Abstract
Introduction:
Benefits and harms of tenofovir disoproxil fumarate (TDF) in HIV-infected, antiretroviral treatment (ART)-naïve patients of any age have not been systematically reviewed since recent milestone trials were published.
Methods:
We searched MEDLINE, EMBASE, CENTRAL, SCI, LILACS, WHO GHL, and ClinicalTrials.gov for randomized controlled trials (RCTs) comparing TDF-based treatments with any other ART-regimen (last search 01/2015). Trial characteristics and results were extracted, risks of bias systematically assessed, and treatment effects synthesized in meta-analyses using random-effects models.
Results:
We included 22 RCTs (8297 patients). We found no differences between groups for mortality, AIDS, fractures, CD4 cell count, and virological failure; and inconclusive information due to inadequate reporting for cardiovascular events, renal failure, proteinuria, rash, and quality of life. Tenofovir disoproxil fumarate-based regimens significantly reduced total cholesterol (mean difference − 18.42 mg/dl; 95% confidence interval [CI] − 22.80 to − 14.0), LDL-cholesterol ( − 9.53 mg/dl; − 12.16 to − 6.89), HDL-cholesterol ( − 2.97 mg/dl; − 4.41 to − 1.53), and triglycerides ( − 29.77 mg/dl; − 38.61 to − 20.92), bone mineral density (BMD) (hip: − 1.41%; − 1.87 to − 0.94), and glomerular filtration rate (eGFR) ( − 3.47 ml/minute; − 5.89 to − 1.06) over 48 weeks of follow-up. Effects were similar in trials comparing fixed-dose TDF/FTC-based regimens with ABC/3TC-based regimens. We found no influence of baseline viral load on virological failure.
Discussion:
Moderate-quality evidence suggests similar effects of TDF-based treatment regimens and other ART on virological failure. Tenofovir disoproxil fumarate-based regimens are associated with a more favorable lipid profile, but with increased risk of reduced BMD and eGFR. Improved reporting quality is vital to allow assessment of clinical outcomes in future trials.
Introduction
Tenofovir disoproxil fumarate (TDF) is a recommended first-line drug as part of a combined antiretroviral therapy (ART) for HIVCitation1,Citation2 and commonly used in a fixed-dose co-formulation with emtricitabine (TDF/FTC). Several large randomized controlled trials (RCTs) evaluated the comparative effectiveness of TDF-based treatment regimens in ART-naïve, HIV-infected patients, with some trials using the fixed-dose combination of abacavir/lamivudine (ABC/3TC) as the comparator.Citation3–Citation5 Although TDF is widely used, there is no systematic review and meta-analysis that includes recent milestone trials and covers both efficacy and safety outcomes of TDF-based regimens in treatment-naïve patients.Citation6–Citation8
In a comprehensive systematic review and meta-analysis of RCTs in ART-naïve patients, we assessed the comparative effectiveness of TDF-based treatments, including commonly used fixed dose co-formulations, on various patient-important outcomes and surrogate markers.
Methods
Inclusion criteria
We included RCTs in ART-naïve HIV-infected patients of any age. Eligible RCTs compared TDF-based treatments with any other ART without TDF. We separately analyzed trials comparing TDF/FTC-based regimens with ABC/3TC-based regimens. We excluded trials on HIV infection prevention and short-time treatments.
Identification of evidence
We systematically searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), LILACS, Science Citation Index, and the WHO Global Health Library with support of an information specialist and without restrictions on language or publication status (last search 01/2015; see supplementary material 1 for search strategy, available online at www.maneyonline.com/doi/suppl/10.1179/1945577115Y.0000000004). We also searched clinicaltrials.gov (last search 04/2014), conference proceedings of major international HIV meetings,Citation9–Citation13 and screened reference lists of all eligible articles. We perused results published on clinicaltrials.gov for supplemental information, and we systematically contacted authors when analytical details were unclear or missing.
Two reviewers independently screened titles, abstracts, and trial registries. One reviewer screened conference proceedings and reference lists. Full texts of potentially eligible articles were obtained and two reviewers independently determined eligibility. Discrepancies were resolved by discussion or with a third reviewer.
Data extraction
We extracted characteristics of the study, patients, interventions, and the following predefined outcomes: mortality, AIDS-defining events, virological failure, fractures, cardiovascular events, renal failure, rash, quality of life, CD4 cell count, HDL-, LDL-, total cholesterol, triglycerides, estimated glomerular filtration rate (eGFR), proteinuria, bone mineral density (BMD), and body fat change. Data were extracted by one reviewer and verified by a second reviewer using pre-tested electronic extraction forms. Virological failure data were independently extracted by two reviewers. Disagreements were resolved by consensus.
Study outcomes
For outcomes reported as changes from baseline, we primarily combined results observed 48 weeks after randomization ( ± 4 weeks) to reflect mid-term effects. For clinical events, we primarily used the latest time-point that clearly had 80% or more of randomized patients per treatment group under observation (to limit bias due to incomplete patient data). In sensitivity analyses, we used the other time-point (i.e. the latest or 48 weeks).
Virological failure was analyzed only at 48 weeks. We used snapshot analyses if available, otherwise time-to-loss-of-virological-response analyses (TLOVR). We accepted any approach for dealing with missing data but we preferred “missing = failure” analyses.
Rash was evaluated only using trials comparing TDF/FTC with ABC/3TC in HLA-B*-5701-negative patients to reflect modern treatment circumstances.Citation1,Citation2
We used ITT-analyses that included all randomized patients, where available, otherwise we used “modified” ITT-analyses.Citation14,Citation15
Risk of bias assessment
Two reviewers independently assessed the risk of bias using the Cochrane risk of bias tool.Citation16 We deemed the overall risk of bias low for trials with low risk in all subdomains: (1) adequate randomization process; (2) treatment blinding; (3) outcome assessment blinding; (3) reporting bias (i.e. high risk for studies published only as abstract; other sources of reporting bias were not explored); (4) attrition bias.Citation16
Analysis
We calculated summary relative risks or mean differences (with 95% confidence intervals, CI),Citation17 using random-effects models. We used Peto's approach for outcomes with event rates below 1%.Citation16 For unspecified time-points of outcome assessment, we used the mean or median study follow-up.Citation16 If the number of patients evaluated at 48 weeks was unclear, we imputed it by subtracting from the number of randomized patients the median proportion of lost patients in the remaining studies of that meta-analysis. We used absolute changes from baseline per group reported as mean with standard deviations (SD), where available.Citation16 Missing means or SDs were converted or approximated from other given statistics or imputed from the remaining studies in that meta-analysis.Citation16 We used 0.5 as continuity correction.Citation16
When studies compared more than two treatments, we included all comparisons separately if possible without double-counting patients. In five cases, we excluded outdated or very uncommonly used regimens.Citation18–Citation23
We assessed the between-study heterogeneity using the I2-metric (with 95%CI).Citation24 In sensitivity analyses, we used Peto's approach and Mantel–Haenszel fixed-effect models without continuity correction for event rates between 1 and 10%. We conducted none of the other pre-specified sensitivity/subgroup analyses (publication status, funding source, risk of bias, children, women, pregnant women, breastfeeding women, patients with renal disease) due to the low number of pertinent comparisons per subset. However, we conducted a post hoc subgroup analysis on virological failure by viral load at treatment initiation (above or below 100 000 HIV-RNA copies/ml). We used Stata 13.1 (Stata Corp, College Station, TX, USA) for all analyses. P-values are two-tailed.
Results
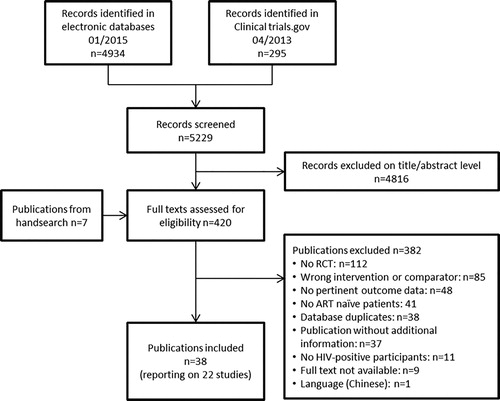
Of 5229 references, 420 full texts were screened and 22 trials with 8297 randomized ART-naïve participants were included (; Table 1). One trial (A5224s) was a substudy of another trial (A5202).
Table 1. Characteristics of included studies
Tenofovir disoproxil fumarate/FTC-based regimens were compared with ABC/3TC-based regimens in six trials (see Supplemental Digital Content one for details on characteristics of included studies). Most trials were small with < 100 patients per group (13 of 22). Relevant treatment effects were measured after 12–184 weeks of follow-up, with 19 of 22 studies providing outcome data observed at 48 weeks of follow-up or later.
Risk of bias assessment
We deemed the overall risk of bias high for 17 of 22 trials, mainly due to open study designs. The risk was low for two studies and unclear for three studies (see Supplemental Digital Content 1 for overview over risk of bias assessment). The randomization process was clearly adequate in 10 trials and unclear in the remaining cases. Five trials were double-blinded and 15 open-label. Detection bias was deemed low for 20 trials. Two trials were published as abstract only with high risk of reporting bias. Attrition bias was deemed high or unclear in 12 trials due to large proportions of randomized patients not or not clearly included in analyses.
Outcomes
Clinical events and deaths
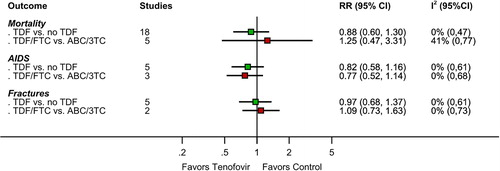
Clinical events and deaths were rare in all studies. There were 106 deaths among 7582 patients in 18 trials (median follow-up 48 weeks; range 16–184 weeks), 127 cases of AIDS among 4480 patients in five trials (median follow-up 130.5 weeks; range 48–144), and 126 fractures among 4007 patients in five trials (median follow-up 138 weeks; range 48–144). Effects were imprecise with wide confidence intervals (). The RR for death was 0.88 (95%CI 0.60 to 1.30; , see Supplemental Digital Content 2 for graph) with TDF-based regimens versus other regimens. The RR for AIDS was 0.82 (95%CI 0.58 to 1.16; , see Supplemental Digital Content 2 for graph). The RR for fractures was 0.97 (95%CI 0.68 to 1.37; , see Supplemental Digital Content 2 for graph). Effects were similar in studies comparing TDF/FTC with ABC/3TC. We found no between-study heterogeneity.
Figure 2. Results of meta-analyses for effects of tenofovir-based versus non-tenofovir-based regimens on the clinical outcomes mortality, AIDS, and fractures. RR: relative risk. 3TC: lamivudine; ABC: abacavir; FTC: emtricitabine; TDF: tenofovir disoproxil fumarate.
We found no pertinent outcome data on quality of life in any study. Data on cardiovascular events, renal failure, proteinuria, and rash were very inconsistently reported using very heterogeneous definitions and were therefore not pooled (see Supplemental Digital Content 1 for qualitative summary).
Virological failure
Virological failure at 48 weeks was reported in 16 trials. The RR for being free of virological failure (HIV-1-RNA levels < 50 copies/ml) was 1.03 (95%CI 0.99–1.07) with TDF-based regimens versus other regimens (; see Supplemental Digital Content 2 for graph). Similar effects were found in trials comparing TDF/FTC with ABC/3TC (RR 1.02; 0.95–1.10; , see Supplemental Digital Content 2 for graph). Between-study heterogeneity was moderate to high ().
Figure 3. Results of meta-analyses for effects of tenofovir-based versus non-tenofovir-based regimens on the outcome free of virological failure. Relative risk (RR) below 1 indicates a higher risk for virological failure; 2 studies using slightly different thresholds were included in < 50 cells/ml analysis (RADAR used 48 copies/ml, PROGRESS used 40 copies/ml). 3TC: lamivudine; ABC: abacavir; FTC: emtricitabine; TDF: tenofovir disoproxil fumarate.
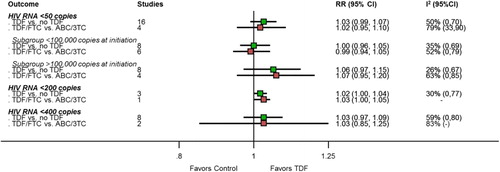
We found no influence of baseline viral load, effects were similar in patients with HIV-RNA levels below or above 100 000 copies/ml (, see Supplemental Digital Content 2 for graph) and interaction tests were non-significant but of limited validity due to heterogeneity (P = 0.14 across all studies and P = 0.12 across TDF/FTC vs ABC/3TC comparisons). We found similar results using other thresholds for virological failure ().
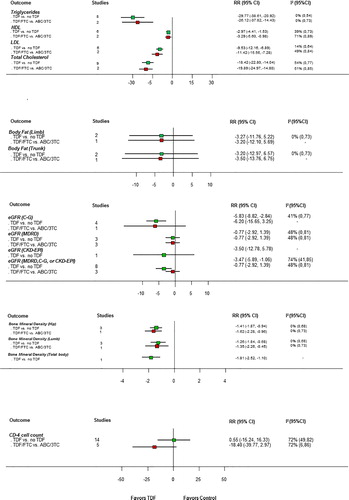
CD4 cell count
CD4 cell count changes over 48 weeks were reported in 14 trials. Tenofovir disoproxil fumarate-based regimens had no different effect versus other regimens: mean difference (95%CI): 0.55 cells/ml ( − 15.24 to 16.33) (, see Supplemental Digital Content 2 for graph). Between-study heterogeneity was high. For trials comparing TDF/FTC-based regimens with ABC/3TC-based regimens, we found a trend toward lower CD4 cell counts with TDF/FTC-based regimens (mean difference − 18.40; 95%CI − 39.77 to 2.97; Web appendix 2; test for interaction P < 0.01 but of limited validity due to heterogeneity).
Lipid level
Lipid level changes (LDL-, HDL-, and total-cholesterol; triglycerides) over 48 weeks were reported in eight trials. All lipid levels (including HDL-cholesterol) significantly decreased with TDF-based regimens versus other regimens: mean difference (95%CI): LDL-cholesterol − 9.53 mg/dl ( − 12.16 to − 6.89); HDL-cholesterol − 2.97 mg/dl ( − 4.41 to − 1.53); total cholesterol − 18.42 mg/dl ( − 22.80 to − 14.04); triglycerides − 29.77 mg/dl ( − 38.61 to − 20.92) (, see Supplemental Digital Content 2 for graph). There was low to moderate between-study heterogeneity. Effects were similar in trials comparing TDF/FTC-based regimens with ABC/3TC-based regimens.
Estimated glomerular filtration rate
Estimated glomerular filtration rate (eGFR) effects over 48 weeks were reported in eight trials (, see Supplemental Digital Content 2 for graph). The Cockcroft-Gault formula was used in five trials, the Modification of Diet in Renal Disease (MDRD) formula in three trials, and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula in one trial. One small trial (Epzicom-TruvadaCitation25) used the Cockcroft–Gault and MDRD formula.
Renal function significantly decreased over 48 weeks with TDF-based regimens versus other regimens: mean difference (95%CI): − 3.47 ml/minute ( − 5.89 to − 1.06) (, see Supplemental Digital Content 2 for graph; we used MDRD-based results from Epzicom-TruvadaCitation25 but effects were similar with Cockcroft-Gault). Across the three trials comparing TDF/FTC with ABC/3TC, we found no statistically significant decrease with TDF-based regimens, but (see below) two of the three trials (90.8% of the evidence contributing to the summary effect) reported only MDRD-based results: mean difference (95%CI): − 0.77 ml/minute ( − 2.92 to 1.39) (; see Supplemental Digital Content 2 for graph).
We observed high heterogeneity for the overall estimate, which was reduced by combining trials with identical eGFR measurement methods. Effects were significantly larger in trials using the Cockcroft–Gault formula ( − 5.83 ml/minute; 95%CI − 8.82 to − 2.84; see Supplemental Digital Content 2 for graph) than in other trials (P < 0.01 for interaction). We found no eGFR decrease when combining only MDRD-based results ( − 0.77 ml/minute; 95%CI − 2.92 to 1.39; see Supplemental Digital Content 2 for graph).
Bone mineral density
Bone mineral density (BMD) changes over 48 weeks were reported in four trials (, see Supplemental Digital Content 2 for graph). Three trials measured BMD at the hip and lumbar spine, respectively, and one trial (RADARCitation26) reported effects on total and subtotal (total minus head) BMD (). All analyses consistently showed a greater relative decrease with TDF-containing treatments: mean difference (95%CI): hip: − 1.41% ( − 1.87 to − 0.94); lumbar spine: − 1.26% ( − 1.84 to − 0.68) (, see Supplemental Digital Content 2 for graphs).
Body fat
Body fat changes over 48 weeks were reported in two studies. The pooled effects on relative changes of trunk and limb fat showed no significant treatment difference (, see Supplemental Digital Content 2 for graph). Final values for total limb at 48 weeks (not change from baseline) were reported in one study (934) and were significantly higher with TDF but the results are based on only 19% of the randomized patients (data not shown).
Sensitivity analyses
All sensitivity analyses confirmed the main findings (data not shown).
Discussion
This analysis of 22 RCTs found no different comparative effects of TDF-based and non-TDF-based treatments on mortality, AIDS-defining events, fractures, CD4 cell count, and virological failure. Tenofovir disoproxil fumarate-based regimens were associated with more favorable lipid levels, but reduced BMD and eGFR.
Effects were similar in trials comparing TDF/FTC versus ABC/3TC-based regimens, in particular for virological failure in relation to baseline viral load (below or above 100 000 copies/ml), consistent with previous research.Citation6 Effects on CD4 cell counts seem to be less favorable with TDF/FTC versus ABC/3TC-based regimens, but the formally statistically significant test of interaction needs to be cautiously interpreted due to heterogeneity.
We found no relevant difference for clinical outcomes and mortality, but none of the trials considered clinical outcomes or mortality as primary endpoints, events were rare, and effect estimates were imprecise. Most clinical events were only casually reported – typically nonsystematically, together with other adverse effects, without standardized definitions and sufficient information to calculate risk ratios, making a combined analysis unfeasible.
We found no evidence for an increased fracture risk but CIs were wide. It remains therefore unclear if the BMD-decrease with TDF-based regimens translates to a moderately increased fracture risk. The observational evidence is inconsistent.Citation27,Citation28
Consistent with our findings, a previous meta-analysis of RCTs and observational studies (published up to 2009 and including naïve and non-naïve patients) found a larger eGFR decline with TDF-regimes over a median duration of 48 weeks when analyzed using the Cockcroft–Gault formula ( − 3.92 ml/minute; 95%CI − 2.13 to 5.70) compared to MDRD-based analyses ( − 2.56 ml/minute; 95%CI − 0.57 to 5.69).Citation7 This systematic analysis detected no significant risk differences for renal failure, but several individual observational studies indicated that the eGFR changes associated with TDF-based regimens may translate to an increased risk of chronic kidney disease.Citation8
We found clinically relevant decreases of total cholesterol, LDL-cholesterol, and triglycerides with TDF-regimens. These favorable changes, however, are contrasted by a HDL-cholesterol decrease. We observed similar lipid profile changes across trials comparing TDF/FTC versus ABC/3TC, suggesting more favorable effects on cardiovascular risk profiles with TDF versus abacavir, but it remains unclear if this translates to patient-relevant differences in clinical outcomes. Several observational studies indicated a moderately increased risk of myocardial infarction with abacavir versus TDF.Citation29–Citation34
Our comprehensive analysis considered numerous predefined patient-important outcomes and clinically relevant surrogates, used a highly sensitive literature search, applied established methods of evidence synthesis, and used diverse approaches to address potential bias. We kept a broad scope on the entire spectrum of TDF-containing regimens and specifically focused on clinically relevant populations and treatment comparisons (i.e. TDF/FTC vs ABC/3TC).
However, there are limitations. First, in most studies, the co-administered ART-compounds were different between TDF-based regimens and comparator regimens. E.g. the most frequent second reverse transcriptase inhibitors were those in commonly used fixed-dose ART-combinations, thus FTC in the TDF-based regimens and 3TC in the comparator regimens; the most frequent third compound was EFV in the TDF-based regimens and a boosted protease inhibitor in the comparator regimens. Thus, the treatment effects need to be interpreted as effects of combined ART-regimens and may not be attributed to TDF alone. Specific impact of co-administered ART-compounds remains speculative. Second, we assessed biomarker changes from baseline to a fixed mid-term time-point (48 weeks). Only few results were consistently reported for later time-points and many studies failed to observe substantial proportions of the randomized population with longer follow-up. Our approach allowed a consistent clinical interpretation of changes over time and reduced statistical heterogeneity. Longer follow-up would not change the interpretation of the early safety signals from surrogate outcomes (eGFR, BMD). Second, incomplete, inconsistent, and unclear reporting required various imputations and assumptions, despite systematic author requests, which remained unanswered in some cases. This reduced the quality of evidence. It probably has also increased the heterogeneity in some analyses, which, however, does not invalidate the credibility of the combined effects.Citation35 The substantial heterogeneity in virological failure analyses might be explained by diverse and non-standardized approaches (TLOVR or snapshot analyses; various approaches to address missing outcome information or treatment switches); the reported data did not allow us to use a single consistent approach across all studies (e.g. missing = failure). Third, we did not consider outcomes defined by their assumed drug relationship (i.e. “drug-related” adverse events), or outcomes limited to events that led to withdrawal (i.e. “study withdrawal due to adverse event”) because of potential bias due to complex reasons for withdrawal,Citation16,Citation36 and because we deemed any judgment about the causal relationship between drug and outcome highly subjective, in particular, in unblinded trials. Fourth, with respect to lipid changes, most studies did not report concomitant lipid-modifying treatment (e.g. statins). Finally, most studies had a high overall risk of bias, mainly because of lack of blinding. High attrition rates further enhanced this problem (with loss-to-follow-up rates from 5 to 60% at 48 weeks), but sensitivity analyses with limited attrition rates are reassuring.
Acknowledging these limitations, we conclude that there is moderate-quality evidence for similar comparative effects of TDF-containing treatments and other ART on virological failure. TDF-based regimens may offer some advantage in patients with increased cardiovascular risk due to more favorable effects on lipid profiles. However, in patients with increased risk of chronic kidney disease, TDF should be carefully selected and the potential benefits thoroughly balanced.
Because ART is life-long, long-term tolerability and safety are paramount, in particular, given the comparable antiviral effectiveness. Yet, the reported evidence from 22 trials was insufficient to assess the translation of biomarker changes to clinical outcomes. This is to some degree due to rarity of events, but moreover, reporting of outcomes and adverse events was frequently not standardized, not systematic, and not transparent. This creates important knowledge gaps and makes treatment decisions difficult.Citation37 Better reporting of RCTs in this area is inevitable to efficiently use research resources, facilitate comparative effectiveness research, and optimize evidence-based decision-making in HIV care.
Acknowledgements
The authors thank Kübra Oezoglu for her outstanding administrative assistance and support with literature management. We thank all study authors and the staff of the trial sponsors for providing very helpful information on their study results.
Disclaimer Statements
Contributors
LGH and HCB conceived and designed the study; LGH coordinated the review; all authors collected the data; LGH, HE and HCB analyzed the data and interpreted the results; LGH wrote the first draft and all authors made revisions on the manuscript and approved the final version of the paper. HCB is the guarantor.
Funding
The Basel Institute for Clinical Epidemiology & Biostatistics received financial support from Gilead Sciences for the submitted work. The Basel Institute for Clinical Epidemiology & Biostatistics is supported by Santésuisse and the Gottfried and Julia Bangerter-Rhyner-Foundation. The funders had no role in study design, data collection and analysis, the preparation of the manuscript or the decision to publish. Gilead Sciences commented on the manuscript before submission.
Conflict of interest
HCB received travel grants, honoraria, and unrestricted research grants from Gilead, Bristol-Myers-Squibb, and ViiV Healthcare. MS received travel grants, and is member of advisory boards of abbvie, Bristol–Myers Squibb, Gilead, Janssen, Merck Sharp & Dohme, and ViiV. All other authors declare no competing interests.
Ethical approval
Not required for this study.
References
- European AIDS Clinical Society (EACS). EACS Guidelines Version 7. 2014;. http://www.eacsociety.org/Portals/0/140601_EACS%20EN7.02.pdf. Accessed December 08, 2014.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. : Department of Health and Human Services; 2014;. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed Decmber 08, 2014.
- Smith KY, Patel P, Fine D, Bellos N, Sloan L, Lackey P, et al. Randomized, double-blind, placebo-matched, multicenter trial of abacavir/lamivudine or tenofovir/emtricitabine with lopinavir/ritonavir for initial HIV treatment. AIDS. 2009;23(12):1547–1556.
- Daar ES, Tierney C, Fischl MA, Sax PE, Mollan K, Budhathoki C, et al. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1: a randomized trial. Ann Intern Med. 2011;154(7):445–456.
- Walmsley SL, Antela A, Clumeck N, Duiculescu D, Eberhard A, Gutiérrez F, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369(19):1807–1818.
- Cruciani M, Mengoli C, Malena M, Serpelloni G, Parisi SG, Moyle G, et al. Virological efficacy of abacavir: systematic review and meta-analysis. J Antimicrob Chemother. 2014;69(12):3169–3180.
- Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51(5):496–505.
- Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of renal disease among people living with HIV: a systematic review and meta-analysis. BMC Public Health. 2012;12:234.
- HIV drug therapy in the Americas congress 13-15 January 2013, São Paulo Brazil. J Int AIDS Soc. 2013;16(Suppl 1):18720.
- 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention, 30 June-03 July 2013. Kuala-Lumpur, Malaysia, 2013.
- 14th European AIDS Conference, 16-19 October 2013. Brussels, Belgium, 2013.
- 11th international congress on drug therapy in HIV infection 11-15 November 2012, Glasgow UK. J Int AIDS Soc. 2012;15(Suppl 4):18060–18301.
- XIX international AIDS conference 22-27 July 2012, Washington DC USA. J Int AIDS Soc. 2012;15(Suppl 3)
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Bürgi E, Scherer M, et al. The effects of excluding patients from the analysis in randomised controlled trials: meta-epidemiological study. BMJ. 2009;339:b3244.
- Tierney JF, Stewart LA. Investigating patient exclusion bias in meta-analysis. Int J Epidemiol. 2005;34(1):79–87.
- The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. www.cochrane-handbook.org.
- Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826.
- Campbell TB, Smeaton LM, Kumarasamy N, Flanigan T, Klingman KL, Firnhaber C, et al. Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: a randomized clinical trial in diverse multinational settings. PLoS Med. 2012;9(8):e1001290.
- Matthews GV, Avihingsanon A, Lewin SR, Amin J, Rerknimitr R, Petcharapirat P, et al. A randomized trial of combination hepatitis B therapy in HIV/HBV coinfected antiretroviral naive individuals in Thailand. Hepatology. 2008;48(4):1062–1069.
- Menezes CN, Crowther NJ, Duarte R, Van Amsterdam D, Evans D, Dickens C, et al. A randomized clinical trial comparing metabolic parameters after 48 weeks of standard- and low-dose stavudine therapy and tenofovir disoproxil fumarate therapy in HIV-infected South African patients. HIV Med. 2014;15(1):3–12.
- Menezes CN, Duarte R, Dickens C, Dix-Peek T, Van Amsterdam D, John MA, et al. The early effects of stavudine compared with tenofovir on adipocyte gene expression, mitochondrial DNA copy number and metabolic parameters in South African HIV-infected patients: a randomized trial. HIV Med. 2013;14(4):217–225.
- Phanuphak N, Ananworanich J, Teeratakulpisarn N, Jadwattanakul T, Kerr SJ, Chomchey N, et al. A 72-week randomized study of the safety and efficacy of a stavudine to zidovudine switch at 24 weeks compared to zidovudine or tenofovir disoproxil fumarate when given with lamivudine and nevirapine. Antivir Ther. 2012;17(8):1521–1531.
- Torti C, Cologni G, Quiros-Roldan E, Antinori A, Orani A, Tirelli V, et al. Lopinavir/r+zidovudine+lamivudine versus efavirenz+tenofovir+lamivudine as a first-line HAART: a pilot RCT (the Si.S.Ther. trial). 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention; 2007;. Abstract no. CDB2462007.
- Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335(7626):914–916.
- Nishijima T, Takano M, Ishisaka M, Komatsu H, Gatanaga H, Kikuchi Y, et al. Abacavir/lamivudine versus tenofovir/emtricitabine with atazanavir/ritonavir for treatment-naive Japanese patients with HIV-1 infection: a randomized multicenter trial. Intern Med. 2013;52(7):735–744.
- Bedimo RJ, Drechsler H, Jain M, Cutrell J, Zhang S, Li X, et al. The RADAR study: week 48 safety and efficacy of RAltegravir combined with boosted DARunavir compared to tenofovir/emtricitabine combined with boosted darunavir in antiretroviral-naive patients. Impact on bone health. PLoS One. 2014;9(8):e106221.
- Bedimo R, Maalouf NM, Zhang S, Drechsler H, Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS. 2012;26(7):825–831.
- Hansen AB, Gerstoft J, Kronborg G, Larsen CS, Pedersen C, Pedersen G, et al. Incidence of low and high-energy fractures in persons with and without HIV infection: a Danish population-based cohort study. AIDS. 2012;26(3):285–293.
- Hemkens LG, Bucher HC. HIV infection and cardiovascular disease. Eur Heart J. 2014;35(21):1373–1381.
- Belloso WH, Orellana LC, Grinsztejn B, Madero JS, La Rosa A, Veloso VG, et al. Analysis of serious non-AIDS events among HIV-infected adults at Latin American sites. HIV Med. 2010;11(9):554–564.
- Martin A, Bloch M, Amin J, Baker D, Cooper DA, Emery S, et al. Simplification of antiretroviral therapy with tenofovir-emtricitabine or abacavir-lamivudine: a randomized, 96-week trial. Clin Infect Dis. 2009;49(10):1591–1601.
- Young J, Xiao Y, Moodie EE, Abrahamowicz M, Klein MB, Bernasconi E, et al. The effect of cumulating exposure to abacavir on the risk of cardiovascular disease events in patients from the Swiss HIV cohort study. J Acquir Immune Defic Syndr. 2015;69(4):413–421.
- Choi AI, Vittinghoff E, Deeks SG, Weekley CC, Li Y, Shlipak MG. Cardiovascular risks associated with abacavir and tenofovir exposure in HIV-infected persons. AIDS. 2011;25(10):1289–1298.
- Desai M, Joyce V, Bendavid E, Olshen RA, Hlatky M, Chow A, et al. Risk of cardiovascular events associated with current exposure to HIV antiretroviral therapies in a US veteran population. Clin Infect Dis. 2015;61(3):445–452.
- Ioannidis JP, Patsopoulos NA, Rothstein HR. Reasons or excuses for avoiding meta-analysis in forest plots. BMJ. 2008;336(7658):1413–1415.
- Ioannidis JP, Evans SJ, Gøtzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141(10):781–788.
- Glasziou P, Altman DG, Bossuyt P, Boutron I, Clarke M, Julious S, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet. 2014;383(9913):267–276.
- Sax PE, Tierney C, Collier AC, Daar ES, Mollan K, Budhathoki C, et al. Abacavir/lamivudine versus tenofovir DF/emtricitabine as part of combination regimens for initial treatment of HIV: final results. J Infect Dis. 2011;204(8):1191–1201.
- Sax PE, Tierney C, Collier AC, Fischl MA, Mollan K, Peeples L, et al. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. N Engl J Med. 2009;361(23):2230–2240.
- McComsey G, Kitch D, Sax PE, Tebas P, Tierney C, Jahed NC, et al. Peripheral and central fat changes in subjects randomized to abacavir-lamivudine or tenofovir-emtricitabine with atazanavir-ritonavir or efavirenz: ACTG study A5224s. Clin Infect Dis. 2011;53(2):185–196.
- McComsey GA, Kitch D, Daar ES, Tierney C, Jahed NC, Tebas P, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS clinical trials group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203(12):1791–1801.
- Post FA, Moyle GJ, Stellbrink HJ, Domingo P, Podzamczer D, Fisher M, et al. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults: 48-week results from the ASSERT study. J Acquir Immune Defic Syndr. 2010;55(1):49–57.
- Stellbrink HJ, Orkin C, Arribas JR, Compston J, Gerstoft J, Van Wijngaerden E, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51(8):963–972.
- Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292(2):191–201.
- Izzedine H, Hulot JS, Vittecoq D, Gallant JE, Staszewski S, Launay-Vacher V, et al. Long-term renal safety of tenofovir disoproxil fumarate in antiretroviral-naive HIV-1-infected patients. Data from a double-blind randomized active-controlled multicentre study. Nephrol Dial Transplant. 2005;20(4):743–746.
- Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354(3):251–260.
- Pozniak AL, Gallant JE, DeJesus E, Arribas JR, Gazzard B, Campo RE, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naive patients: virologic, immunologic, and morphologic changes – a 96-week analysis. J Acquir Immune Defic Syndr. 2006;43(5):535–540.
- Arribas JR, Pozniak AL, Gallant JE, Dejesus E, Gazzard B, Campo RE, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/lamivudine and efavirenz in treatment-naive patients: 144-week analysis. J Acquir Immune Defic Syndr. 2008;47(1):74–78.
- Mills A, Mildvan D, Podzamczer D, Fätkenheuer G, Leal M, Than S, et al. Maraviroc once-daily nucleoside analog-sparing regimen in treatment-naive patients: randomized, open-label pilot study. J Acquir Immune Defic Syndr. 2013;62(2):164–170.
- Mills A, Mildvan D, Podzamczer D, Fätkenheuer G, Leal M, Than S, et al. Once-daily maraviroc in combination with ritonavir-boosted atazanavir in treatment-naive patients infected with CCR5-tropic HIV-1 (study A4001078): 96-week results. Paper presented at: J Int AIDS Soc. Conference: 19th International AIDS Conference Washington, DC United States. [Conference Start: 20120722 Conference End: 20120727. Conference Publication: (var.pagings), 15, pp 52-53, 2012. Date of Publication: 24 Oct 2012].
- Pinola M, Lazzarin A, Antinori A, Carosi G, Giovanni DP, Moroni M. Lopinavir/ritonavir + tenofovir dual therapy versus lopinavir/ritonavir-based triple therapy in HIV-infected antiretroviral naive subjects: the kalead study. J Antivir Antiretrovir. 2010;2(4):056–062.
- Maitland D, Moyle G, Hand J, Mandalia S, Boffito M, Nelson M, et al. Early virologic failure in HIV-1 infected subjects on didanosine/tenofovir/efavirenz: 12-Week results from a randomized trial. AIDS. 2005;19(11):1183–1188.
- Moyle G, Higgs C, Teague A, Mandalia S, Nelson M, Johnson M, et al. An open-label, randomized comparative pilot study of a single-class quadruple therapy regimen versus a 2-class triple therapy regimen for individuals initiating antiretroviral therapy. Antivir Ther. 2006;11(1):73–78.
- Raffi F, Babiker AG, Richert L, Molina JM, George EC, Antinori A, et al. Ritonavir-boosted darunavir combined with raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults infected with HIV-1: 96 week results from the NEAT001/ANRS143 randomised non-inferiority trial. Lancet. 2014;384(9958):1942–1951.
- Nozza S, Galli L, Antinori A, Chiappetta S, Mazzotta F, Zaccarelli M, et al. Maraviroc 150 mg QD plus lopinavir/ritonavir, a NRTI-sparing regimen for HIV-infected naive patients: 48-weeks final results. J Int AIDS Soc. 2012;15(6):18232.
- Reynes J, Lawal A, Pulido F, Soto-Malave R, Gathe J, Tian M, et al. Examination of noninferiority, safety, and tolerability of lopinavir/ritonavir and raltegravir compared with lopinavir/ritonavir and tenofovir/emtricitabine in antiretroviral-naive subjects: the progress study, 48-week results. HIV Clin Trials. 2011;12(5):255–267.
- Reynes J, Trinh R, Pulido F, Soto-Malave R, Gathe J, Qaqish R, et al. Lopinavir/ritonavir combined with raltegravir or tenofovir/emtricitabine in antiretroviral-naive subjects: 96-week results of the PROGRESS study. AIDS Res Human Retroviruses. 2013;29(2):256–265.
- Torti C, Quiros-Roldan E, Regazzi M, Antinori A, Patroni A, Villani P, et al. Early virological failure after tenofovir + didanosine + efavirenz combination in HIV-positive patients upon starting antiretroviral therapy. Antivir Ther. 2005;10(4):505–513.