Abstract
Background
Gut manipulation during surgery may induce local oxidative stress. Supplemental inspired oxygen increases arterial and tissue oxygen partial pressures. This study examined whether a 0.80 fraction of inspired oxygen (FiO2) attenuates the oxidative stress, reducing xanthine oxidase (XO) activity in colonic mucosa as a possible mechanism during colon surgery.
Methods
Twenty-four cancer patients who underwent elective colon resection were randomly assigned to either 0.30 FiO2 or 0.80 FiO2 during surgery. Malondialdehyde (MDA) and oxidized glutathione (GSSG) levels were measured in blood plasma. We also determined the enzymatic activities of XO and xanthine dehydrogenase (XDH) in the colonic mucosa after resection of the piece of colon.
Results
Oxygen partial pressure in arterial blood was higher in the 0.80 FiO2 group than in the 0.30 FiO2 group (P < 0.001). MDA and GSSG levels measured in blood plasma as well as MDA measured in colonic mucosa were lower in the 0.80 FiO2 group than in the 0.30 FiO2 group. Otherwise, XO enzymatic activity and XO/(XO + XDH) ratio in colonic mucosa were lower in the 0.80 FiO2 group than in the 0.30 FiO2 group.
Conclusions
XO may be a major source of reactive oxygen species in patients during colon surgery. Inspiring 0.80 oxygen during colon surgery increases arterial partial pressure and this treatment was associated with reduced XO activity and levels of oxidative stress in colonic mucosa.
Introduction
Intestinal tissue is metabolically very active and has a poor tolerance for even short periods of hypoxia or ischaemia.Citation1 The special architecture of intestinal villi's microcirculation may increase the susceptibility of intestinal epithelium to hypoxic damage due to insufficient perfusion, which increases the dependency on oxygen uptake.Citation2,Citation3 Other factors may increase the relative intestinal hypoxia that can occur with normal arterial oxygen saturation during abdominal surgery, including: surgical stress, surgical retractors, increased intra-abdominal pressure, intestinal mobilization, operative disruption of blood vessels, and splanchnic vasoconstriction.Citation4 On the other hand, laparotomy and mild intestinal manipulation leads to activation of xanthine oxidase (XO) in enterocytes. In brief, in ischaemic tissues the phosphorylation potential fall enhances ADP catabolism with increased production of hypoxanthine and xanthine. Intracellular proteases are stimulated by Ca2+ overload and acidosis and convert xanthine dehydrogenase (XDH) into XO. This results in oxidative damage to the intestinal mucosa by generation of superoxide anions.Citation5–Citation7
Fractional inspired oxygen (FiO2) is a major determinant of arterial oxygen partial pressure (PaO2) and tissue oxygen tension (as well as haemoglobin levels and cardiac output). Consequently, a high PaO2 improves oxygenation during hypoxia in intestinal epithelial cells in poorly perfused areas.Citation8–Citation10 Perioperative administration of highly concentrated inspired oxygen (0.80 FiO2 throughout surgery and for a postoperative period) may enhance PaO2 and oxygen tissue tension and reported a reduction in the risk of wound infection in patients undergoing colon surgery.Citation4,Citation11,Citation12 In contrast, other studies hypothesized that supplemental perioperative oxygen increased the production of reactive oxygen species,Citation13 or that it did not result in a difference in risk of surgical site infection after abdominal surgery.Citation14 However, we recently found a decrease in oxidative stress levels in patients undergoing colon surgery with supplemental perioperative oxygen (0.80 FiO2), with a decrease in blood levels of oxidative stress markers from lipoperoxidation (malondialdehyde, MDA) and glutathione oxidation (oxidized glutathione, GSSG).Citation15 We do not have data on the possible oxidative stress mechanisms in these patients. For this reason, this work is a complement of the previously cited article.Citation15
We initiated this study to determine the effect of a high concentration of oxygen in the inspired gas mixture on oxidative stress mechanisms during colon surgery. The objective was to establish the effect of two different oxygen concentrations (0.30 FiO2 or 0.80 FiO2) on oxidative stress marker levels. Specifically, to determine whether supplemental oxygen with 0.80 FiO2 protected against oxidative stress during colon surgery. We have analysed the MDA levels, as a lipoperoxidation marker, as well as the XDH and XO activities in the colonic mucosa, as these activities represent a major source of free radicals during intestine manipulation or ischaemia–reperfusion.
Materials and methods
Study subjects
Twenty-four consecutive cancer patients undergoing elective colon resection were randomly assigned to receive 0.30 FiO2 (n = 12) or 0.80 FiO2 (n = 12) after obtaining informed consent. Surgeons and data analysers were blinded to the treatment regimen. Ages ranged from 18 to 80 years. The study was carried out in accordance with the Helsinki Declaration of 1975 and subsequent reviews. The Ethical Committee of the hospital approved the study. Exclusion criteria were fever, existing signs of infection, diabetes mellitus (type 1 or 2), pregnancy, drug addiction, intake of antioxidants vitamins, and malnutrition (serum albumin under 3.3 g/dl, white cell count under 2500 cells/mm3, or body weight loss over 20% in the last 2 months).
Protocol
The night before surgery all patients underwent a standard bowel preparation with electrolyte solution.Citation11,Citation12 In addition, 2 days before surgery patients were on a liquid diet without residues or supplemental antioxidant vitamins. All patients were given prophylactic intravenous antibiotics (metronidazole combined with amoxicillin-clavulanic acid) 60 minutes before the surgical incision and for 48 hours postoperatively (every 8 hours), according to the hospital protocol.
Anaesthesia
Anaesthesia was induced intravenously with sodium thiopental (3–5 mg/kg) and fentanyl (2 µg/kg). Endotracheal intubation was facilitated with rocuronium bromide (0.6 mg/kg). Anaesthesia was subsequently maintained with sevoflurane (2%), fentanyl, and rocuronium bromide. After induction of anaesthesia, patients were randomly assigned to receive either 0.30 FiO2 or 0.80 FiO2 throughout surgery. During surgery, the lungs were ventilated in a volume-controlled mode (8–10 ml/kg tidal volume) and patients were hydrated with Ringeŕs solution (15 ml/kg/hour).Citation11,Citation12 Standard anaesthesia monitoring, including arterial oxygen saturation, blood pressure, heart rate, and end tidal CO2 were measured at 5 minutes intervals during anaesthesia.
Measurements
Arterial blood samples for analysis were obtained from a radial artery line 20 minutes after induction of anaesthesia (‘before surgery’ in ) and immediately after the surgery procedure ended (‘after surgery’ in ). The arterial blood gases: pH, partial oxygen pressure (PaO2), and partial carbon dioxide pressure (PaCO2) were analysed with an ABL-3 (Radiometer, Copenhagen, Denmark).
Once the surgeon removed the piece of colon, a sample was taken from the healthy proximal border that included the colonic mucosa. These samples were frozen in liquid nitrogen and stored at −80°C until they were processed. All frozen colonic mucosa samples were extracted at ice temperature. A 200 mg aliquot was weighed, homogenized (Ultraturrax T8, Labortecnik, Staufen, Germany) in 4 ml/g phosphate buffer, and centrifuged (15 000 g for 30 minutes at 4°C). The supernatant was removed and eluted through a 5 × 1.44 cm2 Sephadex G-25 column equilibrated with phosphate buffer. The eluted was fractionated into aliquots, which were individually tested for XO and XDH activities and protein concentration in colonic mucosa.
All chemicals were provided at the highest available purity degree from Sigma-Aldrich (St Louis, MO, USA), Merk (Darmstad, Germany), and Boehringer Mannheim (Mannheim, Germany).
MDA levels were measured in blood plasma and colonic mucosa. Briefly, lipoperoxides from lipid peroxidation of cellular polyunsaturated fatty acid were hydrolysed by boiling in dilute phosphoric acid (0.44 M). MDA is one of these hydrolysis products. Then, thiobarbituric acid (TBA) was added to the hydrolysis products, which reacts specifically with MDA to form MDA-(TBA)2 adduct. Next, plasma (or colonic mucosa homogenate) proteins were precipitated with methanol and removed by centrifugation. The protein-free extract was fractioned by a high-performance liquid chromatography (HPLC) method to separate the MDA-(TBA)2 adduct from interfering chromogens and quantified spectrophotometrically (Shimadzu SPD 10 AV, Shimadzu Scientific Instruments Inc., Columbia, MD, USA) with UV light at 532 nm.Citation16
GSSG concentration was measured by an HPLC method in whole blood, with detection at 365 nm which was developed to measure GSSG in the presence of large excess of reduced glutathione, using N-ethylmaleimide to prevent reduced glutathione (GSH) oxidation.Citation17
Determinations of XO and XDH activities in the colonic mucosa were carried out by fluorometric assays. In brief, the XO-catalysed conversion of pterin (2-amino-4-isoxanthopterin hydroxypteridine) to isoxanthopterin was the basis for this fluorometric assay (excitation: 345 nm and emission: 390 nm). XO is assayed in the presence of pterin only, while combined XDH plus oxidase activity is determined with methylene blue, which replaces NAD+ as an electron acceptor. XDH activity was calculated by subtracting the XO activity from the total activity (XDH + XO).Citation18 This assay has been successfully applied where XO and XDH activities are too low to detect spectrophotometrically. To express XDH-to-XO conversion, we used the ratio XO/(XO + XDH). The protein concentration in colonic mucosa was measured by the method of Lowry using bovine serum albumin as the standard.Citation19
Statistical analysis
Results are expressed in tables (as median and range) and figures (as scatter plot and median). Differences were analysed with two-tailed tests for non-parametric data: Mann–Whitney U and Wilcoxon tests. P values <0.05 were considered statistically significant. Pearson's correlation coefficients were determined between different parameters of oxidative stress in colonic mucosa: XO enzymatic activity and XO/(XO + XDH) ratio as independent variables and MDA as a dependent variable. The Statistical Package for the Social Sciences (version 11.0) was used for analysis (SPSS, Chicago, IL, USA) and Excel (Microsoft Office 2003, Redmond, WA, USA).
Results
No differences were found between the study groups in clinical and physical data (). Only intra-operative oxygen saturation on pulse oxymetry (SpO2) values were higher (P < 0.001) in patients receiving 0.80 oxygen (0.80 FiO2 group) compared with patients who received 0.30 oxygen (0.30 FiO2 group) ().
Table 1. Demographic data of the two groups of cancer patients
Table 2. Intraoperative values of clinical and physical status of the two groups of patients
Twenty minutes after the induction of anaesthesia and immediately after the end of surgery, both SpO2 and PaO2 values from arterial blood gas analysis were significantly higher in the 0.80 FiO2 patient group compared with the 0.30 FiO2 patient group (P < 0.001). No differences were found between the study groups in other parameters, such as PaO2/FiO2, PaCO2, pH, haemoglobin, and haematocrit (see and ).
Figure 1. Effects of FiO2 on arterial blood gas analysis (PaO2 and PaCO2) in the two groups (0.30 FiO2 and 0.80 FiO2) of cancer patients, before surgery and after surgery. Values expressed as scatter plot and median (horizontal bars). Differences were analysed with two-tailed tests for non-parametric data: Mann–Whitney U and Wilcoxon tests. P values <0.05 were considered statistically significant. (A and C), PaO2 values in the 0.80 FiO2 group were significantly higher (P < 0.001) than in the 0.30 FiO2 group's values, before and after surgery. (B and D), in PaCO2 values, no differences were found between the study groups, before and after surgery.
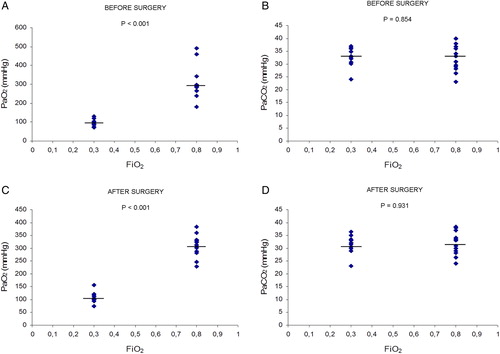
Table 3. Effects of FiO2 on arterial blood gas analysis in the two groups of cancer patients
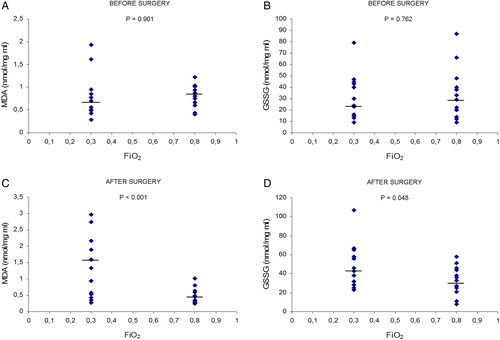
summarizes the results of oxidative stress markers measured in blood plasma. Blood plasma levels of MDA after surgery were lower in the 0.80 FiO2 group than in the 0.30 FiO2 group (P < 0.001). In addition, in the 0.30 FiO2 group, blood plasma levels of MDA after surgery were higher than before surgery (P < 0.029). However, in the 0.80 FiO2 group, blood plasma levels of MDA after surgery were lower than before surgery (P < 0.010). Blood plasma levels of GSSG after surgery were lower in the 0.80 FiO2 group than in the 0.30 FiO2 group (P < 0.048). In addition, in the 0.30 FiO2 group, blood plasma levels of GSSG after surgery were higher than before surgery (P < 0.039).
Figure 2. Effects of FiO2 on levels of oxidative stress markers like MDA and GSSG in arterial blood of the two groups (0.30 FiO2 and 0.80 FiO2) of cancer patients, before surgery and after surgery. Values expressed as scatter plot and median (horizontal bars). Differences were analysed with two-tailed tests for non-parametric data: Mann–Whitney U and Wilcoxon tests. P values <0.05 were considered statistically significant. (A and B), MDA and GSSG values respectively – for these markers no differences were found between the study groups before surgery. (C and D), MDA and GSSG, respectively, in the 0.30 FiO2 group were significantly higher than in the 0.80 FiO2 group after surgery.
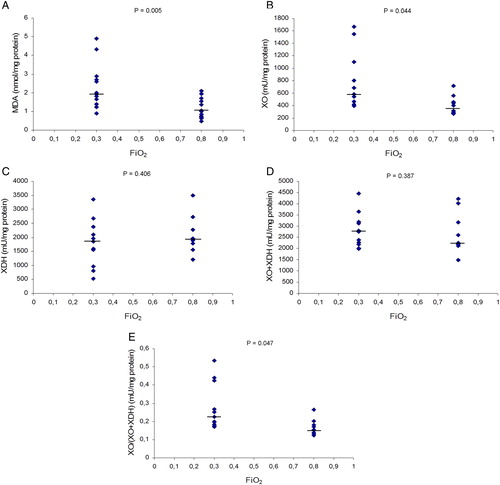
summarizes the results measured in colonic mucosa from removed piece of colon in the two groups of cancer patients. Tissue values of MDA after surgery were lower in the 0.80 FiO2 group than in the 0.30 FiO2 group (P = 0.005). In colonic mucosa, tissue values of XO activity were higher in the 0.30 FiO2 group than in the 0.80 FiO2 group (P = 0.044), and tissue values of XO/(XO + XDH) ratio, to express XDH-to-XO conversion, were higher in the 0.30 FiO2 group than in the 0.80 FiO2 group (P = 0.047).
Figure 3. Effects of FiO2 on MDA levels, XO activity, XDH activity, XO + XDH activities, and XO/(XO + XDH) ratio in colonic mucosa from excised colon in the two groups of cancer patients. MDA, malondialdehyde; XO, xanthine oxidase; XDH, xanthine dehydrogenase. Values expressed as scatter plot and median (horizontal bars). Differences were analysed with two-tailed tests for non-parametric data: Mann–Whitney U and Wilcoxon tests. P values <0.05 were considered statistically significant. (A) MDA values were significantly lower in the 0.80 group than in the 0.30 group. (B) XO activity was significantly lower in the 0.80 group than in 0.30 group. (C) On XDH activity no differences were found between the study groups. (D) On XO + XDH activities no differences were found between the study groups. (E) On XO/(XO + XDH) ratio, values were significantly lower in 0.80 group than 0.30 group.
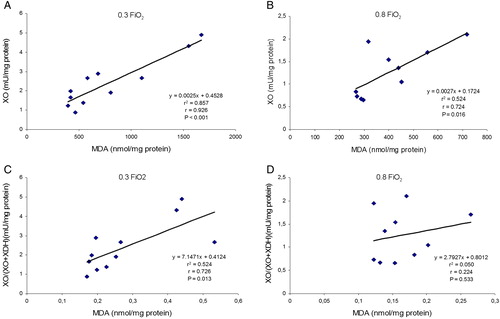
Significant and positive correlations were found between MDA tissue levels (as a dependent variable) and XO enzymatic activity and XO/(XO + XDH) ratio (as independent variables) in both groups. The Pearson's correlation coefficient (r) values were higher in the 0.30 FiO2 group than in the 0.80 FiO2 group (see ).
Figure 4. Effects of FiO2 on the correlations between MDA levels and XO activity and XO/(XO + XDH) ratio in colonic mucosa from removed piece of colon in the two groups of patients. MDA, malondialdehyde; XO, xanthine oxidase; XDH, xanthine dehydrogenase. r, Pearson's correlation coefficient. XO enzymatic activity and XO/(XO + XDH) ratio as independent variables and MDA as a dependent variable. P values <0.05 were considered statistically significant. (A) correlation between MDA levels and XO activity was very significant in 0.30 FiO2 group. (B) correlation between MDA levels and XO activity was significant in 0.80 FiO2 group and the r value was lower than 0.30 FiO2 group. (C) correlation between MDA levels and XO/(XO + XDH) ratio on 0.30 FiO2 group was statistically significant. (D) correlation between MDA levels and XO/(XO + XDH) ratio in 0.80 FiO2 group was not statistically significant and the r value was lower than 0.30 FiO2 group.
Discussion
This study shows that inhalation of 0.80 oxygen during colon surgery results in an increase in arterial oxygen partial pressures, which results in a decrease of XO enzymatic activity and XO/(XO + XDH) ratio and MDA tissue levels in the colonic mucosa. These results may explain, at least in part, a possible mechanism of oxidative stress induced by ischaemia–reperfusion in these patients.
MDA is a final product of fatty acid peroxidation in cell membranes and a classical marker of oxidative stress in tissues and blood.Citation16 We recently reported a reduction of MDA blood plasma levels and other blood oxidative stress marker levels, such as GSSG, in patients undergoing colon surgery with supplemental oxygen (0.80 FiO2).Citation15 In the present study, we again observed an increase of MDA and GSSG levels in the blood plasma, as well as in the colonic mucosa, of patients who inspired 0.30 FiO2 during colon surgery. However, patients who inspired 0.80 oxygen during the same study period did not show these increased oxidative stress markers. Therefore, administration of 0.80 FiO2 in ventilated patients undergoing colon surgery may protect against the peroxidation of fatty acids in the colonic mucosa by oxidative stress.
Splanchnic vasoconstriction induced by surgical stress is known to influence intestinal hypoxia during the operative period. Tissue hypoxia triggers a sequence of events that may ultimately lead to cellular dysfunction and XO activation with generation of superoxide anions, which are very reactive oxygen species.Citation5–Citation8,Citation20–Citation22 XO is derived from XDH in those hypoxia situations, and XO is a major source of superoxide anions during reperfusion.Citation20,Citation22 In this study we found that XO enzymatic activity and XO/(XO + XDH) ratio, to express XDH-to-XO conversion, in colonic mucosa during colon surgery were lower in patients who inspired 0.80 FiO2 than in patients who inspired 0.30 FiO2.
A transmural gradient of PaO2 exists across the colon with mucosal PaO2 far lower than serosal PaO2. Both serosal and mucosal PaO2 decrease during hypovolemia in shocked rats.Citation23 However, our patients were well hydrated and they did not have hypovolemia. Therefore, it is reasonable that a hyperoxic status and an increased PaO2 improve oxygen delivery to the places most in need of oxygen, such as the colon mucosa.
Arterial blood values (SpO2 and PaO2) were significantly higher in patients who received 0.80 FiO2 compared to patients receiving 0.30 FiO2. Although we have not used a Clark type polarographic oxygen electrode for measurement of tissue oxygen tension, we assumed that oxygen tension was also higher in the intestinal mucosa in patients receiving 0.80 FiO2.Citation8–Citation10 In rats it has been reported that a marked degrees of tissue hypoxia can be tolerated before major histological damage. Non-viable bowel had significantly impaired tissue oxygenation when compared with viable bowel.Citation24
It is possible that the high PaO2 may also have had an effect on the blood cells, because recently it has been shown that 80% oxygen may decrease the production of TNF alpha and increase production of reactive oxygen species in human leukocytes, and exerts significant effects on multiple cellular and immunological parameters, providing a potential mechanism for benefits from the use of supplemental oxygen.Citation25
This increase in oxygen tissue could explain a decrease in the activity of XO and XO/(XO + XDH) ratio in the group of patients who breathed 0.80 FiO2 since XDH is activated by hypoxia and XO is activated by reperfusion. This mechanism might explain, at least in part, the increase of oxidative stress in patients who inspired 0.30 FiO2.Citation15 Furthermore, the degree of oxidative stress, expressed as tissue levels of MDA in colonic mucosa, correlated positively and very significantly with the activity of XO and XO/(XO + XDH) ratio. These correlation coefficients were higher in the group of patients who breathed 0.30 FiO2 than in those who breathed 0.80 FiO2.
We could not find respiratory complications in our patients during the study period. Administration of 0.80 FiO2 during surgery and 2 hours after surgery did not worsen pulmonary function or cause atelectasis, as confirmed by chest computed tomographic scans.Citation11,Citation26 Furthermore, no changes were observed in the PaO2/FiO2 ratio, which rules out respiratory distress.
In relation to the clinical implications of supplemental oxygen and hyperoxia, recently it has been shown that hyperoxia has a suppressive effect on radical superoxide generation in blood, with improvement of oxidative stress, inflammatory response, and early endothelial damage in rats subjected to cerebral ischaemia–reperfusion.Citation27 In this context, it also has been recently demonstrated that hyperoxic preconditioning of rats in vivo protects against ischaemia–reperfusion myocardial damage by inhibition of mitochondrial transition pore opening and permeability cytochrome c release.Citation28
In summary, the results of our study showed an increase in oxidative stress marker levels in blood and colonic mucosa occur when 0.30 FiO2 is used during colon surgery, possibly through an increase in XO enzymatic activity in the colonic mucosa. The administration of 0.80 FiO2 prevented oxidative stress, with a reduction of lipid peroxidation and glutathione oxidation; this may be due to decreases in XO enzymatic activity and XO/(XO + XDH) ratio in the colonic mucosa. No signs of lung alteration were observed when the high concentration of oxygen was used during surgery. Our results support the administration of 0.80 FiO2 during colon surgery, because it prevented oxidative stressCitation15 and can be effective in the reduction of surgical wound infections, return of bowel function and duration of hospitalization. The primary defence against surgical pathogens is oxidative killing by neutrophils and oxidative killing is a function of tissue oxygen partial pressure.Citation11,Citation12,Citation29,Citation30 Moreover, supplemental oxygen appears to confer few risks to the patients and little associated cost.
Acknowledgement
The authors thank Maria Dolores Royo for her generous and skilful technical assistance and Koen J. Mateboer for proofreading the English manuscript.
References
- Beuk RJ, Heineman E, Tangelder GJ, Kurvers HA, Bonke HJ, Egbrink MG. Effects of different duration of total warm ischemia of the gut on rat mesenteric microcirculation. J Surg Res 1997;73:14–23.
- Aranow JS, Fink MP. Determinants of intestinal barrier failure in critical illness. Br J Anaesth 1996;77:71–81.
- Shepherd AP, Kiel JW. A model of countercurrent shunting of oxygen in the intestinal villus. Am J Physiol 1992;262:H1136–H42.
- Greif R, Laciny S, Rapf B, Hickle RS, Sessler DI. Supplemental oxygen reduces the incidence of posoperative nausea and vomiting. Anesthesiology 1999;91:1246–52.
- Anup R, Aparna V, Pulimood A, Balasubramanian KA. Surgical stress and the small intestine: role of oxygen free radicals. Surgery 1999;125:560–9.
- Thomas S, Anup R, Prabhu R, Balasubramanian KA. Effect of surgical manipulation of the rat intestine on enterocyte populations. Surgery 2001;130:479–88.
- Thomas S, Pulimood A, Balasubramanian KA. Heat preconditioning prevents oxidative stress-induced damage in the intestine and lung following surgical. Br J Surg 2003;90:473–81.
- Gottrup F, Firmin R, Rabkin J, Halliday B, Hunt TK. Directly measured tissue oxygen tension and arterial oxygen tension assess tissue perfusion. Crit Care Med 1987;15:1030–6.
- Hopf HW, Jensen JA, Hunt TK. Calculation of subcutaneous tissue blood flow. Surg Forum 1988;39:33–6.
- Ratnaraj J, Kabon B, Talcott MR, Sessler DI, Kurz A. Supplemental oxygen and carbon dioxide each increase subcutaneous and intestinal intramural oxygenation. Anesth Analg 2004;99:207–11.
- Greif R, Akça O, Horn E-P, Kurz A, Sessler DI. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med 2000;342:161–7.
- Belda FJ, Aguilera L, García de la Asunción J, Alberti J, Vicente R, Ferrandiz L, et al. Supplemental perioperative oxygen and the risk of surgical wound infection. A randomized controlled trial. J Am Med Assoc 2005;294:2035–42.
- Pryor KO, Fahey TJ, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgical population. J Am Med Assoc 2004;291: 89–7.
- Meyhoff CS, Wetterslev J, Jorgensen LN, Henneberg SW, Høgdall C, Lundvall L, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery. J Am Med Assoc 2009;302:1543–50.
- García-de-la-Asunción J, Belda FJ, Greif R, Barber G, Viña J, Sastre J. Inspired supplemental oxygen reduces markers of oxidative stress during elective colon surgery. Br J Surg 2007;94:475–7.
- Wong SHY, Knight JA, Hopfer SM, Zaharia O, Leach CN, Sunderman FW. Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin Chem 1987;33:214–20.
- Asensi M, Sastre J, Pallardó FV, García de la Asunción J, Estrela JM, Viña J. A high-performance liquid chromatography method for measurement of oxidized glutathione in biological samples. Anal Biochem 1994;217:323–8.
- Beckman JS, Parks DA, Pearson JD, Marshall PA, Freeman BA. A sensitive fluorometric assay for measuring xanthine dehydrogenase and oxidase in tissues. Free Radic Biol Med 1989;6:607–15.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951;193:265–75.
- Biffl WL, Moore EE. Splanchnic ischaemia/reperfusion and multiple organ failure. Br J Anaesth 1996;77:59–70.
- Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol 1986;250:G749–53.
- McCord J. Oxygen-derived free radicals in post-ischemic tissue injury. N Engl J Med 1985;312:159–63.
- Zabel DD, Hopf HW, Hunt TK. Transmural gut oxygen gradients in shocked rats resuscited with heparan. Arch Surg 1995;130:59–63.
- Sheridan WG, Lowndes RH, Williams GT, Young HL. Determination of a critical level of tissue oxygenation in acute intestinal ischemia. Gut 1992;33:762–6.
- Qadan M, Battista C, Gardner SA, Anderson G, Akca O, Polk H. Oxygen and surgical site infection. A study of underlying immunologic mechanisms. Anesthesiology 2010;113:369–77.
- Akça O, Podolsky A, Eisenhuber E, Panzer O, Hetz H, Lampl K, et al. Comparable postoperative pulmonary atelectasis in patients given 30% or 80% Oxygen during and 2 hours after colon resection. Anesthesiology 1999;91:991–8.
- Fujita M, Tsuruta R, Kaneko T, Otsuka Y, Kutsuna S, Izumi T, et al. Hyperoxia suppresses excessive superoxide anion radical generation in blood, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats: laboratory study. Shock 2010;34:299–305.
- Petrosillo G, Di Venosa N, Moro N, et al. In vivo hyperoxic preconditioning protects against rat-heart ischemia/reperfusion injury by inhibiting mitochondrial permeability transition pore opening and cytochrome c release. Free Radic Biol Med 2011;50:477–83.
- Babior BM, Oxygen-dependent microbial killing by phagocytes. N Engl J Med 1978;298:659–68.
- Allen DB, Maguire JJ, Mahdavian M, Wicke C, Marcocci L, Scheuenstuhl H, et al. Wound hypoxia and acidosis limit neutrophyl bacterial mechanisms. Arch Surg 1997;132:991–6.