Abstract
Background:
Raltegravir (RAL) plus tenofovir/emtricitabine (TDF/FTC) is a recommended initial antiretroviral regimen. A substantial proportion of persons diagnosed with HIV infection and starting antiretrovirals in the U.S. are African-American (AA); however, the effects of this regimen on metabolic parameters have largely been studied in white patients.
Methods:
Single-arm, open-label study of untreated AA HIV-infected patients administered RAL with TDF/FTC for 104 weeks. Changes in fasting lipids, insulin resistance, visceral adipose tissue (VAT), abdominal subcutaneous adipose tissue (SAT), limb and trunk fat, and bone mineral density (BMD) were assessed at weeks 56 and 104.
Results:
Thirty (85% men) participants were included. Median entry characteristics included age of 38 years, CD4 323 cells/mm3, HIV RNA level 29 245 copies/ml, and body mass index 28.1 kg/m2. At 56 and 104 weeks, significant increases in VAT, trunk fat, limb fat, and overall fat were observed. Bone mineral density decreased by 1.5% by week 104.There were no significant changes in non-HDL-cholesterol, fasting triglycerides, or insulin resistance. A median CD4 cell count increase of 318 cells/mm3 (IQR 179, 403; full range 40, 749) (P < 0.001) was observed. Assuming missing = failure, 78 and 70% had HIV RNA levels < 40 copies/ml at weeks 56 and 104, respectively. There were no treatment-related discontinuations and no new antiretroviral resistance mutations were detected.
Conclusions:
In this cohort of AAs, initiation of RAL with TDF/FTC was associated with significant general increases in fat. Significant changes in lipids or insulin resistance were not observed and there was a small decline in BMD. Therapy was well tolerated and effective. These results are consistent with findings of studies of initial antiretroviral therapy in racially diverse cohorts and inform treatment selection for AA patients starting therapy for HIV infection.
Introduction
A variety of metabolic abnormalities including altered body shape, generalized fat gain, dyslipidema, glucose intolerance, and declines in bone density have been observed following the initiation of antiretroviral therapy.Citation1–Citation3 Associations between HIV therapies and many of these metabolic complications have been observed to vary by antiretroviral agent and regimen.Citation4–Citation14
Raltegravir (RAL), an inhibitor of HIV-1 integrase, is approved for the treatment of HIV infection and, in combination with tenofovir/emtricitabine (TDF/FTC), is recommended as an initial therapy in the US Department of Health and Human Services HIV guidelines for the treatment of adolescents and adults.Citation15 However, in contrast to agents from other antiretroviral classes, there has been limited long-term study of the effect of RAL on metabolic parameters, particularly in African-Americans (AA), despite shifts in the US HIV epidemic toward persons of color.Citation16 Racial differences in changes in metabolic parameters among those HIV-infected treated with antiretroviral therapies have been described.Citation17–Citation23 In addition, antiretroviral metabolism and tolerability have been associated with race in cohort studies and clinical trials.Citation24,Citation25 Given the limited data on the metabolic effects of RAL on metabolic parameters in AAs, we conducted a longitudinal cohort study that exclusively enrolled antiretroviral naïve AA men and women and administered RAL in combination with TDF/FTC to assess on-treatment changes in body shape, serum lipids, glucose tolerance, and bone density.
Methods
Study design and population
This is a single-arm, open-label study of treatment naïve HIV-infected individuals who self-identified as being AA who were administered the antiretroviral combination RAL 400 mg twice daily orally plus fixed dose TDF/FTC at 300 mg/200 mg once daily. The primary aim of the study was to determine RAL pharmacokinetics in this population compared to those derived from white patients – results that have been previously been published.Citation26 A secondary objective was to describe the metabolic changes of participants experienced while receiving this antiretroviral combination. In addition to being AA, inclusion criteria included having had less than seven cumulative days of prior exposure to antiretroviral therapy, a plasma HIV RNA level >1,000 copies/ml at the time of screening, an estimated glomerular filtration rate by Modification of Diet in Renal Disease (MDRD) of at least 60 ml/min/1.73 m2, and hepatic transaminase levels no more than three times the upper limit of normal. A genotypic resistance assay was performed as part of routine clinical care and those with detected resistance to TDF or FTC were excluded.
Subjects were recruited at four HIV care clinics in North Carolina, including the University of North Carolina (UNC) Infectious Diseases Clinic in Chapel Hill, the Wake Forest University Health Sciences Infectious Diseases Clinic in Winston-Salem, the Durham County Early Intervention Clinic in Durham, and the Wake County Early Intervention Clinic in Raleigh. All subjects had to be able to provide informed consent. The institutional review boards at UNC and Wake Forest Health Sciences approved the study protocol.
Following informed consent, all subjects attended a baseline visit followed by study visits at weeks 2, 4, 8, 12, 24, 40, 56, 72, 96, and 104 to assess for safety and tolerability as well as pharmacokinetics.
Evaluations
Assessment of metabolic parameters, including body shape were conducted at the baseline, week 56 and 104 study visits. Visceral Adipose Tissue (VAT) and abdominal Subcutaneous Adipose Tissue (SAT) were assessed using abdominal computerized tomography (CT) performed at both UNC and Wake Forest. A single 10 mm thick slice was prescribed at the level of the L4 disc space from lateral topogram. Data were acquired using 120 kVp and weight-dependent mAs. Data were reconstructed using a soft-tissue filter. Using the Matlab (Natick, MA, USA) software package for data analysis, the areas of SAT and VAT were calculated from the single image for each patient. Adipose tissue was defined as pixels of CT attenuation ranging between − 190 and − 30 HU. Three contours were drawn around the skin surface, the peripheral border of the abdominal wall musculature, and the central border of the abdominal wall musculature, respectively. The sum of all voxels with attenuation within the predefined fat range between the skin surface and peripheral border was defined as SAT. The sum of all fat attenuation voxels within the central border of the abdominal wall musculature was defined as VAT.
Whole body dual-energy X-ray absorptiometry (DXA) was performed at UNC and Wake Forest to assess trunk and limb fat volumes, as well as bone mineral density (BMD). Participants were assessed using the same machine for each of their DXA scans.
Fasting lipids, glucose, and insulin levels were collected at each of the metabolic study visits and transported to UNC for analysis by the hospital clinical laboratory. Insulin and glucose values were used to quantify insulin resistance using the Homeostasis Model of Assessment-Insulin Resistance (HOMA-IR).
All subjects had a physical examination and routine safety laboratories collected as part of clinical care including chemistries (creatinine, electrolytes, hepatic transaminases) and hematology that were performed at the clinical laboratories used at each of the study clinics.
Statistical Methods
Median change from baseline was calculated for participants with at least >1 post-baseline assessment; repeated measures ANOVA and paired sample post hoc analyses with Bonferroni correction were utilized to analyze outcome measures; P value is significant if < 0.025 for pairwise comparisons. To test for differences in CD4 cell counts over time, the Sign test was performed. For missing data, the last observation was carried forward and sensitivity analyses were performed, treating missing data as failure. Missing HIV RNA levels were assumed to be a detectable value.
Results
Participant characteristics and disposition
Of the 39 participants enrolled in the study, 30 had at least two metabolic assessments during the course of the study and were included in the metabolic analyses. The characteristics of participants are listed in . Notably, the participants were mostly male and generally overweight at initiation of HIV therapy. Two participants were lost to follow-up before week 104 and in accordance with the intent to treat, missing = failure analysis plan, were considered treatment failures. None of the evaluated participants who remained available for study follow-up changed from the study regimen of RAL, TDF/FTC.
Table 1. Participant characteristics
Body shape changes
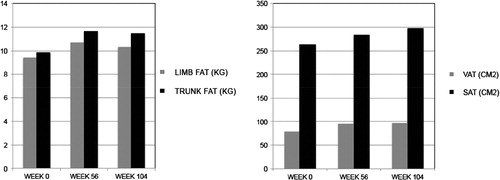
Visceral adipose tissue as measured by single slice CT scan increased 21% from a median baseline of 78.8 cm2 at 56 weeks (P = 0.022) and 22% from baseline at 104 weeks (P = 0.002) (, ). Subcutaneous adipose tissue by CT scan also increased at each of these time points: 11% from a median baseline of 262.6 cm2 at week 56 (P = 0.59) and 15% from baseline at week 104 (P = 0.003). Overall trunk fat increased 18% from a median baseline of 9.8 kg at 56 weeks (P = 0.01) and 17% from baseline by week 104 (P = 0.002). The ratio of VAT to SAT did not change significantly during the study.
Table 2. Changes from baseline in body composition and bone density
Limb fat by DXA scanning increased over the course of the study – by 14% from a median baseline of 9.4 kg at week 56 (P = 0.003) and by 10% by week 104 (0.004). Overall, a gain in total body fat (DXA) from a median of 20.2 kg of 16% at week 56 (P = 0.007) and 12% at week 104 (P = 0.002) was observed, corresponding to an increase in weight from baseline of 3.17 kg at 56 weeks (P = 0.007) and 2.93 kg at 104 weeks (P = 0.002).
Bone mineral density
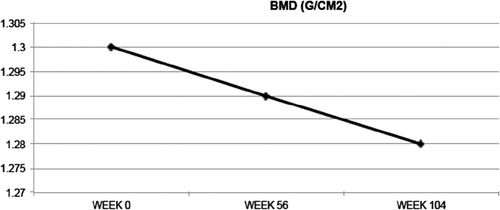
Bone mineral density declined slightly but statistically significantly from a baseline 1.30–1.28 g/cm2 (1.5%) by 104 weeks (P = 0.009) (, ).
Lipids and HOMA-IR
There were no significant changes from baseline in non-HDL cholesterol or fasting triglyceride levels during the study follow-up (). Similarly, there was no significant change in the median HOMA-IR observed over this period.
Table 3. Changes in fasting lipids and insulin resistance
Immunologic and Virologic Response and Tolerability
The median CD4 cell count values at baseline and at 104 weeks were 323 cells/mm3 (interquartile range [IQR] 248, 423) and 588 (IQR 438, 790) cells/mm3, respectively, with the median change from baseline to week 104 of 318 cells/mm3 (IQR 179, 403; full range 40, 749) (P < 0.001). Using last observation carried forward, 80 and 75% of participants had a plasma HIV RNA level of < 40 copies/ml at week 56 and 104, respectively, and 93 and 98% had HIV RNA levels < 200 copies/ml at these time points. Assuming patients with missing values had detectable HIV RNA levels, the proportion with an HIV RNA level of < 40 copies/ml at 56 and 104 weeks was 78 and 70%, and < 200 copies/ml were 88 and 90%, respectively. No new antiretroviral resistance mutations were detected during the course of the study.
There were no discontinuations of the study regimen due to intolerance. Three participants developed Grade III neutropenia and one of these also a Grade III low hemoglobin, all ascribed to be unrelated to study drug. There were no cases of renal tubulopathy or discontinuation/adjustment of study treatment for renal issues.
Discussion
Two years after the initiation of antiretroviral therapy, the combination of RAL plus TDF/FTC in AA patients was associated with generalized gains in fat, a small reduction in bone density, and no significant change in lipids or insulin resistance. The rate of viral suppression was high and produced significant increases in CD4 cell counts.
Our results are concordant with those from other studies examining the metabolic effects of RAL plus TDF/FTC in those initiating antiretroviral therapy. In the STARTMRK trial comparing initial treatment of HIV infection with RAL or efavirenz in combination with TDF/FTC, mean total and LDL cholesterol increases over 96 weeks were significantly greater in the efavirenz arm compared to those assigned to RAL; mean total cholesterol and LDL-cholesterol increased 10 mg/dl and 7 mg/dl, respectively among those assigned to RAL, approximately a third of the increase in these levels seen in the efavrienz arm.Citation4 Mean triglyceride levels declined by 4 mg/dl in those assigned RAL but rose 40 mg/dl on average in the efavirenz group. In the RAL group, truncal fat increased from baseline to week 96 by 21.6% and appendicular fat by 17%. There were no differences between the treatment groups in changes from baseline in trunk or appendicular fat volume. Notably, only 10% of those studied in the substudy were Black.
More recently, the U.S. AIDS Clinical Trials Group (ACTG) reported results of the A5257 trial comparing RAL with the ritonavir-boosted protease inhibitors darunavir and atazanavir in treatment-naïve patients also receiving TDF/FTC.Citation27 Both LDL cholesterol and triglyceride levels increased from baseline in the protease inhibitor arms but slightly declined in those assigned RAL. Total body BMD declined by 1.7% over 96 weeks in those in the RAL arm (1.6% in the darunavir arm and 2.7% in the atazanavir arm).Citation28 In a body composition, substudy from baseline to week 96, significant increases in all fat depots (including VAT, SAT, peripheral fat, and trunk fat) were seen and were not significantly different between the three study arms. Among the RAL-assigned subjects, VAT increased by 30%, SAT by 25%, peripheral fat by 20%, and trunk fat by 29%. In that trial, 42% of the participants were AA but subgroup analyses based on race have not been reported.
There are fewer data on the metabolic impact of regimens that include integrase inhibitors other than RAL. Elvitegravir requires pharmacological boosting with cobicistat, an agent that has been demonstrated to increase total- and LDL-cholesterol levels as well as triglycerides.Citation29 Cobicistat also leads to an increase in plasma tenofovir levels, possibly increasing the effects of that nucleotide analog on BMD.
Dolutegravir appears to have similar effects on lipids as RAL.Citation30 There are limited data available describing body shape, bone density, and glucose metabolism during dolutegravir therapy.
The magnitude of the general increase in fat seen in our study, as mentioned above, has been also observed in more diverse cohorts enrolled in other trials of initial therapy and in clinical practice.Citation31 This may be a so-called “return to health” phenomenon as the gains are seen across regimens using different agents and antiretroviral classes. Likewise, the 1–2% decline in bone density we observed in this group of AA patients is similar to that seen in ACTG 5257 in the RAL plus TDF/FTC arm where more than half of participants were white.Citation27 Decline in BMD with the initiation of antiretroviral therapy has been well described and may be due to immune reconstitution with possible activation of osteoclasts responsible for bone remodeling.Citation21 Tenofovir-containing regimens produce greater declines in BMD than others, indicating an added effect of this agent on bone.Citation21
In our study as expected, the antiretroviral regimen was well tolerated with few treatment-related adverse events reported and no treatment discontinuation due to toxicity. These findings also echo those of the A5257 trial, which found RAL in combination with TDF/FTC to be superior to protease inhibitor based therapy, largely as a consequence of better tolerability of the integrase inhibitor.Citation27
There are limitations to our study. Foremost, the small sample size was relatively small but the number of patients enrolled and followed in this single-arm study was sufficient for the analysis of the metabolic outcome variables of interest. In addition, the study did not include a control arm. As described above, data from other studies conducted among racially diverse populations are available and permit general comparisons. We found changes in metabolic parameters including body shape, lipids, and bone density on this AA cohort were very similar to those previously reported. Adherence to study therapy over the course of the investigation was monitored only by self-report and, therefore, suboptimal adherence may have been missed, complicating the evaluation of drug effect on the metabolic outcomes. That the vast majority of participants achieved and maintained viral suppression suggests that there was substantial and persistent exposure to the study regimen during the study. Bone mineral density was assessed with whole body DXA scanning as that was the modality used to measure body fat. Assessment of hip and spine BMD may be more sensitive for changes that could be associated with fracture risk. AIDS Clinical Trials Group trial 5257 examined changes in total BMD and found similar magnitude of change following initiation of RAL plus TDF/FTC.Citation27
In summary, among HIV-infected AA men and women, initial treatment with RAL plus TDF/FTC for up to 2 years was associated with general increases in fat without significant changes from baseline in serum lipids or in glucose tolerance. A small decline in total BMD was observed. Therapy was well tolerated, with few discontinuations and was effective with high rates of viral suppression. These results suggest that the metabolic effects of RAL plus TDF/FTC among AA patients are similar to those reported in racially diverse cohorts and inform the use of this regimen in this population.
Acknowledgements
The authors are grateful to the study participants and also thank the study coordinators, Suzanne Blevins, Donna Pittard, and Sandra Hauser as well as Catherine Kronk for data management.
Disclaimer Statements
Contributors
All authors were involved in the planning, implementation and analyses of this study. The manuscript was written by LY, BH, and DW.
Funding
This study was sponsored and funded by Merck, which markets raltegravir under the brand name Isentress. The study was designed, managed, and analyzed by the authors alone. The opinions expressed in the manuscript represent the collective views of the authors and do not necessarily reflect the official position of Merck or the institutions affiliated with the academic authors.
Conflicts of interest
Drs. Young, Hyslop, Yueh, and Napravnik report no conflicts. Dr. Wohl has research grants that have been awarded to his university from Gilead and Merck. He has received honoraria for participation on advisory boards from Gilead and Janssen. Dr. Wilkin has research grants that have been awarded to her university from Gilead, Janssen, Pfizer, Merck, and GlaxoSmithKline.
Ethics approval
This study was approved by the University of North Carolina Institutional Review Board.
References
- Srinivasa S, Grinspoon SK. Metabolic and body composition effects of newer antiretrovirals in HIV-infected patients. Eur J Endocrinol. 2014;170(5):R185–R202.
- Walker Harris V, Brown TT. Bone loss in the HIV-infected patient: evidence, clinical implications, and treatment strategies. J Infect Dis. 2012;20(Suppl 3):S391–S398.
- Krishnan S, Schouten JT, Atkinson B, Brown T, Wohl D, McComsey GA, et al. Metabolic syndrome before and after initiation of antiretroviral therapy in treatment-naive HIV-infected individuals. J Acquir Immune Defic Syndr. 2012;61(3):381–389.
- Lennox JL, Dejesus E, Berger DS, Lazzarin A, Pollard RB, Ramalho Madruga JV, et al. Raltegravir versus efavirenz regimens in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr. 2010;55(1):39–48.
- Molina JM, Andrade-Villanueva J, Echevarria J, Chetchotisakd P, Corral J, David N, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. J Acquir Immune Defic Syndr. 2008;53(3):323–332.
- Ferrer E, del Rio L, Martinez E, Curto J, Domingo P, Ribera E, et al. Impact of switching from lopinavir/ritonavir to atazanavir/ritonavir on body fat redistribution in virologically suppressed HIV-infected adults. AIDS Res Human Retroviruses. 2011;27:1061–1065.
- Podzamczer D, Andrade-Villanueva J, Clotet B, Taylor S, Rockstroh JK, Reiss P, et al. Lipid profiles for nevirapine vs. atazanavir/ritonavir, both combined with tenofovir disoproxil fumarate and emtricitabine over 48 weeks, in treatment-naive HIV-1-infected patients (the ARTEN study). HIV Med. 2011;12:374–382.
- Arathoon E, Schneider S, Baraldi E, Lim PL, Opravil M, Van De Casteele T, et al. Effects of once-daily darunavir/ritonavir versus lopinavir/ritonavir on metabolic parameters in treatment-naive HIV-1-infected patients at week 96: ARTEMIS. Int J STD AIDS. 2013;24:12–17.
- Aberg JA, Tebas P, Overton ET, Gupta SK, Sax PE, Landay A, et al. Metabolic effects of darunavir/ritonavir versus atazanavir/ritonavir in treatment-naive, HIV type 1-infected subjects over 48 weeks. AIDS Res Human Retroviruses. 2012;28:1184–1195.
- Sax PE, DeJesus E, Mills A, Zolopa A, Cohen C, Wohl D, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379:2439–2448.
- Rockstroh JK, DeJesus E, Henry K, Molina JM, Gathe J, Ramanathan S, et al. A randomized, double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus coformulated emtricitabine and tenofovir DF for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr. 2013;62:483–486.
- Erlandson KKD, Tierney C, Sax P, Daar E, Tebas P, Melbourne K, et al. Change in lean body mass and association with bone mineral density change in subjects randomized to abacavir/lamivudine or tenofovir/emtricitabine with atazanavir/ritonavir or efavirenz: ACTG A5224s. 20th Conference on Retroviruses and Opportunistic Infections, Atlanta, March 3–6. [Abstract 825], 825. 2013.
- Molina JM, Cahn P, Grinsztejn B, Lazzarin A, Mills A, Saag M, et al. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet. 2011;378:238–246.
- Cohen CJ, Andrade-Villanueva J, Clotet B, Fourie J, Johnson MA, Ruxrungtham K, et al. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority. Lancet. 2011;378:229–237.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. http://aidsinfo.nih.gov/ContentFiles/Adultand Adolescent GL.pdf. Accessed November 2, 2014..
- Centers for Disease Control and Prevention. HIV Surveillance Report, 2012.Vol. 24.http://www.cdc.gov/hiv/library/reports/surveillance/. Published November 2014.Accessed November 2, 2014..
- Foulkes AS, Wohl DA, Frank I, Puleo E, Restine S, Wolfe ML, et al. Associations among race/ethnicity, ApoC-III genotypes, and lipids in HIV-1-infected individuals on antiretroviral therapy. PLoS Med. 2006;3(3):e52.
- Kumar PN, Rodriguez-French A, Thompson MA, Tashima KT, Averitt D, Wannamaker PG, et al. A prospective, 96-week study of the impact of trizivir, combivir/nelfinavir, and lamivudine/stavudine/nelfinavir on lipids, metabolic parameters and efficacy in antiretroviral-naive patients: effect of sex and ethnicity. HIV Med. 2006;7(2):85–98.
- Gibert CL, Shlay JC, Sharma S, Bartsch G, Peng G, Grunfeld C, et al. Racial differences in changes of metabolic parameters and body composition in antiretroviral therapy-naive persons initiating antiretroviral therapy. J Aquir Immune Defic Syndr. 2009;50(1):44–53.
- Nicholaou MJ, Martinson JJ, Abraham AG, Brown TT, Hussain SK, Wolinsky SM, et al. HAART-associated dyslipidemia varies by biogeographical ancestry in the multicenter AIDS cohort study. AIDS Res Hum Retroviruses. 2013;29(6):871–879.
- Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51(5):554–561.
- Andany N, Raboud JM, Walmsley S, Diong C, Rourke SB, Rueda S, et al. Ethnicity and gender differences in lipodystrophy of HIV-positive individuals taking antiretroviral therapy in Ontario, Canada. HIV Clin Trials. 2011;12(2):89–103.
- Ribaudo HJ, Liu H, Schwab M, Schaeffeler E, Eichelbaum M, Motsinger-Reif AA, et al. Effect of CYP2B6, ABCB1, and CYP3A5 polymorphisms on efavirenz pharmacokinetics and treatment response: an AIDS Clinical Trials Group study. J Infect Dis. 2010;202(5):717–722.
- Haas DW, Gebretsadik T, Mayo G, Menon UN, Acosta EP, Shintani A, et al. Associations between CYP2B6 polymorphisms and pharmacokinetics after a single dose of nevirapine or efavirenz in African americans. J Infect Dis. 2009;199(6):872–880.
- Curran A, Martinez E, Saumoy M, del Rio L, Crespo M, Larrousse M, et al. Body composition changes after switching from protease inhibitors to raltegravir: SPIRAL-LIP substudy. AIDS. 2012;26(4):475–481.
- Wohl DA, Dumond JB, Blevins S, Pittard D, Ragan D, Wang R, et al. Raltegravir pharmacokinetics in treatment-naive patients is not influenced by race: results from the raltegravir early therapy in African-Americans living with HIV (REAL) study. Antimicrob Agents Chemother. 2013;57(2):784–788.
- Lennox JL, Landovitz RJ, Ribaudo HJ, Ofotokun I, Na LH, Godfrey C, et al. Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann Intern Med. 2014;161(7):461–471.
- Brown T, Moser C, Currier J, Ribaudo H, Rothenberg J, Dube MP, et al. Bone density changes after antiretroviral initiation with protease inhibitors or raltegravir. Conference on Retroviruses and Opportunistic Infections, March 3-6, Boston. [CROI 2014, Abstract 779LB], 2014.
- Gallant JE, Koenig E, Andrade-Villanueva J, Chetchotisakd P, DeJesus E, Antunes F, et al. Cobicistat versus ritonavir as a pharmacoenhancer of atazanavir plus emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV type 1-infected patients: week 48 results. J Infect Dis. 2013;208(1):32–39.
- Raffi F, Rachlis A, Brinson C, Arasteh K, Górgolas M, Brennan C, et al. Dolutegravir efficacy at 48 weeks in key subgroups of treatment-naive HIV-infected individuals in three randomized trials. AIDS. 2015;29(2):167–174.
- Tate T, Willig AL, Willig JH, Raper JL, Moneyham L, Kempf MC, et al. HIV infection and obesity: where did all the wasting go? Antivir Ther. 2012;17(7):1281–1289.