Abstract
AFN-1252, a potent enoyl-ACP reductase (FabI) inhibitor, is under development for the treatment of Staphylococcus aureus infections. The activity of AFN-1252 against two isolates of S. aureus, MSSA 26213 and MRSA S186, was studied in an in vitro pharmacodynamic model simulating AFN-1252 pharmacokinetics in man. Reductions in bacterial viable count over the first 6 hours were generally 1–2 logs and maximal reductions in viable count were generally achieved at fAUC/MIC ratios of 100–200. Maximum reductions in viable count against MSSA 29213 and MRSA S186 were approximately 4 logs, achieved by 450 mg q12h (fAUC/MIC = 1875) dosing at 28 hours. Staphylococcal resistance to AFN-1252 did not develop throughout the 48-hour experiments. As multidrug resistance continues to increase, these studies support the continued investigation of AFN-1252 as a targeted therapeutic for staphylococcal infections.
Introduction
The increasing prevalence of methicillin-resistant Staphylococcus aureus (MRSA) to newer antibacterial agents, in both community and healthcare settings, is a serious worldwide concern as therapeutic options are limited. Hence, there is a need for new antimicrobials possessing potent activity against this pathogen.
Bacterial fatty acid biosynthesis (FASII) is a relatively new and unexploited target for antimicrobial treatment of S. aureus.Citation1 AFN-1252, a potent inhibitor of staphylococcal enoyl-acyl carrier protein (enoyl-ACP) reductase (FabI), a critical enzyme required for bacterial FASII, is being developed by Affinium Pharmaceuticals, Inc. (Toronto, ON, Canada), in both oral and intravenous formulations, for the treatment of S. aureus infections. In vitro, AFN-1252 exhibits targeted and highly potent activity against Staphylococcus spp., with typical minimum inhibitory concentration (MIC90) values of 0·008–0·015 mg/l, up to 3 log reductions in bacterial viable count over 24 hours and low potential for resistance development.Citation2–Citation4 Murine studies indicate a long elimination half-life 5–7 hours and excellent efficacy in models of infection.Citation5,Citation6 Pharmacokinetic studies in man indicate the potential for once or twice daily dosing.Citation7
The objective of this study was to utilize an in vitro pharmacodynamic model simulating human pharmacokinetics to evaluate potential therapeutic regimens of AFN-1252 against S. aureus, including both methicillin-susceptible S. aureus (MSSA) and MRSA.
MATERIALS AND METHODS
Bacterial isolates, antibiotics, media, and susceptibility testing
Bacterial S. aureus isolates utilized were ATCC 29213, a standard reference MSSA and S186, a clinical MRSA isolate obtained from the bloodstream of an infected patient at the Buffalo Veterans Affair Health System of Western New York. Stock solutions of AFN-1252, provided by Affinium Pharmaceuticals, Inc., were prepared in 100% dimethyl sulphoxide and diluted at least 100-fold in Mueller–Hinton broth (Difco Laboratories, Detroit, MI, USA) supplemented with calcium (25 μg/ml) and magnesium (12·5 mg/l) for MICs determinationsCitation8 and use in the in vitro pharmacodynamic model. Bacterial quantification of all samples was determined using tryptic soy agar with 5% sheep blood (Becton-Dickinson, Mississauga, ON, Canada).
In vitro pharmacodynamic model
The in vitro pharmacodynamic model was as previously described.Citation8 Human pharmacokinetic profiles of AFN-1252 were based on phase 0 studies.Citation9 Simulated therapeutic regimens of AFN-1252 were based on free drug area under the concentration time curve to MIC ratio over MIC (fAUC/MIC) as detailed in . The initial bacterial inoculum was adjusted spectrophotometrically to achieve a final concentration of ∼106 colony-forming units/ml. Bacterial samples were taken at 0, 2, 4, 6, 8, 24, 28, 32, and 48 hours and the viable counts were determined. To investigate the impact of escalating exposures on selection of resistant isolates, samples taken at 0, 24, and 48 hours were also plated on tryptic soy agar containing 4× and 6× MICs of AFN-1252 to detect and amplify resistant subpopulations.
Table 1. Simulated AFN-1252 doses and corresponding free drug area under the concentration time curve to minimum inhibitory concentration (MIC) ratio over MIC (fAUC/MIC) parameters in the in vitro pharmacodynamic model
Results
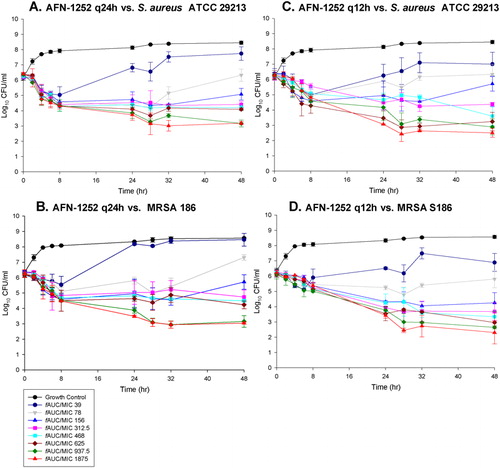
The AFN-1252 MICs for MSSA 29213 and MRSA S186 were both 0·008 μg/ml. The activity of AFN-1252 in the in vitro pharmacodynamic model is shown in . For all dosage regimens, reductions in viable count over the first 6 hours were generally 1–2 logs and maximal reductions in viable count (e.g. −2 to −3 logs) generally achieved at fAUC/MIC of 100–200. Greater reductions in viable count were observed with q12h regimens than corresponding q24h regimens, against both MSSA 29213 and MRSA S186 at 24 and 48 hours. The greatest reductions in viable count against MSSA 29213 and MRSA S186 were approximately 4 logs, achieved by 450 mg q12h (fAUC/MIC = 1875) dosing at 28 hours. Further analysis of the pharmacodynamic responses revealed an excellent correlation in the Hill modelCitation10,Citation11 between log reduction in viable count and fAUC/MIC fits (R2 values 0·998–0·999). AFN-1252 did not develop resistance at any time point throughout the 48-hour experiment as no growth was present on AFN-1252-containing agar.
Discussion
FabI is the sole form of enoyl-ACP reductase present in S. aureus, S. epidermidis, and other staphylococci. No alternative enzyme or rescue pathway, e.g. exogenous fatty acids, for FabI in staphylococci has been identified suggesting that FabI is essential to cell viability in Staphylococcus spp and therefore has the potential to become a significant new target for the treatment of staphylococcal infections.Citation4
AFN-1252 is a highly potent and specific inhibitor of FabI with exquisite activity against staphylococci in extensive MIC studies,Citation2–Citation4 and superior activity to linezolid in the MRSA murine thigh lesion model.Citation5 Pharmacokinetic studies in human volunteers indicate a good safety profile and the potential for once or twice a day dosing.Citation7 Rate of kill studies demonstrate that AFN-1252 typically achieves a 1–2 log reduction in viable count within 24 hours and therefore is not bacteriostatic but does not meet the CLSI criteria of bactericidal.Citation12 Other studies have also indicated that AFN-1252 can achieve a >2 log reduction in bacterial count over more than 24 hoursCitation4 and hence its action may be best described as ‘slowly bactericidal’. Although other triclosan-based FabI inhibitors CG400462, CG400549,Citation13 and MUT056399Citation14 are under investigation, they appear to be less potent than AFN-1252 and are less advanced.
In this current investigation, we studied the bactericidal activity and pharmacodynamics of AFN-1252, using an in vitro pharmacodynamic model, against two strains of S. aureus to determine optimal therapeutic regimens including comparisons of once and twice daily dosing. As a result of these studies, the pharmacodynamic profile of AFN-1252 was adequately characterized with the fAUC/MIC well related to antibacterial killing, which is also in agreement with what others have shown.Citation15 Twice daily dosing achieved marginally greater reductions in bacterial viable counts than once daily dosing, approaching the CLSI definition of bactericidal. Both dosing regimens resulted in bactericidal activity starting at 28 hours. It will be interesting to perform further pharmacokinetic/pharmacodynamic studies using hollow fibre and animal infection models to see if this affects the degree of bactericidal activity.
We would like to thank Nachum Kaplan, Barry Hafkin, and The Micron Group for critical review of this manuscript. This study was presented in part at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, 27–30 September 2006. Affinium Pharmaceuticals, Inc. provided research support of this study, but was not involved with the study design, completion, data analysis, or the conclusions provided in the manuscript.
References
- Heath RJ, Rock CO. Fatty acid biosynthesis as a target for novel antibacterials. Curr Opin Investig Drugs. 2004;5:146–53.
- Karlowsky JA, Laing NM, Baudry T, Kaplan N, Vaughan D, Hoban DJ, et al.. In vitro activity of API-1252, a novel FabI inhibitor, against clinical isolates of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 2007;51:1580–1.
- Kaplan N, Yethon J, Albert M, Bardouniotis E, Thalakada R, Walsh N, et al.. In vitro characterization of API-1252, a novel inhibitor of bacterial fatty acid biosynthesis, against drug resistant staphylococci. Proceedings of the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco, CA, USA. Poster F1-0754.
- Kaplan N, Albert M, Awrey D, Bardouniotis E, Berman J, Clarke T, et al.. AFN-1252 — mode of action, in vitro activity and in vivo efficacy of a selective anti-staphylococcal FabI inhibitor. Antimicrob Agents Chemother. 2012;56(11):5865–74.
- Banevicius MA, Deryke CA, Kaplan N, Vaughan D, Nicolau DP. In vivo pharmacodynamic profiling of API-1252 against Staphylococcus aureus in a murine thigh model. Proceedings of the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco, CA, USA. Poster F1-0759.
- Weis WJ, HafkinB, Kaplan N, Pulse M, Nguyen P, Renick P, et al.. Oral pharmacokinetics and effficacy of AFN-1252 in a murine septicaemia infection model with S. aureus. Proceedings of the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2010 Sep 12–15; Boston, MA, USA. Poster F1-833.
- Kaplan N, Hafkin B. Tolerability, safety and pharmacokinetics of multiple oral doses of AFN-1252 in healthy subjects. Proceedings of the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco, CA, USA. Poster F1-1349.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, 15th informational supplement M100-S15. Wayne, PA: CLSI; 2005.
- Kaplan N, Flanner H, Hafkin B. Correlation of AFN-1252 phase 0 microdosing and phase I pharmacokinetics. F1-2006. Proceedings of the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2009 Sep 12–15; San Francisco, CA, USA. Poster F1-2009.
- Harigaya Y, Bulitta JB, Forrest A, Sakouluas G, Lesse AJ, Mylotte JM, et al.. Pharmacodynamics of vancomycin at simulated epithelial lining fluid concentrations against methicillin-resistant Staphylococcus aureus (MRSA): implications for dosing in MRSA pneumonia. Antimicrob Agents Chemother. 2009;53:3894–901.
- Tsuji BT, von Eiff C, Kelchlin PA, Forrest A, Smith PF. Attenuated vancomycin bactericidal activity against Staphylococcus aureus hemB mutants expressing the small-colony-variant phenotype. Antimicrob Agents Chemother. 2008;52:1533–7.
- Clinical and Laboratory Standards Institute. Methods for determining bactericidal activity of antimicrobial agents; approved guideline M26A. Wayne, PA: CLSI; 1999.
- Park HS, Yoon YM, Jung SJ, Kim CM, Kim JM, Kwak JH. Antistaphylococcal activities of CG400549, a new bacterial enoyl-acyl carrier protein reductase (FabI) inhibitor. J Antimicrob Chemother. 2007;60:568–9.
- Escaich S, Prouvensier L, Saccomani M, Durant L, Oxoby M, Gerusz V, et al.. The MUR056399 inhibitor of FabI is a new antistaphylococcal compound. Antimicrob Agents Chemother. 2011;55:4692–7.
- Banevicius MA, Deryke CA, Kaplan N, Vaughan D, Nicolau DP. In vivo pharmacodynamic profiling of API-1252 against Staphylococcus aureus in a murine thigh model. Proceedings of the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco, CA, USA. Poster F1-0759.