Abstract
Mustard seeds (MS), which are consumed in considerable amounts by the Japanese people that, interestingly, have the longest life expectancy in the world, are known to contain a number of yet not fully defined but quite powerful anti-oxidants. A suspension of extracted MS was found to suppress oxidized-LDL-induced macrophage respiratory burst in vitro, to prevent growth, and to induce apoptotic death of SW480 cells (a human colon cancer cell line), while no such effects were found for normal 3T3 cells. A diet enriched with MS decreased plasma levels of the lipid peroxidation product malonaldehyde in mice exposed to the colon cancer-inducer azoxymethane (AOM). Such a diet also dose-dependently enhanced the activity of several anti-oxidant enzymes, such as superoxide dismutase (SOD), catalase, and GSH-peroxidase and, moreover, reduced AOM-mediated formation of colon adenomas by about 50%. Further studies are required to detail and explore the beneficial effects of MS and their rich content of powerful anti-oxidants.
Introduction
Since the Tang dynasty (618–907), the seeds of the Sinapis Alba Linn plant, commonly called white or yellow mustard, have been used in China as a spice as well as a medicine. It is now included in the Pharmacopoeia of the People's Republic of China. In Chinese traditional medicine, mustard seeds (MS) are considered an excellent food preservative, a potent anti-cancer agent, and a protector against prostatic hyperplasia.Citation1 Recently, components of MS were shown to possess anticancer activity by decreasing the incidence of 7,12-dimethylbenz(a)anthracene-induced skin cancers and their trans-placental and trans-lactational spreading in Swiss albino mice.Citation2 Gagandeep et al.Citation3 reported a preventive efficacy of dietary mustard seed on benzo(a)pyrene [B(a)P]- and 3-methylcholantrene-induced tumorigenesis in mice fore-stomach and uterine cervix, respectively. However, since we hold limited knowledge regarding the use of MS in the prevention or control of colon cancers, experimental studies are needed to elucidate their efficacy and molecular mode of action, thus providing a basis for later human intervention.
Here, we investigated the effect of feeding MS to Kunming mice by analyzing azoxymethane (AOM)-induced colon tumors for signs of proliferation, apoptosis, and beta-catenin expression. We report a clear inhibitory effect of MS on colon tumorgenesis in the form of a significantly reduced proliferation activity together with enhanced apoptosis. A diet rich in MS enhanced the anti-oxidant status of the animals and depressed peroxidative damage in a dose-dependent way, suggesting that MS might be an effective natural protective agent against colorectal cancer. We also found that an emulsion of extracted mustard seeds (EMS) had the ability to significantly reduce production of reactive oxygen species (ROS) from peritoneal macrophages when exposed to oxidized LDL (ox-LDL), to prevent growth and to induce apoptotic death of SW480 cells (a human colon cancer cell line), while no such effects were found for normal 3T3 cells. These observations may be of importance for the further evaluation and explanation of the anti-tumorigenic effect of MS.
Materials and methods
Extracted mustard seed
EMS were prepared as previously reported.Citation4 Briefly, MS were extracted in ether and ethanol to remove lipids. The sediment was then spun down and dried to a powder that is referred to as EMS of which a 1.5% suspension was prepared in phosphate-buffered saline (PBS) and injected to animal, intra-peritoneal (i.p.). After 3 days, peritoneal macrophages were collected and exposed to ox-LDL (see below) for an assay of ROS.
Mustard seeds
Fresh MS were crushed using a mechanical blender (Ultra-Turrax model T25, Labotechnik, Staufen, Germany) and added to the standard chow at the desired concentration (5 or 10%) and pellets were formed and stored under dry and strict sanitary conditions at room temperature in a store room of the animal house of the Southern Medical University until the end of the experiments.
Reactive oxidative species (ROS) assay
The production of ROS by mice peritoneal macrophages, obtained from mice that were initially exposed to EMS (see above), and then to ox-LDL in vitro (see above) was assessed by measuring oxidation of dihydrorhodamine 123 (DHR 123; Molecular Probes).Citation5,Citation6 Cells were exposed to 100 µg/ml ox-LDL (Sigma, St Louis, MO, USA), and fluorescence was analyzed on a Becton-Dickinson FACScan machine using CellQuest software.
Cytotoxic effect of EMS on normal and malignant cells
SW480 and NIH/3T3 cells were grown in 25 cm2 flasks at standard culture condition. Cells were incubated for 72 hours with indicated concentration of EMS dissolved in complete medium, cell numbers and their viability were determined in a hemocytometer following staining with trypan blue. Cell viability was measured according to the instruction of the manufacturer of the Cell Counting Kit-8 (Dojindo, Kumamoto, Japan)Citation7 and expressed as the means ± SD in percentage of the control viability (=100%). Growth inhibition ratio (IR%) was calculated according to the formula: IR% = ((the absorbance of the blank control group − the absorbance of the experimental group)/the absorbance of the blank control group) × 100.
Caspase-3 assay
Attached and floating cells were harvested, washed twice in PBS, and stored at −20°C. Caspase-3 activity was detected using the caspase-3 Assay Colorimetric Kit (Keygen Biotech Co., Nanjing, China).Citation8 The assay is based on the hydrolysis of the peptide substrate acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA) by caspase-3, resulting in the release of a pNA moiety. The concentration of the released pNA was calculated from the absorbance values at 405 nm and a standard curve from a defined pNA solution. Results were adjusted to the total protein content, and activities were expressed as nmol pNA/mg protein.
Animal experiments
Four weeks old Kunming mice with an average body weight (BW) of 20 g were provided by the Southern Medical University animal center. The mice were maintained in an air-conditioned animal facility at 12-hour light/dark cycles and provided standard mice chow and tap water ad libitum. All animals were cared for according to the principles expressed in ‘Guide for the care and use of laboratory animals in China’. The study was reviewed and approved by the Animal Care Committee of the China Southern Medical University.
Altogether 60 mice were randomly divided into four groups of 15 mice each. Group I received standard diet and injections of saline. In the other three groups, each mouse received i.p. injections of AOM (10 mg/kg body wt) once a week for 3 weeks.Citation9 Group II was fed a 5% MS-enriched diet, group III a 10% MS diet, and group IV was exposed to a standard diet and AOM. The animals were given the described mustard seed diet 2 weeks before start of the AOM injections, and this diet was continued until the end of the experiment. Mice were sacrificed by ether inhalation 33 weeks after their last injection of AOM or saline and the body weights were measured.
Tumor assays
To evaluate the modifying effects of dietary MS on the development of AOM-induced tumor, all animals were killed in 3 weeks after the last AOM injection. Venous blood samples were collected from the hearts using a tube containing heparin sodium. The tubes were centrifuged for 10 minutes at 3000 × g and the plasma was stored at −20°C until analysis. Colon was removed from each animal, flushed with a 0.9% NaCl-solution, and cut open longitudinally. The total number of tumors per colon, their location along the bowel (mm from the anus), and their size (largest diameter) were recorded. The tumors were fixed in 10% formalin (Fisher Scientific Co., Fair Lawn, NJ, USA), freshly made from paraformaldehyde, serially sectioned, and stained with hematoxylin and eosin (Invitrogen, California, USA) for histological examination. Slides were coded and then read by a pathologist blinded to the analysis using a model H-600 transmission electron microscope (Electronic Stock Limited Company, Japan). If tumor cells invaded the submucosa, the tumor was diagnosed as an adenocarcinoma. When tumor cells did not invade the submucosa, the tumor was diagnosed as an adenoma (low- and high-grade intraepithelial neoplasias).Citation10
Immunostaining for proliferative cell nuclear antigen (PCNA)
Cell proliferation was measured using the methods described by Zheng et al.Citation11,Citation12 Tumor sections were incubated with a primary antibody against the PCNA (1:100; Bioworld Technology Inc., Minnesota, USA) for 60 minutes. A streptavidin–biotin complex peroxidase kit (LSAB kit, Dako, Japan) was then applied and peroxidase activity was developed with the substrate 3,3′-diaminobenzidine tetrahydrochloride (DAB, Dako).
Apoptosis assay
Nuclear morphology was studied and documented by fluorescence microscopy and the TUNEL (terminal transferase dUTP nick end labeling) assay was performed.Citation13
Assays for the activity of superoxide dismutase (SOD), catalase (CAT), GSH-peroxidase (Px), and levels of lipid peroxidation products (LPO) in plasma
Blood samples were collected from the hearts using a tube containing heparin sodium. The tubes were centrifuged for 10 minutes at 3000 × g using a centrifuge (Eppendorf 5417R, Hamburg, Germany) and plasma was collected. SOD, CAT, GSH-Px, malonaldehyde, and LPO were assayed with colorimetric Diagnostic Reagent Kits (Nanjing Jiancheng Bioenginerring, Nanjing city, P.R. China) according to the manufacturer's instructions.
Statistical analyses
Results were expressed as means ± SD and analyzed using the SPSS13.0 software. The ANOVA test followed by the Student–Newman–Keuls method was used and tumor incidences were compared using χ2 test. *: P < 0.05, **: P < 0.01; and ***: P < 0.001.
Results
Sinapis Alba Linn is an anti-oxidant
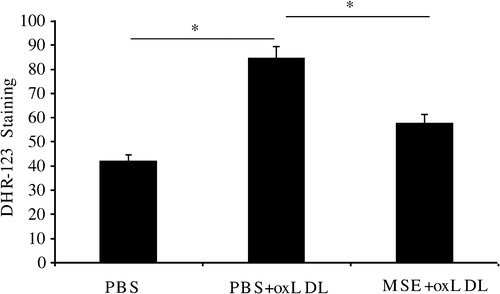
Mustard seed extract has been described as an anti-oxidant.Citation14 To analyze its presumed anti-oxidant effect, 1.5% EMS was administrated (or not) (i.p.) to Kunming mice and the oxidative burst of later collected peritoneal macrophages, induced by ox-LDL, was assayed by fluorescent staining with DHR123. shows that EMS significantly inhibits macrophages-mediated oxidation.
Figure 1. EMS suppresses ox-LDL-induced oxidative burst in peritoneal macrophages. EMS (1.5% in PBS; 1 ml; i.p.) was administrated daily for 3 days, using PBS as a vehicle control. On the 5th day, peritoneal macrophages were collected, washed in PBS, and activated by exposure to 100 µg/ml ox-LDL for 10 minutes. The resulting oxidative burst was analyzed by exposure to 10 µM DHR-123 using FACS. Values from three independent experiments are expressed as means ± SD. The ox-LDL in PBS group significantly differed from the control (PBS only) group (*P < 0.05), whereas the EMS + ox-LDL group did not (n = 15).
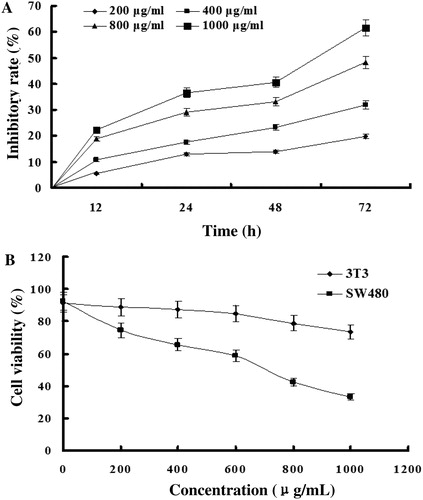
EMS inhibits growth of cultured colon cancer cells and induces cell death without showing significant effects on normal 3T3 cells
To test the effect of EMS on colon cancer cells, we explored SW480 human colorectal carcinoma cells with respect to proliferation rate and survival. A shows that the extract inhibits cellular proliferation dose dependently. We also assessed immortalized fibroblastic 3T3 cells. B shows that EMS is much less toxic to normal fibroblasts than to colon cancer cells.
Figure 2. EMS inhibits proliferation of colon cancer cells, while 3T3 cells are not affected. (A) Colon cancer cells – SW480 line – were exposed to 200, 400, 800, and 1000 µg/ml of EMS suspended in complete culture medium, for 72 hours. The growth inhibitory effect was assayed as described in the Materials and methods section. A significant dose-dependent growth inhibition was observed over the 72 hours observation period. (B) SW480 and 3T3 cells were exposed to the indicated amounts of mustard seed extract for 72 hours and cell proliferation was determined by the CCK-8 assay (n = 3). As shown, an extract of MS significantly inhibits proliferation of colon cancer cells, while 3T3 fibroblasts are not affected.
EMS activates caspase-3 with ensuing apoptosis of SW480 cells
To confirm that EMS induces apoptosis in colon cancer cells, we then assayed the activity of caspase-3 in SW480 cells exposed to EMS. SW480 cells were seeded at a density of 5 × 105 per well in six-well plates and were allowed to adhere for 24 hours. The time course of caspase-3 activation was evaluated in an initial set of experiments. Cells were exposed to different concentrations of EMS for 24 hours. shows that the activity of caspase-3 increased in a dose-dependent manner.
Table 1. Caspase-3 activity in SW480 cells exposed to different amounts of EMS for 24 hours
MS reduces the AOM-induced formation of colon tumors
Since the above in vitro studies showed EMS to possess anti-tumor effects, we then tested this effect also in vivo. shows that MS significantly and dose-dependently reduced the number of tumors, although not their size. is a sample image of normal colon tissue and colon tumors (adenomas). All tumors were carefully studied by light microscopy following semi-serial sectioning and found to be adenomas with varying degree of atypia. No invasion of the muscularis mucosae was found and, therefore, no true adenomcarcinoms.
Figure 3. Light microscopy images of AOM-induced colonic adenoma. The mice were randomly divided into four groups and fed standard chow or a diet supplemented with different amounts of MS. AOM was simultaneously administered by i.p. injection once a week for 3 weeks at a dose of 10 mg/kg body weight. After 35 weeks of feeding with either normal chow or a diet enriched with MS (5 or 10%), colon tissue were collected, fixed, and sectioned. The occurrence and type of tumors was analyzed by HE staining. The illustrations shown are from animals exposed to AOM only. Arrows indicate adenomas, most of which showed severe atypia, although no infiltrating carcinomas were found (×100). The tumor incidence of the AOM group was 86.7% (, indicating that 10 mg/kg body weight of AOM was sufficient to induce adenomas in Kuming mice at a high rate.
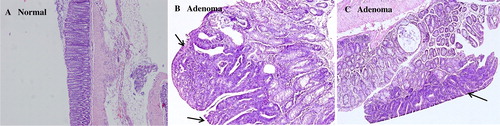
Table 2. Effect of dietary MS on AOM-induced colon tumorigenesis in mice
MS enhances the activity in plasma of anti-oxidant enzymes and decreases LPO
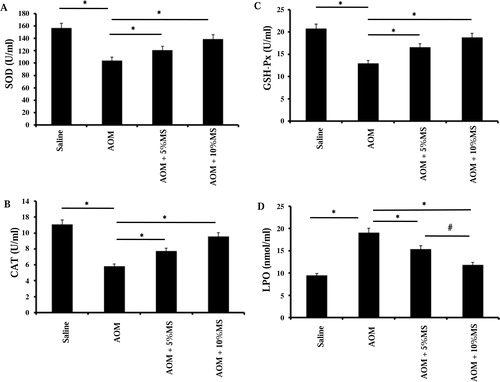
To find out whether MS enhances anti-oxidative systems in vivo, we assayed plasma from animals fed 5 or 10% MS. A shows that MS dose-dependently counteracts the AOM-induced reduction in SOD activity, whereas B and C shows a similar effect on CAT and GSH-Px. The level of the oxidative stress product LPO was significantly decreased in plasma, which suggests MS to contain potent anti-oxidants (D).
Figure 4. MS enhances the activity of plasma SOD, CAT, and Gpx as well as it suppresses lipid peroxidation (LPO) in AOM-exposed mice. The animals were randomly divided into four groups and fed normal chow or a diet supplemented with MS (5 or 10%). AOM (10 mg/kg body weight i.p.) was given once a week for 3 weeks. Plasma was collected after 35 weeks of feeding the animal with either. SOD activity (A), CAT activity (B), GSH-Px (Gpx) activity (C), and LPO (D) were assayed as described in the Materials and methods section. Significant increases (*P < 0.05) in SOD, CAT, GSH-Px, and a decrease in LPO were observed in the 10% MS group (n = 15). The results suggest that one of the ways that MS decrease the tumorogenic effects of AOM is by inducing enhanced activities of anti-oxidative enzymes.
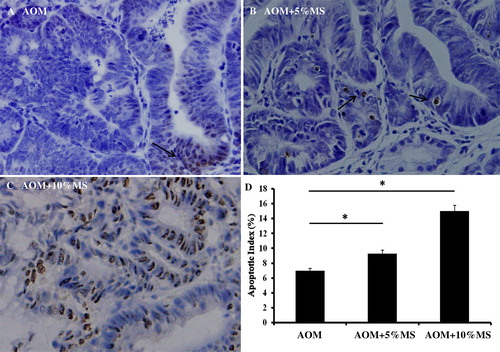
MS inhibits proliferation and induces apoptosis in vivo on colon tumors induced by AOM
Since an emulsion of EMS inhibits colon cancer cell proliferation in vitro, we then explored its effect also in vivo. The PCNA was initially immuno-demonstrated, whereas the TUNEL assay was then performed. As shown in and , MS-containing food inhibits colon cell proliferation and induces apoptosis.
Figure 5. MS inhibits AOM-induced proliferation of colon tumors in vivo. Tumor cell proliferation was analyzed by immunocytochemical demonstration of the PCNA. Proliferative indices (D) were significantly lower in tumors of MS-treated mice. Each group contained 15 mice (n = 15). The result indicates that MS might as well inhibit AOM tumorogenesis by inducing immuno-responses.
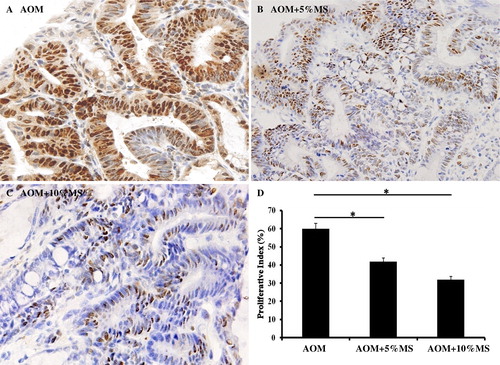
Figure 6. MS provokes apoptosis in AOM-induced adenomas in vivo. Tumor cell apoptosis was analyzed by demonstrating the terminal deoxynucleotidyl transferase enzyme (TUNEL). The data show apoptotic indices to be significantly higher in tumors of MS-treated mice. Each group comprised 15 mice. Also these results suggest that MS might enhance tumor immunity.
Discussion
Plant-based food contains numerous non-nutritive, bioactive compounds known as ‘phytochemicals’. A rapidly increasing number of epidemiological studies show a significant reduction in a variety of cancers among persons that eat a diet rich in fruits and vegetables.Citation15–Citation17 These findings have created the foundation for an exploration and development of functional foods and nutraceuticals. Studies have shown highly significantly diminished amounts of 2-thiobarbituric acid reactive substances in serum from people on diets rich in, for example, canola and mustard.Citation18 To confirm that MS possess a high anti-oxidant capacity, we explored a commonly used system to evaluate oxidative stress, which is based on copper-ox-LDL and its capacity to induce macrophages to produce ROS. Our results demonstrate that mustard seeds contain effective anti-oxidants, since it reduced the oxidative burst that was induced in mouse peritoneal macrophages by ox-LDL.
Moreover, cultured colon carcinoma cells were found to be growth depressed when exposed to a suspension of lipid-EMS that also increased apoptoic cell death. Interestingly, 3T3 cells, which are immortalized but otherwise normal were not affected. In vivo studies on mice also revealed that MS induce enhanced SOD, CAT, and GSH-Px activity and lower plasma LPO, indicating a systemic effect. Caspase-3, which is an executioner caspase in programmed cell death,Citation19 was activated by a suspension of EMS in the human colon cancer cell line SW480, whereas normal fibroblast from the 3T3 cell line, again, were not affected. This suggests that MS specifically inhibits tumor cell proliferation. Since the molecular mechanism behind this phenomenon is still obscure, we, as a start, tested whether perhaps phosphorylation of p53 or activation of beta-cantenine had taken place but found no indication of that (data not shown).
Our further findings, however, suggest that MS contain powerful biomolecules that counteracts the effects of oxidizing species, perhaps by upregulating anti-oxidative defensive mechanisms, depresses proliferation of colon cancer cells, induces apoptotic cell death of such cells and, thus, may be an excellent additive to food that not only acts as a splendid spice and a preservative but also as a protector against colon cancer.
In conclusion, we found that MS is an efficient anti-oxidants, which not only prevents AOM-induced colon carcinogenesis, but also induces programmed cells death to colon tumor cells by activating the caspase cascade and thereby apoptosis. Thus, MS might be considered a potential anti-tumor food.
Acknowledgments
Supported by the National Natural Science Foundation of China (81071549), the Guangdong Natural Science Foundation (9151040701000025), the Guangdong Science and Technology Foundation (2010B06090054), the Nanfang Hospital Foundation (2009B011), the Guangdong Talent Person Import Foundation, the Southern Medical University Talent Person Import Foundation, and the China Chunhui Plan. The authors Haifeng Yuan and Minggu Zhu contributed equally to this work.
References
- Liu L, Liu T, Li G. Isolation and determination of p-hydroxybenzoylcholine in traditional Chinese medicine Semen sinapis Albae. Anal Bioanal Chem 2003;376:854–8.
- Qiblawi S, Kumar A. Chemopreventive action by an extract from Brassica compestris (var Sarason) on 7,12-dimethylbenz(a)anthracene induced skin papillomagenesis in mice. Phytother Res 1999;13:261–3.
- Gagandeep , Dhiman M, Mendiz E. Chemopreventive effects of mustard (Brassica compestris) on chemically induced tumorigenesis in murine forestomach and uterine cervix. Hum Exp Toxicol 2005;24:303–12
- Wu GX, Lin YX, Ou MR. An experimental study(I) on the inhibition of prostatic hyperplasia with extract of seeds of Brassica alba. Zhongguo Zhong Yao Za Zhi 2002;27:766–8.
- Scalia D, Lacetera N, Bernabucci U, Demeyere K, Duchateau L, Burvenich C. In vitro effects of nonesterified fatty acids on bovine neutrophils oxidative burst and viability. J Dairy Sci 2006;89:147–54.
- Yuan Q, Liu YY, Sun K, Chen CH, Zhou CM, Wang CS, et al. Improving effect of pretreatment with yiqifumai on LPS-induced microcirculatory disturbance in rat mesentery. Shock 2009;32:310–6.
- Niu G, Yin S, Xie S. Quercetin induces apoptosis by activating caspase-3 and regulating Bcl-2 and cyclooxygenase-2 pathways in human HL-60 cells. Acta Biochim Biophys Sin 2011;43:30–7.
- Wang Z, Tang X, Li Y. 20-Hydroxyeicosateraenoic acid inhibits the apoptotic responses in pulmonary artery smooth muscle cells. Eur J Pharmccol 2008;588:9–17.
- Kim M, Miyamoto S, Yasui Y, Oyama T, Murakami A, Tanaka T. Zerumbone, a tropical ginger sesquiterpene, inhibits colon and lung carcinogenesis in mice. Int J Cancer 2009;124:264–71.
- Roderick R, Turner MD, Dean T. Colorectal carcinoma nodal staging. Arch Pathol Lab Med 2003;127:673–9.
- Zheng Y, Kramer PM, Olson G, Lubet RA, Steele VE, Kelloff GJ, et al. Prevention by retinoids of azoxymethane-induced tumors and aberrant crypt foci and their modulation of cell proliferation in the colon of rats. Carcinogenesis 1997;18:2119–25.
- Zheng Y, Kramer PM, Lubet RA, Steele VE, Kelloff GJ, Pereira MA. Effect of retinoids on AOM-induced colon cancer in rats: modulation of cell proliferation, apoptosis and aberrant crypt foci. Carcinogenesis 1999;20:255–60.
- Vanamala J, Leonardi T, Patil BS, Taddeo SS, Murphy ME, Pike LM, et al. Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis 2006;27:1257–65.
- Wilhelm J, Vytasek R, Ostadalova I, Vajner L. Evaluation of different methods detecting intracellular generation of free radicals. Mol Cell Biochem 2009;328:167–76.
- McMichael AJ. Food, nutrition, physical activity and cancer prevention. Authoritative report from World Cancer Research Fund provides global update. Publ Health Nutr 2008;11:762–3.
- Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc 2008;67:253–6.
- Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res 2007;55:224–36.
- Shahidi F. Antioxidant factors in plant foods and selected oilseeds. Biofactors 2000;13:179–85.
- Kuribayashi K, Mayes PA, El-Deiry WS. What are caspases 3 and 7 doing upstream of the mitochondria? Cancer Biol Ther 2006;5:763–5.