Abstract
AFN-1252 is a novel inhibitor of FabI, an essential enzyme in fatty acid biosynthesis in Staphylococcus spp. AFN-1252 exhibits typical MIC90 values of ⩽0·015 μg/ml against diverse clinical isolates of S. aureus, oral absorption, long elimination half-live and efficacy in animal models. We now report high binding (∼95%) to serum proteins of mouse, rat, dog and humans, associated with an eight-fold increase in minimal inhibitory concentration (MIC) and which may be responsible for the long elimination half-lives on pharmacokinetic studies. Unlike daptomycin, AFN-1252 activity is not reduced in the presence of lung surfactant. AFN-1252 exhibits a short post-antibiotic effect of 1·1 hours against methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) following a 4-hour exposure period. The AFN-1252 unique spectrum of activity is not compromised by interactions with major antibiotic classes, but demonstrates synergy with low concentrations of gentamicin against MSSA and MRSA. These studies support the continued investigation of AFN-1252 as a targeted therapeutic for staphylococcal infections.
Introduction
The increasing prevalence of multidrug-resistant staphylococcal infections necessitates new agents with novel mechanism of action and ideally oral therapies that can be used to treat these infections. There is also interest in the potential of targeted therapies to treat emerging drug-resistant strains, while providing for fewer adverse effects and less collateral damage, such as disruption of normal flora or resistance pressure on off-target bacteria.
The bacterial fatty-acid (FASII) biosynthetic pathway, which involves several discrete enzymes, has been identified as new antibacterial target.Citation1–Citation5 The bacterial FASII pathway is different to that of mammals which is dependent upon a single proteinCitation6 and hence is a priori reason why inhibitors of bacterial fatty acid biosynthesis should be selective and safe in clinical use.
Enoyl-ACP reductase (FabI) catalyzes the elongation of the acyl chain and is the last step in the bacterial FASII pathway and its inhibition disrupts both saturated and unsaturated fatty acid biosynthesis, preventing bacterial repair and growth. FabI is the sole form of enoyl-ACP reductase present in S. aureus, S. epidermidis and other staphylococci.Citation7 No alternative enzyme or rescue pathway for FabI in staphylococci has been identified, suggesting that FabI is essential to cell viability in Staphylococcus spp. and hence that FabI inhibition could be considered as a targeted anti-staphylococcal therapy.
The mechanism of action of AFN-1252, a potent inhibitor of FabI, has been confirmed using biochemistry, macromolecular synthesis, genetics and co-crystallization studies.Citation7 Primary in vitro studies demonstrate, excellent potency against clinical isolates of S. aureus (typical MIC90 0·015 μg/ml) and coagulase-negative staphylococci (typical MIC90 0·12 μg/ml) regardless of their susceptibility to other classes of antibacterials or clinical origin.Citation7–Citation9 A low propensity for spontaneous resistance developmentCitation7 is predicted and a time-dependent reduction in viability of both methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) is seen in vitro. AFN-1252 was orally available in mouse pharmacokinetic studies and a single oral dose of 1 mg/kg AFN-1252 was efficacious in a mouse model of septicemia providing 100% protection from an otherwise lethal peritoneal infection of S. aureus Smith. An ED50 of 0·15 mg/kg indicated that AFN-1252 was 12 to 24 times more potent than linezolid in the model.Citation7
The current manuscript summarizes further in vitro studies, in particular the impact of serum proteins, lung surfactants and combination studies with representatives of other major antibiotic classes upon the activity of AFN-1252 in minimal inhibitory concentration (MIC) and rate of kill studies.
Methods
All AFN-1252 (Affinium Pharmaceuticals, Toronto, ON, Canada) solutions were prepared from the tosylate anhydrate [lots: GJP-F-49(5), GJP-F-62(2)] or monohydrate [lot MAN-G-133 (3)] salts with concentrations adjusted and reported as free base equivalents, based on the relevant certificates of analysis.
S. aureus ATCC 29213 (MSSA 29213) and ATCC 43300 (MRSA 43300) were used as standard assay strains. Other MSSA and MRSA strains were clinical isolates from laboratory collections.
MIC determination
MIC testing of antimicrobial agents was performed according to the Clinical and Laboratory Standards Institute (CLSI) guidelines using the broth microdilution procedure.Citation10
Serum protein-binding assays
AFN-1252 binding to serum proteins was investigated in three biophysical and one microbiological study covering different techniques, a range of AFN-1252 concentrations and sera from humans and three animal species.
Serum binding determined by equilibrium dialysis
This study evaluated concentration-dependent binding of AFN-1252 to human, mouse, rat and dog serum proteins. A stock solution (2 mg/ml) of AFN-1252 in dimethylsulfoxide (DMSO) was further diluted with DMSO to give working solutions of 40, 200, and 1000 μg/ml for the main assay, and 400 μg/ml for non-specific binding determination. The assay was performed in duplicate with pooled male human, rat (Sprague-Dawley), dog (Beagle) and mouse serum (all Bioreclamation Inc., Westbury, NY, USA). Equal volumes of each working solution were diluted in serum to give final AFN-1252 concentrations of 0·2, 1, 5, and 10 μg/ml. Test solutions were dialyzed against 0·02M phosphate buffered saline (PBS; pH 7·4) at 37°C for 4 hours in an equilibrium dialyzer (Spectrum Laboratories Inc., Rancho Dominguez, CA, USA), using a dialysis membrane with molecular weight cut-off (MWCO) of 12–14 kDa. The concentration of AFN-1252 on each side of the membrane was determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Liquid chromatography was performed using an Agilent Technologies 1100 series binary pump (Wokingham, UK) with Shimadzu SIL-HTC autosampler (Columbia, MD, USA) and Phenomenex Onyx Monolithic C18 column (Macclesfield, UK) at ambient temperature, and mobile phase gradient elution with 0·2% v/v formic acid in water and 0·2% v/v formic acid in acetonitrile at a flow rate of 1·5 ml/min. The injection volume was set at 25 μl and run time at 3·5 minutes. Tandem MS was performed using an MDS Sciex API 4000 (Applied Biosystems, Concord, ON, Canada) and positive electrospray ionization (ESI). Non-specific binding to the apparatus was determined in duplicate, using AFN-1252 at a nominal concentration of 2 μg/ml in PBS dialyzed against PBS at 37°C for 5 hours. The non-specific binding was calculated based on the percentage recovery of AFN-1252 as determined by LC/MS/MS. Using the same procedures, [7-14C]-salicylic acid (Perkin Elmer, Waltham, MA, USA), at 0·2 μg/ml, was included as a control to assess the binding assay conditions; however, post-dialysis samples were analyzed using liquid scintillation counting (Wallac beta counter; Perkin Elmer).
Mouse serum binding determined by ultrafiltration
A master stock solution of 6·4 mg/ml AFN-1252 in 100% DMSO was diluted in 50% DMSO to give working solutions of 400, 200, 150, 100 and 20 μg/ml. Subsequent 1 in 200 dilutions were performed in freshly collected mouse plasma to give the final concentrations of 2, 1, 0·75, 0·5, and 0·1 μg/ml. Each solution was placed in a shaking water bath at 37°C for 10 minutes, then 0·9 ml transferred in triplicate to ultrafiltration devices (Amicon Centrifree® Micropartition, Millipore, Bedford, MA, USA) with MWCO of 30 kDa. Each was centrifuged at 1000g for 30 minutes at 10°C to generate a plasma ultrafiltrate volume of approximately 250 μl. Non-specific binding of AFN-1252 to the filter device was determined using a drug concentration of 0·75 μg/ml. Concentrations of AFN-1252 were determined by high performance LC-MS/MS. The HPLC procedure used an Agilent Zorbax Eclipse XDB-C8 column at 30°C and an injection volume of 30 μl with a 15 minutes run time. Gradient elution mobile phases consisted of acetonitrile and water (9∶1, v/v) with 0·1% formic acid and acetonitrile with 0·1% formic acid, and the flow rate was set at 1 ml/min. Tandem MS was performed using a Bruker Esquire 3000 Plus (Billerica, MA, USA) and positive ESI.
Human, rat, dog and mouse serum binding by ultrafiltration
A working solution of AFN-1252 at 2 mM (750·8 μg/ml) in 50% DMSO was prepared. For each species, 2 μl of working solution was added to 398 μl of serum to give test solutions with an AFN-1252 concentration of 10 μM. Each test solution was incubated at 37°C for 30 minutes, then 4 μl diluted in 36 μl of PBS (Sample 1). The remaining test solutions were loaded into the wells of a MultiScreen filter plate with Ultracel-PPB membrane (Millipore, Billerica, MA, USA) which was then centrifuged at 3300 rpm and 25°C for 45 minutes or until at least 40 μl of ultrafiltrate has been collected for each test solution. For each test ultrafiltrate, a 4-μl aliquot was diluted in 36 μl of PBS (Sample 2), and a further 40 μl was measured into another tube (Sample 3). AFN-1252 analysis was performed using HPLC-MS, as detailed above for the mouse serum study, with an injection volume of 5 μl and flow rate of 20 μl/min. Gradient elution mobile phases were: water with 0·1% formic acid and acetonitrile with 0·1% formic acid.
Effect of serum binding on MIC
The effects of 4% human serum albumin (HSA; Sigma-Aldrich, St. Louis, MO, USA) and 0, 10, 25, 50 or 100% human serum (Bioreclamation Inc., Hicksville, NY, USA) binding on the MICs of AFN-1252 against the standard CLSI reference strain MSSA 29213 was investigated using microdilution procedures based on the CLSI guidelines.Citation10
Effect of serum binding on rate of kill
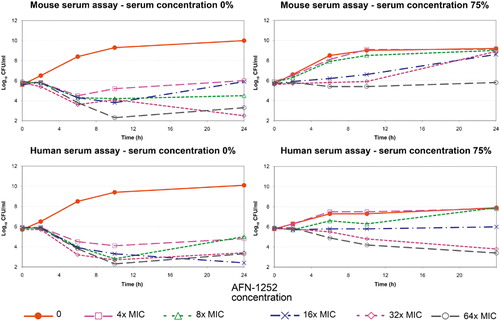
The organism used in the time-kill assay was MRSA 1659, a hospital-acquired MRSA strain with an AFN-1252 MIC of 0·015 μg/ml. A series of flasks were prepared containing Mueller-Hinton II broth (MHB; Becton Dickinson, Sparks, MD, USA), supplemented with AFN-1252 at final concentrations of 0·96, 0·48, 0·24, 0·12 and 0·06 μg/ml (i.e. 64× MIC to 4× MIC) in the presence or absence of 75% pooled human serum (Valley Biomedical, Winchester, VA, USA) or mouse serum (Biologos Inc., Montgomery, IL, USA). At T0, 0·5 ml of bacterial inoculum was added to each vessel which was immediately swirled to mix, and then a 0·5 ml sample removed for the determination of viable count. The vessels were placed in an incubator shaker at 35°C and 150 rpm, and further sampled at 2, 6, 10 and 24 hours after inoculation for determination of viable count. Viable cell counts were carried out by serial 10-fold dilutions in tryptic soy broth and plating out duplicate 50 μl samples on prepared plates of trypticase soy agar and incubating plates for 24 hours at 35°C.
To determine apparent plasma protein binding, the change in log10 CFU/ml T0 to 10 hours was plotted against the AFN-1252 concentration for each test condition. The data were then modelled in WinNonLin V. 5·2·1 with the Emax inhibitory effect model to determine the AFN-1252 concentrations predicted to achieve a change in log10 CFU/ml of 0 (stasis), −0·5, −1 and −1·5. The ratios of AFN-1252 concentrations required to achieve each change in log10 CFU/ml value, in the presence/absence of human or mouse serum, were calculated and used to calculate the plasma protein binding and amount of free drug.
Effect of pulmonary surfactant
In this study, the MICs of AFN-1252, daptomycin and vancomycin, in the presence and absence of surfactant (Survanta, Abbott Laboratories), were determined using CLSI broth microdilution against 11 MSSA and 10 MRSA. Stock solutions of antibiotics were doubly diluted to give a range of working stock solutions at 50× the final solutions, between 1 and 0·001 μg/ml for AFN-1252, and between 64 and 0·06 μg/ml for both vancomycin and daptomycin. Surfactant solutions (in sterile 0·9% saline; 5 μl per assay) were added to achieve a final concentration of 0, 0·1, or 0·8 mg/ml.
Post-antibiotic effect (PAE)
The PAE of AFN-1252 was determined at three concentrations (1×, 32× and 128× the MIC) and two exposure times (1 and 4 hours) using MSSA 29213 and MRSA 43300. For each strain, two sets (one for each challenge time) of eight tubes were set up with AFN-1252 in 4·5 ml of growth medium as follows: Tube 1: 0 μg/ml, sterility control; Tube 2: 0 μg/ml, growth control; Tubes 3–5: 0·032, 0·32 and 1 μg/ml respectively, challenge tubes; Tubes 6–8: 0·000032, 0·00026, and 0·001 μg/ml respectively, controls to monitor for residual compound effect on growth kinetics. An inoculum of 500 μl was added to each of Tubes 2–5, for an initial concentration of 1×106 CFU/ml, and the tubes, together with Tube 1, placed in a shaking incubator at 35°C. After 1 and 4 hours, 5 μl from each of the five tubes were diluted in 5 ml of fresh CAMHB (at T0); at the same time, Tubes 6–8 were inoculated with 500 μl of the non-challenged culture from Tube 2. Each of the eight tubes was sampled for viable cell counts before being placed in a shaking incubator at 35°C. Viable cell counts were performed every 30 minutes for 6 hours by serial 10-fold dilutions (minimum 1000-fold) in 0·9% sterile saline and spot-plating (20 μl) on TSA plates in triplicate. Plates were incubated overnight at 35°C. The limit of detection for this method was 50 CFU/ml. The PAE was calculated using the following formula: PAE = T–C, where T is the time required for the test culture to increase 1 log above the count at T0, and C is the time required for the growth control (Tube 2) to increase 1 log above the count at T0. Each experiment was conducted on four occasions and mean PAE values calculated. Vancomycin was included as control in two experiments.
Activity of AFN-1252 in combination with other antibacterials
The activity of AFN-1252 in combination with representatives of major antibacterial classes was investigated using both checkerboard and rate of kill studies.
Checkerboard assays were performed in 96-well platesCitation11 and fractional inhibitory concentration (FIC) values determined.Citation12 For all of the wells of the microtitration plates that corresponded to an MIC, the sum of the FICs (ΣFIC) was calculated for each well with the equation ΣFIC = FICA+FICB = (CA/MICA)+(CB/MICB), where MICA and MICB are the MICs of drugs A and B alone, respectively, and CA and CB are the concentrations of the drugs in combination, respectively. FIC values were interpreted as follows: synergy (FIC≤0·5); additivity or indifference (FIC>0·5≤2); or antagonism (FIC>2).
Time-kill combination assays were performed as previously described.Citation11 Log phase cells (∼106 CFU/ml) were incubated with varying concentrations of AFN-1252 and combination antibacterial agents for 24 hours at 35°C. At each time point, samples were removed for determination of viable CFU counts. The combination effect in killing at 24 hours was defined as synergy (≧100-fold decrease in CFU/ml), additivity/indifference (<100-fold decrease or increase in CFU/ml) or antagonism (≧100-fold increase in CFU/ml), compared with the more active component alone.
Results
Serum binding of AFN-1252
AFN-1252 was highly bound to plasma proteins from all four species (). Values from equilibrium dialysis exceeded 95% for all serum types and there was no obvious effect of AFN-1252 concentration. Mouse serum binding results from one ultrafiltration assay were marginally higher, at 95 to 98%, than observed with the equilibrium assay. However, results from a second ultrafiltration assay across all four serum types were lower, at 92 to 95%, than corresponding results obtained by equilibrium assays.
Table 1. Determination of serum protein binding of AFN-1252
Effect of serum proteins on MIC
The MIC of AFN-1252 for MSSA 29213 increased 8-fold from 0·004 μg/ml in serum-free CAMHB media to 0·032 μg/ml in CAMHB containing 50% human serum in a serum-concentration dependent manner. No bacterial growth was seen in 100% human serum. Incorporation of 4% human serum albumin into CAMHB was also associated with an 8-fold increase in AFN-1252 MIC.
Effect of serum on rate of kill
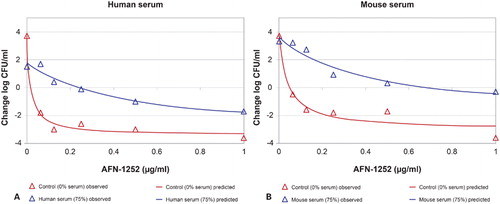
In the absence of serum, AFN-1252 exhibited concentration dependent reduction in the viable count of MRSA 1659, ranging from a 1–2 log10 reduction at 4× MIC to a 4-log reduction at 64× MIC at 10 hours (). In the presence of 75% human serum or mouse serum, bacterial growth rate was reduced and AFN-1252 exhibited less effect. In human serum, 8× MIC resulted in bacteriostatic activity and 64× MIC achieved a 2 log10 reduction at 10 hours. In mouse serum, drug concentrations at 64× MIC achieved bacteriostasis.
Figure 1. Rate of kill of AFN-1252 against MRSA 1659 in the presence and absence of 75% mouse and human serum.
Based on the Emax inhibitory effect model on the change in log10 CFU/ml at 10 hours (), it was calculated that a 13× increase in AFN-1252 concentration would be required to achieve stasis in 75% human serum compared with serum-free media, and increases of between 13× and 18× would be required to achieve the same reductions in viable count activity as seen in serum-free media. The apparent plasma protein binding determinations were similar for human and mouse sera, and ranged between 92 and 94%.
Pulmonary surfactant
AFN-1252 had potent activity against all 20 strains of staphylococci, including 10 MRSA, with MICs of 0·008–0·015 μg/ml in the absence of surfactant (). Similar MICs (0·008–0·03 μg/ml) were observed in the presence of surfactant at either 0·1 or 0·8 mg/ml. Whether in the presence or absence of surfactant, AFN-1252 was several-fold more potent than either vancomycin (MICs of 0·5–1 μg/ml) or daptomycin (MICs of 0·5–2 μg/ml). Vancomycin MICs were generally unaffected by the presence of surfactant while daptomycin MICs were elevated 4- to 16-fold in the presence of surfactant at 1 mg/ml and at least 32-fold in the presence of surfactant at 0·8 mg/ml.
Table 2. In vitro activity of AFN-1252 and comparators against S. aureus in the presence and absence of pulmonary surfactant
Post-antibiotic effect
AFN-1252 exhibited dose-dependent PAEs of 0·1–0·2 hour at 32× MIC and 1·1–1·2 hours at 128× MIC, following 4-hour incubation, against both MSSA 29213 and MRSA 43300 ().
Table 3. Average PAE from four experiments
Combination studies
Checkerboard studies generally showed additivity between AFN-1252 and representatives of the major antibacterial classes against MSSA 29213 and MRSA 43300 (). FIC values of 0·4, indicating synergy, were calculated for combinations of AFN-1252 and gentamicin against both strains. A further study using MSSA 29213, MRSA A6250596 (fluoroquinolone-resistant) and MRSA A7080336 did not detect any synergy or antagonism between AFN-1252 and penicillin, cefuroxime, ceftriaxone, meropenem, levofloxacin, gatifloxacin or azithromycin.
Table 4. Checkerboard analysis of AFN-1252 with S. aureus
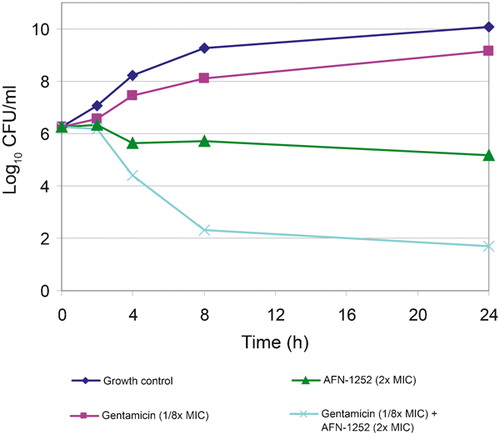
AFN-1252 at 2× MIC resulted in log10 reduction of CFU/ml of −1·1 to −1·53 over 24 hours against MSSA 29213 (). In combination with linezolid at ¼× MIC or 4× MIC, AFN-1252 at 2× MIC had indifferent effect. AFN-1252 also exhibited indifferent effect in combination with vancomycin at ¼× MIC but antagonism with vancomycin at 4× MIC. Linezolid was bactericidal (>3 log reduction in CFU/ml) at 24 hours at concentrations above its MIC; the combination of AFN-1252+linezolid showed indifference at both 4× and ¼× MIC. The combination of AFN-1252 at 2× MIC and gentamicin at ⅛× MIC was synergistic, achieving more than a 4-log reduction in viable count ().
Table 5. Effect of AFN-1252 in combination with linezolid, gentamicin or vancomycin upon reduction in viable count (log CFU/ml) of MSSA 29213 after 24 hours
Discussion
AFN-1252 exhibits exquisite and highly selective activity against Staphylococcus spp., both in vitro and in animal models of infection. The studies described in this manuscript report the effect of serum proteins, lung surfactants and interactions with representatives of other major antibiotic classes, upon the in vitro activity of AFN-1252.
The degree of binding to serum proteins greatly affects the pharmacokinetics, particularly the elimination half-life, and hence pharmacodynamics of a drug. Low serum binding is associated with high levels of free drug but short elimination half-lives and the need for frequent dosing, whereas high serum binding is associated with low levels of free drug and long elimination half-lives as the drug is released from the ‘reservoir’ and the potential for less frequent dosing.Citation13
The extent of protein-binding of AFN-1252 was robustly investigated using different sera and standard equilibrium dialysis and ultrafiltration methods. In all cases, AFN-1252 displayed concentration-independent high serum binding, including values ranging from 92·5 to 98·4% in human serum. Such values relate well to the long elimination half-lives seen in miceCitation7 and early Phase I studies.Citation14 The high serum binding was also evident in MIC studies using MSSA 29213, where AFN-1252 MICs were 8-fold higher in the presence of 50% serum, corresponding to 12·5% free AFN-1252, i.e. approximately 90% binding. Although this latter result confirms the high serum binding, further studies with a genotypically diverse range of strains are desirable.
Protein binding of AFN-1252 was also investigated through its effect in rate of bacterial kill studies in the presence of 75% human or mouse serum. In the absence of serum, AFN-1252 exhibited concentration-dependent reduction in the viable count of MRSA 1659, ranging from a 1–2 log10 reduction at 4× MIC to a 4-log reduction at 64× MIC at 10 hours. In the presence of 75% human serum or mouse serum, bacterial growth rate was markedly reduced and AFN-1252 exhibited less effect. For both human and mouse serum studies, the apparent protein-binding values calculated from the predicted rate of kill using an Emax inhibitory effect model were 92–94%, i.e. within the range obtained from biophysical methods. Time-kill assays in the presence and absence of serum therefore present an alternative approach to determining plasma protein binding values. As with the serum MIC studies, it would be useful to extend this study to a wider range of strains.
AFN-1252 exhibited dose-dependent PAEs against the two indicator strains of MSSA and MRSA. Although the PAE of 0·1–0·2 hour at 32× MIC is small, this increased 6- to 10-fold at 128× MIC. It is to be expected that the long AFN-1252 elimination half-lives seen in animal studies will be further prolonged in humans, in which case PAE may not be a major pharmacodynamic factor.
Decreased efficacy of daptomycin in community-acquired pneumonia is due to inhibition of the drug by pulmonary surfactant.Citation15 This inhibition was readily apparent in vitro with large increases in daptomycin MICs in the presence of a pulmonary surfactant (Survanta®, Abbott Laboratories, Abbott Park, IL, USA) used primarily to increase surface tension in the lungs of premature newborns. We confirmed the marked increase in daptomycin MICs in the presence of lung surfactant at 0·1 mg/ml and 0·8 mg/ml. In contrast to daptomycin, AFN-1252 MICs were not affected when testing in the presence of pulmonary surfactant, indicating that pulmonary surfactant will not impact the activity of AFN-1252 in respiratory infections.
The interaction between AFN-1252 and representatives of other major antibiotic classes was extensively investigated using both checkerboard MICs and time-kill assays against four strains of S. aureus, including the CLSI reference strain MSSA 29213 and three diverse clinical isolates of MRSA. Additivity was most common result in checkerboard MICs, the exceptions being an indication of antagonism in combination with vancomycin against MSSA 29213 in rate of kill studies, but additivity in the checkerboard assay, and synergy, based on FIC values of 0·4, with gentamicin against both MSSA 29213 and MRSA 43300. The synergy with gentamicin was confirmed in time-kill studies against MSSA 29213. As AFN-1252 was selected and developed as an agent specific for Staphylococcus spp., with minimal impact on other flora, it is logical to assume that synergistic or antagonistic interactions would be unwelcome. However, the interaction with gentamicin may be the exception to this rule as gentamicin has a poor safety profile and a combination with AFN-1252 offers the opportunity for increased activity against S. aureus and reduced safety concerns.
In conclusion, these studies confirm the high serum binding of AFN-1252, small PAE, fully retained activity in the presence of lung surfactant and, with the exception of gentamicin, no interaction with other antibiotics. These properties indicate predictable pharmacokinetics and efficacy in clinical use.
We thank Dr George Drusano for assistance in the experimental design and data interpretation of the serum time-kill experiments and Gary E. Zurenko and Roger R. Hinshaw (Micromyx, LLC, Kalamazoo, MI, USA) for their execution. We also thank Dr David Nicolau and Mary Ann Banevicius (Center for Anti-Infective Research & Development Hartford Hospital) for their contributions to the ultrafiltration mouse serum binding assay, and Linh Nguyen, Limei Tao and Alan McIntyre (Charles River Laboratories, Montreal) for the equilibrium dialysis plasma protein binding assays. We finally thank Lauretta Stapert, Dr Chris Pillar and Dr Dean L. Shinabarger (Micromyx, LLC, Kalamazoo, MI, USA) for the lung surfactant MIC assessments. Micron Research Ltd assisted in the preparation of this manuscript.
References
- Miller WH, Seefeld MA, Newlander KA, Uzinskas IN, Burgess WJ, Heerding DA, et al.. Discovery of aminopyridine-based inhibitors of bacterial enoyl-ACP reductase (FabI). J Med Chem. 2002;45:3246–56.
- Heath RJ, White SW, Rock CO. Inhibitors of fatty acid synthesis as antimicrobial chemotherapeutics. Appl Microbiol Biotechnol. 2002;58:695–703.
- Kodali S, Galgoci A, Young K, Painter R, Silver LL, Herath KB, et al.. Determination of selectivity and efficacy of fatty acid synthesis inhibitors. J Biol Chem. 2005;280:1669–77.
- Zhang Y-M, White SW, Rock CO. Inhibiting bacterial fatty acid synthesis. J Biol Chem. 2006;281:17541–4.
- Lu H, Tonge PJ. Inhibitors of FabI, an enzyme drug target in the bacterial fatty acid biosynthesis pathway. Acc Chem Res. 2008;41:11–20.
- Asturias FJ, Chadick JZ, Cheung IK, Stark H, Witkowski A, Joshi AK, et al.. Structure and molecular organization of mammalian fatty acid synthase. Nat Struct Mol Biol. 2005;12:225–32.
- Kaplan N, Albert M, Awrey D, Bardouniotis E, Berman J, Clarke T, et al.. AFN-1252 - Mode of action, in vitro activity and in vivo efficacy of a selective anti-staphylococcal FabI inhibitor. Antimicrob Agents Chemother. 2012;56:5865–74.
- Karlowsky JA, Kaplan N, Hafkin B, Hoban DJ, Zhanel GG. AFN-1252, a FabI inhibitor, demonstrates a Staphylococcus-specific spectrum of activity. Antimicrob Agents Chemother. 2009;53:3544–8.
- Karlowsky JA, Laing NM, Baudry T, Kaplan N, Vaughan D, Hoban DJ, et al.. In vitro activity of API-1252, a novel FabI inhibitor, against clinical isolates of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 2007;51:1580–1.
- Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically – 9th ed. Approved Standard M7-A9. CLSI, Wayne, PA, USA, 2012.
- Eliopoulous GM, Moellering RC. Antimicrobial combinations. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, MD: The Williams & Wilkins Co.; 1996. p. 330–96.
- Meletiadis J, Mouton JW, te Dorsthorst DTA, Verweij PE. Assessing combination of antifungal drugs against yeasts and filamentous fungi: comparison of different drug interaction models. Med Mycol. 2005;43:133–52.
- Merrikin DJ, Briant J, Rolinson GN. Effect of protein binding on antibiotic activity in vivo. J Antimicrob Chemother. 1983;11:233–8.
- Kaplan N, Hafkin B. Tolerability, safety and pharmacokinetics of multiple oral doses of AFN-1252 in healthy subjects. Abstract F1 -1349. 51st ICAAC, Interscience Conference on Antimicrobial Agents and Chemotherapy, 2011 Sep 17–20; Chicago, IL, USA.
- Silverman JA, Mortin LI, Vanpraagh AD, Li T, Alder J. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191:2149.