Abstract
The antioxidant effect of saponarin, which is the main flavone isolated from Gypsophila trichotoma Wend., and its protection against cocaine hepatotoxicity were investigated in male Wistar rats. The animals were treated with cocaine (40 mg/kg i.p.) alone and also after 3 consecutive days of pretreatment with saponarin (80 mg/kg p.o.). After 18 hours the rats were sacrificed by decapitation. The production of thiobarbituric acid reactive substances, reduced glutathione (GSH) and the activity of the following antioxidant enzymes: catalase, superoxide dismutase, glutathione peroxidase, glutathione reductase, and glutathione-S-transferase were assessed in liver homogenate. Administered alone, cocaine induced significant hepatotoxicity manifested with GSH depletion and reduced antioxidant defences. Saponarin pretreatment, however, decreased cocaine toxicity both by increasing GSH levels and antioxidant enzyme activities. The results of this study proved the antioxidant activity of saponarin and its protective effect against cocaine-induced oxidative stress and hepatotoxicity.
Introduction
Flavonoids are a group of natural polyphenolic products with a number of biological and pharmacological activities: antibacterial, antiviral, antioxidant, and antimutagenic effects.Citation1 Numerous medicinal plants contain therapeutic amounts of flavonoids and are used as supplementary therapies for many cardiac, gastroenterological, immunological, etc. disorders.Citation2 Gypsophila trichotoma Wend. (Caryophyllaceae) is a perennial herbaceous plant located in southeast Europe, southwest Asia, Kazakhstan, west Mongolia, Russia, and Turkmenistan. In Bulgaria the plant is found along the Black Sea coast.Citation3 A previous phytochemical study isolated and elucidated the structure of triterpene saponins from G. trichotoma.Citation4 Flavonoids, sterols, and volatile compounds were identified in the aerial parts by high-performance liquid chromatography (HPLC) and gas chromatography (GC) methods.Citation5,Citation6
Saponarin, a naturally occurring apigenin-6-C-glucosyl-7-O-glucoside, is the main flavone isolated from G. trichotoma Wend. (Caryophyllaceae), for which in vitro antioxidant activity was evaluated by a 1,1-diphenyl-2-picryl-hydrazyl (DPPH) test.Citation7 However, in the literature available there are insufficient data about its pharmacological properties. In one of their reviews on flavonoids, Bylka et al.Citation2 mentioned that saponarin has a proven inhibitory activity against a variety of human pathogens. Cos et al.Citation1 reported that flavones, including saponarin, inhibit xanthine oxidase (an important biological source of superoxide radicals) and have superoxide scavenging activity. Regarding these data, investigating the possible in vivo antioxidant activity of saponarin is of a great interest.
Cocaine is a widely abused recreational drug. Although the toxic effects of cocaine are mainly neuronal and cardiovascular, there are a number of data, both experimentalCitation8 and clinicalCitation9,Citation10 regarding its hepatotoxic effects. The oxidative metabolism of cocaine is thought to be responsible for the associated liver injury. In his study, RofaelCitation11 discussed the critical points in cocaine hepatotoxicity. They regarded the production of reactive oxygen species (ROS), such as hydrogen peroxide and superoxide anion radicals, as a result of oxidative metabolism of cocaine, which is critical for cocaine-induced hepatic injury.
On the basis of these data, the aim of the following study was to investigate the possible in vivo antioxidant effect of saponarin, isolated from G. trichotoma Wend., and its protection against cocaine-induced hepatotoxicity in rats.
Materials and methods
Drugs and chemicals
All the reagents used were of analytical grade. Cocaine hydrochloride, as well as other chemicals, 1-chloro-2,4-dinitrobenzene, beta-nicotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt (NADPH), ethylenediaminetetraacetic acid (EDTA), bovine serum albumin (fraction V) reduced glutathione (GSH), oxidized glutathione (GSSG), glutathione reductase (GR), and cumene hydroperoxide were purchased from Sigma Chemical Co. (Taufkirchen, Germany). 2,2′-Dinitro-5,5′dithiodibenzoic acid (DTNB) was obtained from Merck (Darmsstadt, Germany).
Plant material, extraction, and isolation of saponarin
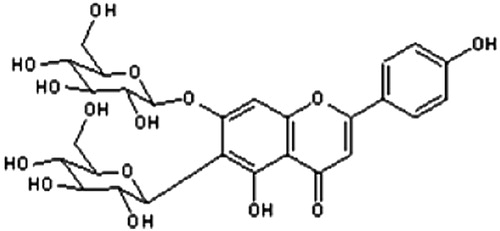
The overground parts of G. trichotoma Wend. (Caryophyllaceae) was collected in August 2008 at the Black Sea coast, Bulgaria. A voucher specimen (SO 103887) was deposited at the Herbarium of the Faculty of Biology, Sofia University. Citation1H NMR (400 MHz) and Citation13C NMR (100.6 MHz) spectra were recorded on Bruker DPX-400 and Bruker AMX-400. High resolution electron impact mass spectrometry (HR-EIMS) was carried out on Varian MAT CH7A. A thin-layer chromatography study was carried out on silica gel plates (Kieselgel G, F254, 60, Merck) with solvent systems n-BuOH/AcOH/H2O (4:1:1), CHCl3/MeOH (9:1), and EtOH/NH3/H2O (80:4:16). The spots were visualized using diphenylboric acid aminoethyl ester/polyethylene glycol (NTS/PEG) reagent (for flavonoids) and anisidine/hydrogen phthalate reagent, followed by heating at 110°C (for sugars). Colon chromatography (CC) was carried out with Diaion HP20. Acid hydrolysis was performed with 7% methanolic HCl for 3 hours. Air-dried powdered plant material (740 g) was exhaustively extracted with 80% methanol. After partial evaporation the aqueous solutions were extracted with CH2Cl2, EtOAc, and n-BuOH successively. The residue from the n-BuOH layer was separated on a Diaion HP20 column, using H2O–MeOH (0 → 100%), to yield saponarin (apigenin 6-C-glucosyl-7-O-glucoside) in a very high quantity (over 2 g) (). Structural assessment of the compound was performed by acid hydrolysis and analysis of MS (ESI-MS: 595.1690 [M + H]+) and Citation1H and Citation13C NMR spectroscopic data.Citation7 The antioxidant activity of saponarin was evaluated by a DPPH test (88.8% inhibition of DPPH radical in the concentration range 0.5 mg/ml).Citation7
For the following in vivo study, saponarin was dissolved, ex tempore, in redistilled water by sonification.
Experimental animals
Male Wistar rats (weighing 200 ± 10 g) were housed under standard laboratory conditions at 20°C, with 12-hour alternating light/dark cycles and free access to food and water. The animals were purchased from the National Breeding Centre, Slivnitza, Bulgaria. All experiments were performed after at least one week of adaptation to this environment. All performed procedures were approved by the Institutional Animal Care Committee and the principles stated in the European Convention for the Protection of Vertebrate Animals used for experimental and other scientific purposes (ETS 123)Citation12 were strictly followed throughout the experiment.
Design of the experiment
The animals were divided into four groups (n = 6). The first group received saponarin at a dose of 80 mg/kg p.o. once a day for 3 days.Citation13 The second group received cocaine at a single dose of 40 mg/kg i.p.Citation14 The third group was treated with saponarin (80 mg/kg p.o. for 3 days), and 2 hours after the last administration the animals were challenged with cocaine (40 mg/kg i.p.). The fourth group comprised control animals, which were treated with saline, involved in the experiment from the very beginning and housed under the same standard laboratory conditions as the treated animals in groups 1–3.
The animals in all groups were sacrificed on the fourth day of the experiment. For all following experiments livers were removed, washed with ice-cold saline solution (0.9% NaCl) blotted dry, weighed and divided into three pieces (stored on ice), 1 g each for assessment of thiobarbituric acid reactive substances (TBARS), GSH levels, and antioxidant enzymes activities. Tissues were homogenized in ice-cold buffers using glass homogenizer (PX-OX 2000).
Preparation of liver homogenate for lipid peroxidation assessment
Lipid peroxidation (LPO) was determined by measuring the rate of production of TBARS (expressed as malondialdehyde equivalents) described by Polizio and PenaCitation15 with slight modifications. Briefly, one volume of homogenate was mixed with one volume 25% trichloracetic acid (TCA) and one volume 0.67% thiobarbituric acid (TBA). Samples were then mixed thoroughly, heated for 20 minutes in a boiling water bath, cooled, and centrifuged at 4000 rpm for 20 minutes. The absorbance of supernatant was measured at 535 nm against a blank that contained all the reagents except the tissue homogenate. Malondialdehyde concentration was calculated using a molar extinction coefficient of 1.56`;105/M/cm and expressed in nmol/g wet tissue.
Preparation of liver homogenate for GSH assessment
GSH was assessed by measuring non-protein sulfhydryls after precipitation of proteins with TCA, using the method by Bump et al.Citation16 Briefly, tissues were homogenized in 5% TCA and centrifuged for 20 minutes at 4000 × g. The reaction mixture contained 0.05 ml supernatant, 3 ml 0.05 M phosphate buffer (pH = 8), and 0.02 ml DTNB reagent. The absorbance was determined at 412 nm and the results are expressed as nmol/g wet tissue.
Preparation of liver homogenates for antioxidant enzyme activity measurement
The livers were rinsed in ice-cold physiological saline and minced with scissors. Ten percent homogenates were prepared in 0.05 M phosphate buffer (pH = 7.4), then centrifuged at 7000 × g and the supernatants then used for antioxidant enzymes assay. The protein content of liver homogenate was measured by the method of LowryCitation17 with bovine serum albumin as a standard.
Catalase activity (CAT) was assessed following the method of Aebi et al.Citation18 Briefly, 10 µl of homogenate was added to 1990 µl of H2O2 solution (containing 6.8 µl of 30% H2O2 + 1983.2 µl 0.05 M phosphate buffer, pH = 7.4). CAT activity was determined by monitoring the H2O2 decomposition which was measured spectrophotometrically by the decrease in absorbance at 240 nm. Enzyme activity was calculated using a molar extinction coefficient of 0.043/mM/cm and expressed as μM/minute/mg.
Superoxide dismutase activity (SOD) was measured according to the method of Misura and Fridovich,Citation19 following spectrophotometrically the autoxidation of epinephrine at pH = 10.4, 30°C, using the molar extinction coefficient of 4.02/mM/cm. The incubation mixture contained 50 mM glycine buffer, pH = 10.4. The reaction is started by the addition of epinephrine. SOD activity is expressed as nmol of epinephrine that are prevented from autoxidation after addition of the sample.
Glutathione peroxidase activity (GPx) was measured by NADPH oxidation, using a coupled reaction system consisting of glutathione, glutathione reductase and cumene hydroperoxide.Citation20 Briefly, 100 µl of enzyme sample was incubated for 5 minutes with 1.5 ml 0.05 M phosphate buffer (pH = 7.4), 100 µl 1 mM EDTA, 50 µl 1 mM GSH, 100 µl 0.2 mM NADPH, and 1 unit glutathione reductase. The reaction was initiated by adding 50 µl cumene hydroperoxide (1 mg/ml) and the rate of disappearance of NADPH over time was determined by monitoring absorbance at 340 nm using an extinction coefficient of 6.22 × 103/M/cm. Results are expressed in nmol/minute/mg.
Glutathione reductase (GR) activity was measured spectrophotometrically at 340 nm according to the method of Pinto et al.Citation21 by following NADPH oxidation and using an extinction coefficient of 6.22 × 103/M/cm. The incubation mixture contained 0.05 M phosphate buffer, pH = 7.4, 2.5 mM GSSG, and 125 µM NADPH at 30°C. Enzyme activity is expressed in nmol/minute/mg.
Glutathione-S-transferase (GST) activity was measured using 1-chloro-2,4-dinitrobenzene (CDNB) as the substrate.Citation22 The incubation mixture containing 1.6 ml 0.05 M phosphate buffer, 100 µl 1 mM GSH, 100 µl 1 mM EDTA, and 100 µl homogenate was incubated for 15 minutes at 37°C. After the incubation, 100 µl 1 mM CDNB was added and the increase in absorbance with time was recorded at 340 nm. Enzyme activity is measured using an extinction coefficient of 9.6 × 103/M/cm and is expressed as nmol of CDNB-GSH conjugate formed/minute/mg protein.
Statistical analysis
Statistical analysis was performed using statistical programme ‘MEDCALC’. Results are expressed as mean ± SEM for six rats in each group. The significance of the data was assessed using the non-parametric Mann–Whitney test. Values of P ≤ 0.05 were considered statistically significant.
Results
The results are shown in –4. Compared to controls, saponarin, administered alone (80 mg/kg p.o. 3 days), increased GSH levels by 35% (P < 0.05) and decreased LPO by 15% (P < 0.05) (). At the same time, no variation in CAT, SOD, GPx, GR, and GST activities was observed ( and ).
Figure 2. TBARS and GSH levels on rat liver in saponarin group (receiving saponarin 80 mg/kg p.o. 3 days), cocaine group (40 mg/kg, single i.p. injection on third day) and saponarin + cocaine group (80 mg/kg p.o. 3 days + 40 mg/kg, single i.p. injection 2 hours after the last saponarin administration). Data are expressed as mean ± SEM of six rats. *P < 0.05 versus control group; +P < 0.05 versus cocaine-treated group.
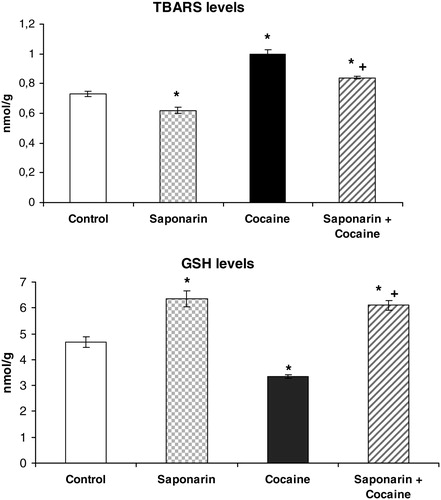
Figure 3. CAT, SOD, and GPx activities on rat liver in saponarin group (receiving saponarin 80 mg/kg p.o. 3 days), cocaine group (40 mg/kg, single i.p. injection on third day) and saponarin + cocaine group (80 mg/kg p.o. 3 days + 40 mg/kg, single i.p. injection 2 hours after the last saponarin administration). Data are expressed as mean ± SEM of six rats. *P < 0.05 versus control group; +P < 0.05 versus cocaine-treated group.
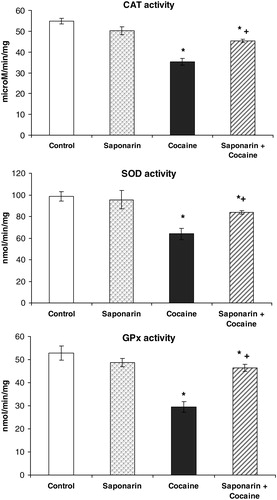
Figure 4. GR and GST activities on rat liver in saponarin group (receiving saponarin 80 mg/kg, p.o. 3 days), cocaine group (40 mg/kg, single i.p. injection on third day) and saponarin + cocaine group (80 mg/kg p.o. 3 days + 40 mg/kg, single i.p. injection 2 hours after the last saponarin administration). Data are expressed as mean ± SEM of six rats. *P < 0.05 versus control group; +P < 0.05 versus cocaine-treated group.
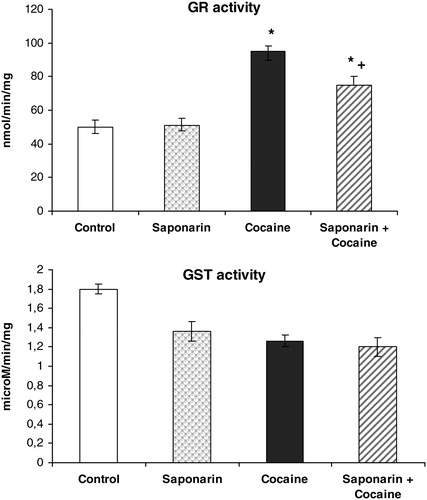
Compared to control animals, acute administration of cocaine (40 mg/kg i.p.) led to significant decreases in GSH levels by 29% (P < 0.05), in CAT activity by 36% (P < 0.05), in SOD activity by 35% (P < 0.05), and in GPx activity by 43% (P < 0.05). At the same time, malondialdehyde (MDA) levels and GR activity were increased by 37% (P < 0.05) and by 91% (P < 0.05), respectively.
Pretreatment with saponarin for three days and a subsequent single i.p. administration of cocaine on the third day produced increase in GSH levels by 30% (P < 0.05), in TBARs levels by 15% (P < 0.05), and in GR activity by 50% (P < 0.05), while the activities of CAT, SOD, and GPx were decreased by 17% (P < 0.05), by 24% (P < 0.05), and by 12% (P < 0.05), respectively, compared to the control group.
In addition, when comparing the data obtained from the saponarin + cocaine group versus the cocaine only group, the following were observed: significant increases in GSH levels by 82% (P < 0.05), in CAT activity by 79% (P < 0.05), in SOD activity by 17% (P < 0.05), and in GPx activity by 58% (P < 0.05), and significant decreases in TBARs levels and GR activity by 16% (P < 0.05) and 21% (P < 0.05), respectively. These results confirm the protective effect of saponarin on enzymatic and non-enzymatic liver antioxidant defensive systems after cocaine damage.
Discussion
Oxidative stress is a common mechanism contributing to initiation and progression of hepatic damage and is caused by different xenobiotics. Cocaine is an alkaloid psychostimulant with a high addictive potential. In experimental animals cocaine has shown severe hepatotoxicity. The mechanism proposed involves ROS which are produced during cocaine bioactivation to norcocaine through N-demethylation by cytochrome P 450 and flavin adenine dinucleotide containing monoxygenases.Citation23 Two major pathways have been postulated to be involved in ROS generation. First, it has been suggested that the nitrosonium ion, generated during the oxidation of norcocaine, or other as yet unidentified reactive metabolites bind covalently to cellular macromolecules. Secondly, as a result of redox cycling between N-hydroxyl norcocaine and norcocaine nitroxide, superoxide anion (O2−) can be produced at the expense of NADPH.Citation24
Since the oxidative stress is regarded as one of the main mechanisms responsible for different types of organ injury, it has been considered that supplementation with antioxidants can exert beneficial effects. A number of biologically active compounds have been isolated and identified as agents with potent antioxidant activities. Recently, flavonoids have received attention due to their hepatoprotective and antioxidant effects. These protective effects could be related to different mechanisms, such as scavenger activity of free radicals that induce LPO and also their ability to stimulate antioxidant defence system.Citation25
Saponarin, a naturally occurring 7-O-glucoside, is the main flavone isolated from G. trichotoma Wend. (Caryophyllaceae) for which in vitro antioxidant activity was evaluated using a DPPH test.Citation7 In the literature there are data on the in vitro antioxidative capacity of saponarin isolated from Gentiana piasezkiiCitation26 and Aloe species (Asphodelaceae).Citation27
In our study, the in vivo antioxidant effect of saponarin, isolated from G. trichotoma Wend. (Caryophyllaceae), on cocaine-induced oxidative liver damage in rats was investigated. Saponarin itself increased levels of the cell protector GSH and inhibited MDA formation. The observed beneficial effect of saponarin on LPO corresponds with the results of Benedet et al.Citation28 that reported an antioxidant activity of saponarin/lutonarin mixture, comparable to those obtained from alpha-tocopherol. Antioxidant molecules (GSH, ascorbic acid, and vitamins A and E), as well as antioxidant enzymes (SOD, CAT, and GPx), protect cells against oxidative stress and free radical damage.Citation29 In our study, acute administration of cocaine induced oxidative stress, manifested by increases in TBARs levels and decreases in GSH levels (see ). GSH has critical physiological functions and protects against oxidative damage. It functions by detoxifying electrophiles and scavenging free radicals consequently producing GSSG.Citation25 The increased GSSG production (which we have not measured in this study) could be a possible reason for the increased activity of GR, which is observed after acute cocaine treatment. Our results are supported by results from other research groups that have shown that cocaine and its oxidative metabolites might produce hepatotoxicity through the generation of ROS, including lipid peroxides and accompanied by GSH depletion.Citation30 The decrease in the activity of SOD, CAT, and GPx (see ) by cocaine indicates a disturbance in the antioxidant defence system. Labib et al.Citation8 reported a significant decrease of antioxidant enzymes activity by cocaine. These data support our results.
In the group receiving saponarin + cocaine, our data demonstrated a significant effect of saponarin as a hepatoprotective and antioxidant agent. Saponarin pretreatment for 3 days prevented the effects observed for cocaine administered alone on liver LPO, GSH levels and CAT, SOD, GPx, and GR activities.
Conclusion
Considering the results of this study, we could conclude that saponarin demonstrates beneficial effects against cocaine-induced hepatotoxicity by modulation of enzymatic and non-enzymatic liver antioxidant defence system. It could be suggested as a potential candidate for adjuvant phytotherapy of mild to moderate liver injury.
References
- Cos P, Ying L, Calomme M, Hu JP, Cimanga K, Van , Poel B, et al. Structure–activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod 1998;61:71–6.
- Bylka W, Matlawska I, Pilewski NA. Natural flavonoids as antimicrobial agents. JANA 2004;7(2):24–31.
- Valev S. In: , Jordanov D. (ed.) Flora Republicae Popularis Bulgaricae. 3rd ed. Sofia: Aedibus Acad Sci Bulgaricae; 1966. p. 388–94.
- Krasteva I, Jenett-Siems K, Kaloga M, Nikolov S. 3-O-Sulfo-triterpenoid saponins from Gypsophila trichotoma. Wend Z Naturforsch 2009;64(3):319–22.
- Krasteva I, Popov I, Pencheva I, Balabanova V, Nikolov S. Phytochemical study of Gypsophila trichotoma Wend. (Caryophyllaceae). Quimica Nova 2008;31(5):1125–6.
- Krasteva I, Yotova M, Popov I, Zdraveva P, Nikolov S. Phytochemical study of leaves and roots of Gypsophila trichotoma Wend. using gas chromatography–mass spectrometry. Pharmacia 2009;56(1–4):3–6.
- Yotova M, Krasteva I, Jenett-Siems K, Zdraveva P, Nikolov S. Secondary metabolites in Gypsophila trichotoma Wend. Pharmacognosy Mag 2010;22 (Suppl):159.
- Labib R, Turkal R, Abdel-Rahman MS. Inhibition of cocaine oxidative metabolism attenuates endotoxin potentiation of cocaine mediated hepatotoxicity. Toxicology 2002;179:9–19.
- Campos FF, Martínez RC, Pérez BE. Fallo hepático fulminante asociado al consumo de cocaína. Ann Med Int 2002;19:365–7.
- Pérez JM, García MD, Leiva SI, García OR. Cocaine-induced hepatotoxicity. Med Clin (Barc) 2008;130(7):279.
- Hany Z. Rofael effect of ketamine pretreatment on cocaine-mediated hepatotoxicity in rats. Tox Lett 2004;152:213–22.
- Council of Europe. European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes. CETS No. 123, 1991 [displayed 2007 May 30]. Available from: http://conventions.coe.int/Treaty/Commun/QueVoulezVous.asp?NT=123&CM=1&CL=ENG).
- Sengupta S, Mukherjee A, Goswami R, Basu S. Hypoglycemic activity of the antioxidant saponarin, characterized as alpha-glucosidase inhibitor present in Tinospora cordifolia. J Enzyme Inhib Med Chem 2009; 24(3):684–90.
- Devi BG, Chan AW. Cocaine-induced peroxidative stress in rat liver: antioxidant enzymes and mitochondria. J Pharmacol Exp Ther 1996;279(1):359–66.
- Polizio AH, Pena C. Effects of angiotensin II type 1 receptor blockade on the oxidative stress in spontaneously hypertensive rat tissues. Regul Pept 2005;128:1–5.
- Bump EA, Taylor YC, Brown MJ. Role of glutathione in the hypoxic cell cytotoxicity of misonidazole. Cancer Res 1983;43:997–1002.
- Lowry OH. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–75.
- Aebi H. Catalase. In: , Bergrenyer HV (ed.) Methods of enzymatic analysis. 2nd ed. New York: Academic Press; 1974. p. 673–84.
- Misura HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247:3170–5.
- Tappel AL. Glutathione peroxidase and hydroperoxydes. Methods Enzymol 1978;52:506–13.
- Pinto MC, Mata AM, Lopez-Barea J. Reversible inactivation of Saccharomyces cerevisiae glutathione reductase under reducing conditions. Arch Biochem Biophys 1984;228:1–12.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249:7130–9.
- Labib R, Turkall R, Abdel-Rahman MS. Oral cocaine produces dose-related hepatotoxicity in male mice. Tox Lett 2001;125:29–37.
- Aoki K, Ohmori M, Takimoto M, Ota H, Yoshida T. Cocaine induced liver injury in mice is mediated by nitric oxide in reactive oxygen species. Eur J Pharmacol 1997;336:43–9.
- Nencini C, Giorgi G, Micheli L. Protective effect of silymarin on oxidative stress in rat brain. Phytomedicine 2007;14:129–35.
- Wu QX, Li Y, Shi YP. Antioxidant phenolic glucosides from Gentiana piasezkii. J Asian Nat Prod Res 2006;8(5):391–6.
- Keyhanian S, Stahl-Biskup E. Phenolic constituents in dried flowers of aloe vera (Aloe barbadensis) and their in vitro antioxidative capacity. Plant Med 2007;73:599–602.
- Benedet JA, Umeda H, Shibamoto T. Antioxidant activity of flavonoids isolated from young green barley leaves toward biological lipid samples. J Agric Food Chem 2007;55(14):5499–504.
- Burton GW, Foster DO, Perly B, Slater TF, Smith IC, Ingold KU. Biological antioxidants. Philos Trans R Soc Lond Biol Sci 1985;311(1152):565–78.
- Kovacic P. Role of oxidative metabolites of cocaine in toxicity and addiction: oxidative stress and electron transfer. Med Hyp 2005;64:350–6.