, Co, Fe, Ni, Mn and Cu or on refractory metals such as V, Cr, Ti, Mo, Nb, Ta, W, Zr and Hf.Citation5,Citation6 The compound forming elements Al and Ti are often added to both families. Here, we offer a critical perspective of the field with the intent of broadening the scope to re-engage the concepts of compositional and microstructural complexity. The main ideas discussed here focus on alloy strategies that include multiphase alloys; increased emphasis on phase equilibria and new strategies and techniques that combine high throughput computations and experiments to rapidly assess the vast number of new alloys suggested by the HEA concept. Basic scientific studies of complex concentrated alloys (CCAs) are essential to this effort but are not discussed here.
Two are better than one
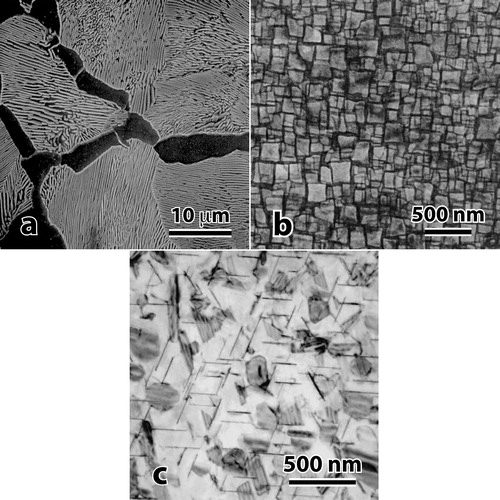
It is often stated that the motivation for HEAs is to avoid intermetallic phases, which might embrittle structural alloys.Citation1–Citation3 Intermetallics can embrittle alloys, but this is not always the case. In fact, the best balance of strength and damage tolerance is found in structural metals that rely on significant volume fractions of intermetallic or ceramic phases. Pearlite is a common microstructural element in steels, where the soft, ductile α solid solution phase is constrained elastically and plastically by surrounding cementite (Fe3C) platelets (). Superalloys give a second microstructural paradigm, where cuboidal Ni3Al precipitates share the face centred cubic (fcc) crystal structure of the disordered γ solid solution parent phase (). By controlling the lattice misfit between the parent and product phases, coherent or semicoherent interfaces are produced that contribute to superalloy properties. The unusual intrinsic behaviour of Ni3Al also contributes to the exceptional properties of superalloys. Aluminium alloys give a third microstructural template, where a number of metastable or stable precipitates are formed in the under aged, peak aged or over aged conditions (). Each of these aging conditions gives a distinct and useful balance of strength and damage tolerance. Intermetallic phases are an essential component in all of these examples. The intermetallic sizes, volume fractions and distributions are carefully controlled to produce both strength and damage tolerance.
Figure 1. Exquisitely engineered multiphase microstructures with exceptional strength and damage tolerance from a pearlitic steel showing co-continuous plates of α-ferrite and Fe3C cementite (with permission from Ref. 7), b nickel based superalloy showing high volume fraction of discrete, coherent cuboid Ni3Al precipitates (with permission from Ref. 8) and c age hardened aluminium alloy showing discrete, coherent laths of Al2Cu and other intermetallic phases (with permission from Ref. 9)
To better understand the success of these microstructures, we consider the five classic strengthening mechanisms in metals: workhardening, grain size (Hall–Petch) hardening, solid solution strengthening, particle cutting and particle bypass (Orowan strengthening). The first three mechanisms are widely used in solid solution alloys such as brass, austenitic stainless steels, the 400 series of Monel alloys and the 5000 series of aluminium alloys. These alloys have good structural properties but are never the best in class and are usually used when some property besides strength is important. These additional properties include environmental resistance, processibility, appearance (for architectural uses) and low cost. Workhardening and solid solution strengthening are not as potent as the remaining mechanisms, and grain refinement induces brittleness when used aggressively, as in the production of nanocrystalline metals.Citation10 None of these three mechanisms are useful above about half the absolute melting temperature of the alloy due to recovery, recrystallisation, diffusion and grain growth. The remaining mechanisms – particle cutting and Orowan strengthening – are among the most potent strengthening techniques and retain effectiveness even at very high temperatures. These approaches can also give both excellent strength and ductility by careful control of intermetallic size, spacing and volume fractions.
The relationships between microstructure and properties are complex, and an exceptional balance of strength and damage tolerance is achieved in steels, aluminium alloys and superalloys via three distinctly different microstructural templates. Nevertheless, common features from these alloys can be used to build a simple strategy for the development of HEAs for structural applications. The maximum use temperature Tuse is a key parameter in this strategy. In each of these three examples, the alloys are a single phase solid solution above Tuse and have a small number of phases (two for pearlitic steels and superalloys, more for aluminium alloys) at and below Tuse. The strengthening phase is generally an intermetallic whose volume fraction is controlled by alloy composition. The size and spacing of the strengthening phase are controlled by dissolving above Tuse, quenching to produce a supersaturated solid solution and then aging at a temperature below Tuse. The solutionising temperature, quench rate, aging time and temperature are key parameters to control in developing appropriate microstructures. The intermetallic phase may be thermodynamically stable (as in superalloys) or metastable (as in pearlite and aluminium alloys), although metastable phases can limit application at the highest temperatures. None of these alloys have first order phase transformations below Tuse, to avoid changes in microstructure or properties during service. The parent and strengthening phases may share a common crystal symmetry, such as fcc and ordered fcc in superalloys, but this is not required. In each of these examples, the parent phase crystal structure is relatively simple and/or close packed (fcc, body centred cubic, hexagonal close packed), but the strengthening phase need not be.
These ideas outline a simple strategy for developing particle strengthened HEAs, which can be considered to be HEA steels or HEA superalloys, for example. The microstructures described here are not inclusive – other microstructural paradigms exist and may also be used to guide a development strategy. For example, microstructures composed of two disordered solid solutions can be used as a template (as for the alloy Ti–6Al–4V). All of these ideas are well founded in scientific knowledge and practical experience and can significantly expand the scope of HEA research. An alloy development strategy based on these ideas has been discussed in detail elsewhere.Citation11
What’s in a name?
The idea of particle strengthened HEAs aggravates a vigorous controversy in the HEA field. Some believe that alloys with two or more phases at low temperatures cannot rightly be called HEAs, even if the alloy is a single phase solid solution at high temperatures. The issue is that elemental partitioning between two or more phases reduces ΔSconf of the system relative to the single phase alloy. The high ΔSconf of these alloys thus becomes hidden at low temperatures but re-emerges when the alloy returns to a single phase solid solution at high temperatures.
The accepted HEA definition (see the first sentence of the Introduction above) simply gives compositional bounds and does not specify the magnitude of ΔSconf, so there should be no argument. These compositional bounds are based on accessing the maximum ΔSconf possible in the alloy while the debate implies that the minimum ΔSconf that is actually achieved should be used to define an HEA. In fact, the entropy of an alloy is not a single value. The magnitude of ΔSconf can change with temperature,Citation12 and it may vary significantly when a first order phase transformation occurs. If an HEA is to be defined by a single value of ΔSconf, then it must be specified at which temperature this value is most meaningful. This depends on the purpose of the study at hand. Using the minimum ΔSconf has the practical difficulty that it cannot be known a priori if an alloy is an HEA unless the stable phases are known at all temperatures before the alloy is made. It could also be argued that the entropic energy −TΔSconf is more important, since this is what competes with formation enthalpies of intermetallic compounds ΔHf (see the following section). However, this product approaches zero at very low temperatures regardless of the magnitude of ΔSconf, so that only alloys with ΔHf≧0 for all possible compounds would be HEAs. Enforcing such a definition would restrict the number of alloys that could be called HEAs to a trivial few. There is no unequivocal ‘right’ or ‘wrong’ answer to the issues posed here, since the ‘best’ answer is subjective and depends on the purpose of the work being performed.
This discussion is not offered to resolve the controversy, but rather to point out that this diverts attention from the major benefit offered by the vast range of unexplored compositions and microstructures. The HEA concept focuses thought and exploration on an immense span of new compositions where the mechanisms, microstructures and properties are uncertain. It is very likely that new scientific models and new alloys of practical benefit will come from these studies. In our opinion, it matters less that an alloy satisfies a subjective initial definition, and it matters more that the work was based on an important new idea, was well performed and has led to a meaningful result. We thus value the idea (vast composition and microstructure space) that inspires the work over the name used to label it.
There is a simple way to avoid this issue altogether. Many names have been used for HEAs, including multicomponent alloys, multiprinciple element alloys, CCAs and baseless alloys. Simply using a different name avoids this complication, since reference to the magnitude of configurational entropy is avoided. This is not a criticism of the HEA label or of the work conducted to date – the fundamental idea is unchanged and retains all of its potency and impact. This suggestion is simply a practical approach to side step an unproductive controversy so that the community can remain focused on exploring, understanding and developing the enormous extent of CCAs.
Predicting and achieving equilibrium
At present, Gibbs free energies give the only accepted approach to predict the stable phases in an HEA. The Gibbs free energies for all possible solid solution and intermetallic phases are compared, and the lowest energy system is the equilibrium system. The Gibbs free energy of solid solution phases is based on the enthalpies and entropies of mixing;1 For ideal and regular solutions
2 The configurational entropy of solid solution phases can thus be easily estimated. The mixing enthalpies ΔHmix can also be approximated and are available for a wide range of atom pairs.Citation13 As a result, ΔGSS is easily approximated. Rather than competing with ΔSconf, note that ΔHmix (which is commonly negative in metallic systems) works together with ΔSconf to increase the stability of disordered solid solution phases.
The Gibbs free energies of intermetallic phases require enthalpies and entropies of formation3 The formation entropy in ordered compounds ΔSf is generally very smallCitation14 and can be approximated as zero. Comparing equations (1) and (3), we see that Hmix and ΔSmix stabilise disordered solid solutions, while ΔHf destabilises them. There are no simple approaches for estimating ΔHf. Experimental data are available,Citation14–Citation19 and values can be calculated using first principles techniques.Citation20 Calculations are complicated by the need to estimate ΔHf for all possible crystal structures for a given pair of atoms and stoichiometry, and then finding the most negative value. High throughput algorithms are under development, but it is not yet a simple matter to generate ΔHf values from first principles calculations. Since the full range of desired ΔHf values are not readily accessible, phenomenological models are used to predict trends in stability of intermetallic and solid solution phases.Citation2,Citation21,Citation22 These models show rough trends but are not expected to give accurate predictions of phase stability.
The most established way to estimate ΔHf, and to predict phase stability, is given by the CALculated PHAse Diagram (CALPHAD) method. This approach is based on experimental measurements of thermodynamic properties and phase equilibria for a wide range of binary and ternary systems. These experimental data are used to generate thermodynamic functions that depend on temperature and composition. These functions, in turn, allow thermodynamic properties and phase equilibria (i.e. phase diagrams) to be predicted at compositions and temperatures where data are not available. Selected databases are available for alloy systems of high interest, based on elements such as Fe (for steels), Ni (for superalloys), Sn (for solders), Al, Ti and others. These databases may have over a dozen elements and are built using data from many, but not all, of the binary and ternary phase diagrams represented in each database. Solution databases are also available for all metallic elements and are built using data from all known binary phase diagrams.
The CALPHAD approach has been used for over 30 years and is considered an industry standard for predicting phase equilibria. However, predictions are most reliable when the alloys being considered fall within the composition range over which the database has been constructed. These databases generally do not extend to the centres of the phase diagrams, and so great caution must be used when extrapolating far from compositions used to construct the databases. Nevertheless, progress can be made. Calculations of equimolar and near equimolar alloys to date show reasonable agreement for the numbers and types of phases present.Citation23 Agreement is less reliable, but still modest, for reaction temperatures, phase compositions and phase volume fractions. Efforts are now underway to more critically assess the limits of calculations using current databases and to establish quantitative credibility criteria to guide the reliability and use of such predictions.Citation24
Experimental observations of phase equilibria in CCAs are essential to validate these credibility criteria and to begin extending the compositional ranges of thermodynamic databases. Measured phase equilibria are also essential to assess the ability of ΔSconf to stabilise disordered solid solutions. Finally, phase equilibria are essential for the development and use of high temperature structural metals. Unfortunately, the vast majority of reported HEA data are for as cast microstructures (see, for example data in Refs. 5 and 6). This is a serious deficiency that casts a pall of uncertainty on any study where the phases present are a key concern. It cannot be overstated how urgently reliable measurements of equilibrated HEA microstructures are needed. This is a major focus for future efforts.
More! Faster!
Equilibrium measurements are time consuming, and the immense composition space of HEAs makes this an almost inconceivable task. Structural materials also demand the careful development of complex yet exquisite microstructures to deliver an exacting balance of properties – another daunting undertaking. Combining the sheer number of alloy compositions with microstructural complexity absolutely demands a completely new approach for exploring, assessing and developing these alloys.
High throughput experiments are a mainstay in many fields.Citation25–Citation28 Typically used to measure functional properties, several issues limit the application of high throughput methods to structural materials. A successful structural material must simultaneously satisfy a demanding balance of over a dozen different properties, and high throughput tools are not fully developed for all of these properties. Some techniques are available now, such as mapping the composition and crystal structures of the phases present. Nanoindentation is easily automated and can give information on both modulus and hardness that are key indicators of strength, and diffusion ‘multiples’ (where >2 elements interdiffuse simultaneously) can accelerate data acquisition for kinetics and phase equilibria.Citation29 However, several key properties are not yet sufficiently parallelised. Nanocalorimetry methods can identify phase transformations,Citation30 but higher temperature capabilities are needed. Conceptual approaches have been discussed for oxidation and corrosion resistanceCitation11 but have not yet been implemented. Melting temperature data are essential to validate CALPHAD predictions and to calibrate alloy use temperatures, but there are currently no high throughput approaches to measure this key property.
By far, the most significant barrier is the lack of high throughput methods to give useful data for essential engineering properties such as yield and ultimate strengths, ductility, damage tolerance, creep strength and fatigue. These properties all depend sensitively on microstructure. Microstructure sets internal length scales that limit the ability to miniaturise the test volume. Miniaturisation is a key feature of highly parallel methods. Thus, structural materials present a major limitation to current high throughput techniques. These barriers are not insurmountable, but new approaches and breakthroughs are needed. A more detailed discussion of high throughput characterisation methods for structural metals is available elsewhere.Citation11
In addition to advancements in high throughput testing, the rapid exploration and development of structural materials demand new approaches for producing materials libraries. Physical vapour deposition techniques are commonly used today to manufacture libraries with controlled composition gradients to measure functional properties. Some structural material properties can also use this method, and the ability to control composition in complex alloys is already being demonstrated.Citation31,Citation32 However, structural material libraries for HEAs may also require new manufacturing methods. In addition to continuous composition gradients, stepped gradients and even discrete ‘dots’, each with a unique ‘composition on demand’, may be needed. Test techniques for structural materials may call for material libraries that are more than an order of magnitude thicker than can currently be produced via physical vapour deposition. Additive manufacturing and three-dimensional printing may give new capabilities for all of these needs. To rapidly evaluate the microstructural complexity of structural materials, libraries of fixed composition but with controlled microstructure gradients are also needed. The Jominy bar is a well known example of such a test, but there are otherwise almost no examples to draw upon. Material libraries of fixed composition, subjected to controlled thermal gradients, can be imagined to rapidly establish the microstructures associated with solution and aging temperatures in the solution treatment and aging heat treatment typically used for two-phase structural materials. Deformation gradients can also be used to give gradients in grain size.
From the very start, the high throughput experiments described above need to be integrated with high throughput computations. A number of computational capabilities have been developed to sufficient maturity, but their application to this problem set has not yet been established and requires thought and validation. The CALPHAD computations for phase equilibria discussed above are one example, others are available for linking microstructure and materials properties. The materials science and engineering communities clearly recognise the value of integrating experiments and computations – this is embodied in the Integrated Computational Materials Engineering and Integrated Computational Materials Science and Engineering activities and the Materials Genome Initiative in the United States. These give a solid foundation upon which progress can be made on the problems defined here. The challenges identified in the present assessment add high throughput as a new requirement for integration of experimental and computational methods.
Potential applications
The present paper focuses on approaches to developing HEAs as structural materials. We describe microstructural design for a balance of strength and damage tolerance and high throughput experiments needed to accelerate development. Structural materials require many other properties to be successful, and some work already reports on the corrosion resistance, oxidation behaviour and wear properties of structural HEAs.Citation5 High entropy alloys also have properties that may be attractive for functional applications. Electrical, thermal and magnetic properties of HEAs have already been measured.Citation5 The HEAs have been considered as diffusion barriers for microelectronics, for hydrogen storage and for applications that require catalytic properties or resistance to irradiation damage.Citation5
It is often concluded that attractive structural and functional properties can be found in HEAs. However, there is much work between measuring a single attractive property in a single alloy and demonstrating credibility for a particular application. A suite of properties must be produced in the same alloy simultaneously. In addition to structural or functional properties, cost and ease of manufacture are key features that must be considered. These have generally not been considered to date. Much of the HEA work seems to be aimed at demonstrating the attractive potential of HEAs as a class of materials, with less emphasis on more focused and extended efforts to develop a particular HEA for a particular application. Such a balance is perhaps appropriate for a new concept, but HEAs have passed their first decade as a new idea and may be reaching a critical juncture. The time may be right for initiating focused efforts to develop particular HEAs for specific uses.
Summary perspectives
The present paper develops an approach for expanding the current work on HEAs to include the full range of compositional and microstructural complexity offered. This expanded scope is essential for the development of new, high performance structural metal alloys. The well known, successful multiphase microstructures of pearlitic steels, superalloys and age hardened aluminium alloys are offered as templates to guide future studies on multiphase compositionally complex alloys.
The vision developed here offers many new challenges that are unique to the development of CCAs as structural metals. The complexity of structural metals previously came primarily from the vast array of possible microstructures – the explosion of compositional complexity in HEAs multiplies this complexity manyfold. A severe lack of phase equilibria data in complex composition space is identified, and new high throughput experimental techniques are needed that can evaluate microstructure sensitive properties. New approaches for producing material libraries with controlled composition and microstructure gradients must be conceived and validated, perhaps using new capabilities offered by additive manufacturing. These high throughput experiments must be integrated with high throughput computations into a single strategy to rapidly explore, evaluate and develop new structural materials over the full range of compositions and microstructures possible in CCAs.
The challenges are daunting. Advancements may be made individually at different institutes, but the community awaits a key integrating opportunity to synergise efforts on these challenges to more rapidly accelerate progress.
Acknowledgements
The author acknowledges useful discussions on this topic with O. Senkov, U. Glatzel, E. George and J. Miller.
References
- Yeh J.-W, Chen S.-K, Lin S.-J, Gan J.-Y, Chin T.-S, Shun T.-T, Tsau C.-H and Chang S.-Y: ‘Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes’, Adv. Eng. Mater., 2004, 6, (5), 299–303.
- Guo S, Ng C, Lu J and Liu CT: ‘Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys’, J. Appl. Phys., 2011, 109, 103505.
- Yeh J.-W: ‘Recent progress in high entropy alloys’, Ann. Chim. Sci. Mat., 2006, 31, (6), 633–648.
- Cantor B, Chang ITH, Knight P and Vincent AJB: ‘Microstructural development in equiatomic multicomponent alloys’, Mater. Sci. Eng. A, 2004, A375–A377, 213–218.
- Murty BS, Yeh J.-W and Ranganathan S: ‘High entropy alloys’; 2014, London, UK, Butterworth-Heinemann.
- Zhang Y, Zuo TT, Tang Z, Gao MC, Dahmen KA, Liaw PK and Lu ZP: ‘Microstructures and properties of high-entropy alloys’, Prog. Mater Sci., 2014, 61, 1–93.
- Howell PR: ‘The pearlite reaction in steels: mechanisms and crystallography’, Mater. Charact., 1998, 40, 227–260.
- Pearson DD, Lemkey FD and Kear BH: ‘Stress coarsening of γ‘ and its influence on creep properties of a single crystal superalloy’, Proc. 4th Int. Symp. on ‘Superalloys’, 513–520; 1980, Warrendale, PA, TMS.
- Hutchinson CR, Fan X, Pennycook SJ and Shiflet GJ: ‘On the origin of the high coarsening resistance of plates in Al–Cu–Mg–Ag alloys’, Acta Mater., 2001, 49, 2827–2841.
- Ma E: ‘Instabilities and ductility of nanocrystalline and ultrafine-grained metals’, Scr. Mater., 2003, 49, 663–668.
- Miracle DB, Miller JD, Senkov ON, Woodward C, Uchic MD and Tiley J: ‘Exploration and development of high entropy alloys for structural applications’, Entropy, 2014, 16, (1), 494–525.
- Christian JW: ‘The theory of transformations in metals and alloys’; 2002, Oxford, UK, Pergamon Press.
- Takeuchi A and Inoue A: ‘Mixing enthalpy of liquid phase calculated by Miedema’s scheme and approximated with sub-regular solution model for assessing forming ability of amorphous and glassy alloys’, Intermetallics, 2010, 18, 1779–1789.
- Franke P and Neuschutz D: ‘Thermodynamic properties of inorganic materials compiled by SGTE’, Landolt-Bornstein Numerical Data and Functional Relationships in Science and Technology: Group IV: Physical Chemistry, 2002, Volume 19; Subvolume B; Binary Systems; Phase Diagrams, Phase Transition Data, Integral and Partial Quantities of Alloys.
- de Boer FR, Boom B, Mattens WCM, Miedema AR and Niessen AK: ‘Cohesion in metals: transition metal alloys (Cohesion and structure)'; 1989, Dordrecht, North Holland.
- Guo Q and Kleppa OJ: ‘Standard enthalpies of formation of some alloys formed between group IV elements and group VIII elements, determined by high-temperature direct synthesis calorimetry II. Alloys of (Ti,Zr,Hf) with (Co,Ni)’, J. Alloys Compd, 1998, 269, 181–186.
- Guo Q and Kleppa OJ: ‘The standard enthalpies of formation of the compounds of early transition metals with late transition metals and with noble metals as determined by Kleppa and co-workers at the University of Chicago – a review’, J. Alloys Compd, 2001, 321, 169–182.
- Hultgren R, Orr RL, Anderson PD and Kelley KK: ‘Selected values of thermodynamic properties of metals and alloys’, 1963, New York, John Wiley.
- Kubaschewski O, Alcock CB and Spencer PJ: ‘Materials thermochemistry’, 6th edn, 1993, Oxford, Pergamon.
- Curtarolo S, Hart GLW, Nardelli MB, Mingo N, Sanvito S and Levy O: ‘The high-throughput highway to computational materials design’, Nat. Mater., 2013, 12, 191–201.
- Tsai M.-H, Tsai K.-Y, Tsai C.-W, Lee C, Juan C.-C and Yeh J.-W: ‘Criterion for sigma phase formation in Cr- and V-containing high-entropy alloys’, Mater. Res. Lett., 2013, 1, (4), 207–212.
- Zhang Y, Zhou YJ, Lin JP, Chen GL and Liaw PK: ‘Solid-solution phase formation rules for multi-component alloys’, Adv. Eng. Mater., 2008, 10, (6), 534–538.
- Zhang F, Zhang C, Chen SL, Zhu J, Cao WS and Kattner UR: ‘An understanding of high entropy alloys from phase diagram calculations’, CALPHAD, 2014, 45, 1–10.
- Senkov O. N, Miller J. D, Miracle DB and Woodward C: ‘Accelerated exploration of multi-principal element alloys with solid solution phases’, Nature Comm., 2015, 6:6529, DOI: 10.1038/ncomms7529.
- Green M, Takeuchi I and Hattrick-Simpers JR: ‘Applications of high throughput (combinatorial) methodologies to electronic, magnetic, optical, and energy-related materials’, J. Appl. Phys., 2013, 113, 231101.
- Potyrailo R, Rajan K, Stoewe K, Takeuchi I, Chisholm B and Lam H: ‘Cominatorial and high-throughput screening of materials libraries: Review of state of the art’, ACS Comb. Sci., 2011, 13, 579–633.
- Potyrailo R and Takeuchi I: ‘Role of high-throughput characterization tools in combinatorial materials science’, Meas. Sci. Technol., 2005, 16, 1–4.
- Rajan K: ‘Combinatorial materials sciences: experimental strategies for accelerated knowledge discovery’, Ann. Rev. Mater. Res., 2008, 38, 299–322.
- Zhao J.-C: ‘Combinatorial approaches as effective tools in the study of phase diagrams and composition–structure–property relationships’, Prog. Mater Sci., 2006, 51, 557–631.
- McCluskey P and Vlassak J: ‘Combinatorial nanocalorimetry’, J. Mater. Res., 2010, 25, (11), 2086–2100.
- Ding S, Liu Y, Li Y, Liu Z, Sohn S, Walker FJ and Schroers J: ‘Combinatorial development of bulk metallic glasses’, Nat. Mater., 2014, 13, 494–500.
- Ludwig A, Zarnetta R, Hamann S, Savan A and Thienhaus S: ‘Development of multifunctional thin films using high-throughput experimentation methods’, Int. J. Mater. Res., 2008, 99, (10), 1144–1149.
