Abstract
Objective
To develop a comprehensive, psychometrically sound, and conceptually grounded patient reported outcomes (PRO) measurement system for individuals with spinal cord injury (SCI).
Methods
Individual interviews (n = 44) and focus groups (n = 65 individuals with SCI and n = 42 SCI clinicians) were used to select key domains for inclusion and to develop PRO items. Verbatim items from other cutting-edge measurement systems (i.e. PROMIS, Neuro-QOL) were included to facilitate linkage and cross-population comparison. Items were field tested in a large sample of individuals with traumatic SCI (n = 877). Dimensionality was assessed with confirmatory factor analysis. Local item dependence and differential item functioning were assessed, and items were calibrated using the item response theory (IRT) graded response model. Finally, computer adaptive tests (CATs) and short forms were administered in a new sample (n = 245) to assess test-retest reliability and stability.
Participants and Procedures
A calibration sample of 877 individuals with traumatic SCI across five SCI Model Systems sites and one Department of Veterans Affairs medical center completed SCI-QOL items in interview format.
Results
We developed 14 unidimensional calibrated item banks and 3 calibrated scales across physical, emotional, and social health domains. When combined with the five Spinal Cord Injury – Functional Index physical function banks, the final SCI-QOL system consists of 22 IRT-calibrated item banks/scales. Item banks may be administered as CATs or short forms. Scales may be administered in a fixed-length format only.
Conclusions
The SCI-QOL measurement system provides SCI researchers and clinicians with a comprehensive, relevant and psychometrically robust system for measurement of physical-medical, physical-functional, emotional, and social outcomes. All SCI-QOL instruments are freely available on Assessment CenterSM.
Introduction
The Spinal Cord Injury-Quality of Life (SCI-QOL) measurement system has been developed over the past 10 years to address the unmet need for comprehensive, conceptually relevant, psychometrically sound, and brief yet precise patient reported outcomes measures (PROs) for use in SCI research and practice. The end result of this work is a set of 19 item response theory (IRT)-calibrated item banks and 3 calibrated scales. Each item bank may be administered as a full bank, short form, or computer adaptive test (CAT), while scales may be administered in fixed-length format only. This manuscript outlines the methodologies used in the five phases of the SCI-QOL development project – namely, (1) subdomain selection, (2) item development, (3) field testing, (4) psychometric analysis and IRT calibration, and (5) testing in a new sample to assess of psychometric properties – and presents the results of graded response model item response theory calibration for each item bank.
Background: 21st century PRO measure development
Across all areas of health outcomes research, new standards have recently been introduced to guide the field of patient reported outcomes (PRO) measurement development efforts. Spearheaded by the Patient Reported Outcomes Measurement Information System (PROMIS), leading measurement experts who specialize in a wide variety of diseases and other health conditions have collaborated over the past 10 years to bring cutting-edge measurement techniques – most notably, those pioneered in the fields of educational and personality measurement – to health care. New Instrument Development and Scientific StandardsCitation1 documents have been developed to outline necessary steps in these development processes.
PROMIS
Multiple federal initiatives have focused on developing health-related quality of life (HRQOL) measures for use in clinical trials. These efforts have focused on universally relevant measures that allow comparison of research findings across medical diseases and conditions. For instance, the National Institutes of Health (NIH) made the development of PROs part of their ‘roadmap’ for medical research in the 21st century, a goal of which is to ‘catalyze changes necessary for transforming new scientific knowledge into tangible benefits for people.’Citation2 The resulting Patient Reported Outcomes Measurement Information System (PROMIS)Citation3 is a universally relevant measurement system with the potential for use in a wide variety of health care studies. PROMIS used state-of-the-art item writing and item pool developmentCitation4,Citation5 procedures that emphasized qualitative feedback and key stakeholder (e.g. patient) participation at multiple phases throughout the instrument development process. Stakeholder involvement helped guide the focus and development of the instrument, ensuring that the content of the resulting measures was conceptually grounded in phenomena deemed relevant and important from patients’ perspectives. Individuals with spinal cord injury (SCI) were included in this early development, making PROMIS one of the very few measurement systems to include people with SCI in the initial domain development.Citation6 PROMIS is also unique from a methodological standpoint,Citation1,Citation7 in that advanced psychometric techniquesCitation8 were used to inform development of a computerized adaptive testing (CAT) platform for instrument administration.
Neuro-QOL
In 2004, the National Institute of Neurological Disorders and Stroke (NINDS) prioritized the development of PROs as part of their efforts to develop common data elements for use in their research studies. Consequently, the Neurological Quality of Life (Neuro-QOL) measurement system was developed using the PROMIS Measurement Standards. The Neuro-QOLCitation9 is a set of PRO item banks developed and validated for individuals with neurological disorders. In addition to adhering to the PROMIS development methodology, the Neuro-QOL incorporated many PROMIS items to facilitate linkage between the measurement systems. Neuro-QOL was designed for use with five neurological conditions: stroke, Parkinson's disease, multiple sclerosis, epilepsy, and amyotrophic lateral sclerosis. The measurement development process did not include individuals with SCI, and as such we found it necessary to develop a related measurement system with direct relevance and applicability to individuals with SCI. This effort is called the SCI-QOL measurement system.Citation10
SCI-QOL
SCI-QOL builds upon the foundation of clearly defined qualitative and quantitative methods and advanced psychometrics at the core of the PROMIS and Neuro-QOL systems. The research presented here describes the methods, measurement design, and results of the phases of this research project. A separate phase of research was conducted in regard to each of the project's specific aims, enumerated below, using a unique (a) sample, (b) set of scientific procedures, and (c) analytic methods. The methods included structured individual interviews with individuals with SCI, formal qualitative research using focus groups with individuals with SCI and clinicians, large-scale calibration field testing across multiple data collection sites, advanced psychometric analyses using IRT, and multisite testing of the newly developed SCI-QOL CAT and short form (SF) instruments.
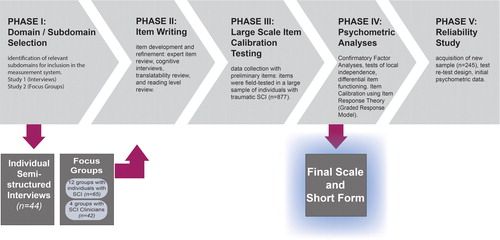
This manuscript provides a detailed description of the methods relevant to most of the manuscripts contained in this special issue. There were five primary aims to our development activities, which occurred and corresponded to five sequential phases of work. Our development goals included: (1) identification of relevant subdomains for inclusion in the measurement system (phase I studies: a – Individual Interviews and b – Focus Groups); (2) item development and refinement (phase II); (3) data collection with preliminary items (phase III); (4) psychometric analyses (factor analyses and item calibration; phase IV); and (5) acquisition of initial psychometric data (phase V). In this manuscript we report on the goals, methodology, and an overview of results in each of the five phases. A guide to the study phases and corresponding project aims is presented in . A glossary of terms is provided as .
Table 1. Glossary of Terms
Phase I: Identification of SCI-QOL domains and subdomains
As described in Tulsky et al.,Citation10 stakeholder feedback is imperative to ensure that the resultant measurement system is conceptually grounded to relevant HRQOL issues as actually experienced by individuals with SCI. We conducted two qualitative studies and consulted with individuals with SCI and other key stakeholders (i.e. SCI clinicians and researchers) to select the domains and specific subdomains to be developed into items banks as part of SCI-QOL measurement system.
Study 1: Individual interviews
Study 1 (Individual interviews) - methods
We conducted IRB-approved semi-structured individual interviews with individuals with SCI to shape the conceptualization of HRQOL in SCI and to identify important themes and even items for inclusion in our new measure. These interviews focused on HRQOL among individuals with traumatic SCI (n = 44). Though largely a sample of convenience, participants were stratified to ensure representation of individuals with different diagnoses (tetraplegia vs. paraplegia), severity of injury (complete vs. incomplete), and time since injury (<1 year, 1–3 years, >3 years). Each interview consisted of open-ended questions about the general nature of HRQOL following injury and typically lasted about 2 hours. Participants were told that they should assume the role of expert, and were encouraged to raise issues resulting from their injuries. Interviewers recorded the responses verbatim and organized them by content area. These responses were used to identify thematic areas of HRQOL not captured in traditional HRQOL scales and to develop initial HRQOL items. Themes generated during Study 1 were used to inform the design and development of Study 2, and several verbatim quotes were used to form the basis of preliminary SCI-QOL items
Study 1 (Individual interviews) - results
Participant Demographic Characteristics. Forty-four community-dwelling individuals with traumatic SCI participated in the individual interviews. The mean age of participants was 42.6 years (SD 13.8). Seventy-three percent of participants were male, which is consistent with the demographic makeup of the overall SCI population (i.e. approximately 80% male).Citation11 Fifty-five percent of the sample self-reported as Caucasian, 32% as Black or African-American, 7% Hispanic, and 7% Asian/Pacific Islander. The majority of participants (59%) were injured in automobile accidents. Fourteen percent of participants were injured by acts of violence, 12% of injuries were sports-related, 12% were sustained from falls, and 5% of injuries were due to other causes. Fourteen percent of the sample was diagnosed with complete tetraplegia, 34% with incomplete tetraplegia, 30% with complete paraplegia, and 23% with incomplete paraplegia. On average, participants were 8.0 years post injury (SD 11.0), with 27% less than one year post injury, 32% between 1–3 years post injury, and 39% were greater than 3 years post injury.
Preliminary HRQOL Domains. Based on direct quotes from participants, study team members drafted a total of 1,289 preliminary HRQOL items across Emotional, Physical-Medical, Physical-Functional, Social/Participation, Environmental Factors, and Sexual Functioning domains. provides counts of preliminary items by domain and subdomain.
Table 2. Preliminary Items Generated from Study 1: Item Generation Interviews
Study 2: Focus groups
Study 2 (Focus groups) - methods
In our second qualitative study, we conducted formal, semi-structured focus groups to identify key domains and subdomains of HRQOLCitation12,Citation13 and to inform the selection and/or development of individual HRQOL items. Participants included individuals with SCI and SCI clinicians who were recruited through four National Institute on Disability and Rehabilitation Research (NIDRR) – funded Model SCI System sites.Citation12,Citation14 Individuals with SCI were included in this study if they had sustained a traumatic SCI and were able to read and understand English. Each site made efforts to recruit individuals with SCI across all levels of injury, and to include representatives from various racial, ethnic, gender, and socioeconomic groups to help ensure a heterogeneous makeup of the overall sample. The SCI professional groups included physiatrists, physical therapists, psychologists, and nurses who work primarily (i.e. ≥50% of the time for a minimum of 3 years) with persons with SCI.
The focus group moderators were the study PI (author DST) and a Ph.D.-level co-investigator (author DV) who had extensive experience (i.e. >20 groups) conducting focus groups related to HRQOL and measurement development. The co-moderators debriefed (i.e. discussed things that went well and things that could be improved for future groups) following each focus group session to help ensure adherence to the focus group guide and method for the remaining groups. Moderators facilitated discussions in a semi-structured manner, providing basic rules and general topics for discussion yet allowing participants to discuss their own stories and perspectives. Participants were encouraged to discuss experiences and issues that affected their QOL and moderators prompted participants to focus on both positive and negative aspects of life with SCI in order to ensure a range of feedback.
After discussing experiences with their own QOL or, in the case of clinicians, the experiences of their patients, focus group participants were asked to define QOL and outline what they perceived to be the most important aspects of QOL for an individual with SCI. Following this general discussion, different patient groups were asked to focus on one specific domain area, e.g. physical health, emotional health, or social participation. A parallel set of focus groups covered physical functioning and activity limitations; the results from these focus groups are reported elsewhere.Citation14,Citation15 Each professional group covered all of the above domains of functioning. Group discussions were audio-recorded and transcribed verbatim.
We used a grounded-theoryCitation16,Citation17 based qualitative approach, as described in Kisala and Tulsky,Citation13 to analyze focus group data. A minimum of two investigators reviewed each transcript independently. Analysis steps included independent transcript review (open coding), development of a hierarchical (axial coding), and application of codes to each segment of transcript text (selective coding) by two independent raters. Raters logged and reconciled disagreements in order to achieve 100% agreement. This final code for each chunk of text was used to calculate the relative frequency of mention for various focus group topics. A detailed description of qualitative analysis results may be found in Tulsky et al.Citation12
Study 2 (Focus groups) - results
A total of 16 focus groups were held across the 4 sites, as follows: 12 groups with individuals with SCI (n = 65) and 4 groups with SCI clinicians (n = 42). The focus group results, participant demographic characteristics, and a more thorough description of their methodology have been published.Citation12,Citation13 Both individuals with SCI and clinicians who work with people with SCI focused on similar issues during the focus group discussions, nominating a variety of subdomains across the larger domains of physical-medical, emotional and social functioning.
Literature review
To be as comprehensive as possible in the selection of subdomains for inclusion, the research team reviewed the literature on each subdomain within the context of SCI, identified key component issues and symptomatology, and identified extant scales where applicable. In domains that overlapped conceptually with PROMIS and Neuro-QOL item banks, the research team used the literature searches conducted by the PROMISCitation3 and Neuro-QOLCitation9 study teams
Additional stakeholder input
Informal interviews with clinicians and researchers with expertise in SCI medicine helped guide the domain selection. Additionally, we held a series of interactive discussions with a regional SCI consumer advisory board that provided input on study methodology and interim results (e.g. preliminary lists of subdomains for inclusion). The advisory board met quarterly and consisted of individuals with SCI, many of whom worked in the area of disability services and/or held leadership positions within the disability community. This advisory board reviewed project progress and provided input on the applicability of each component of the proposed measurement system.
Final subdomain selection
Taking into consideration all of the input described thus far, the SCI-QOL project team selected 30 subdomains for further development (). These included 7 item pools related to physical-medical issues, 8 item pools related to emotional functioning, 6 item pools related to participation and social functioning, and 4 item pools related to sexual functioning. The development of the 5 ‘Spinal Cord Injury-Functional Index (SCI-FI)’ item pools related to physical functioning has been described previously,Citation14,Citation18 therefore these pools are not included among those described here.
Table 3. Subdomains for development, field testing, and analysis
Phase II: item development and refinement
Once it was determined which subdomains would be selected for further development, the goal of the second phase of the study was to develop and refine component items in each topic area. The comments made during the individual interviews and focus groups not only guided selection of relevant domains for inclusion but also contributed directly to the item pools, with participant quotes forming the initial basis of many included items. As described by Kisala and Tulsky,Citation13 the initial step in this process is to define the topic of interest and the scope of the ultimate scale.Citation10,Citation12 Since the goal was to develop an IRT-based scale, we needed to select or write sets of items that address a single underlying construct, with items at various levels of ‘difficulty’ arranged across the construct being measured. For each subdomain, a preliminary item pool was developed using the qualitative feedback from the individual interviews and focus groups to prepare item stems that employed wording used by participants. In subdomains with content overlap with existing measures, extant items were also included, especially items from the Neuro-QOL and PROMIS scales. By using items from PROMIS and Neuro-QOL verbatim, the scores on the SCI-QOL and PROMIS (and SCI-QOL and Neuro-QOL) could be calibrated using IRT-based linking methods to obtain a common metric between the tests. In other words, the common items between the SCI-QOL and PROMIS (or SCI-QOL and Neuro-QOL) serve as anchor items, and through IRT-based linking methods, we can transform SCI-QOL item parameter estimates to the PROMIS or Neuro-QOL metric, enabling direct comparisons with these other PRO measurement scales where relevant. For linkage, only a substantial number of common items need to be used and additional items could be included to ensure content coverage in one of the populations. For SCI-QOL, additional items were written for any relevant area of functioning that was discussed in the SCI medical literature even if it was not described in detail by participants in the qualitative studies.
Qualitative item review process
Each item pool underwent qualitative item review (QIR) to optimize the content, wording, and construct coverage of the included items. The first step in this process involved iterative expert reviews in which members of the investigative team reviewed all items for relevance, redundancy, and wording. Poorly worded items or items that reflected multiple concepts were identified in a team meeting with investigators and reworded. The investigators reviewed each set of items to ensure that they appeared to be related to a similar construct, and also organized the items along a hierarchy of difficulty. Items flagged as not construct representative were removed, gaps in the difficulty continuum were identified, and new items were written to help bridge any gaps in the continuum.
Next, a series of cognitive debriefing interviews were held with individuals with SCI (n = 5 per item) who, in a structured interview format, read each item, responded based on their level of functioning, and then reviewed the meaning of the item and the cognitive processes that led to their response. Participants were asked to discuss the relevance and wording of each item and to identify items that were vague or ambiguous. This process helped ensure that the items selected for testing would be understood as intended by respondents.
Next, we evaluated each item to make sure it would be comprehensible to all participants capable of reading English, regardless of education level. The Lexile Framework™ Citation19 was used to ensure that all items were worded at or below a 5th grade reading level.
For the final QIR step, we conducted a translatability and cultural reviewCitation20 of all newly developed items to ensure that item wording would not preclude translation to Spanish at a later time. A team of translation science experts reviewed each item, identifying specific words or item content that would be difficult to translate or would be culturally inappropriate for Spanish-speaking individuals with SCI, and suggested different ways of stating the item in order to make the final scale more universally relevant for future translation into Spanish.
All item pools were finalized except for one related to environmental factors (i.e. barriers and facilitators to participation such as economic factors). This item pool was removed from the SCI-QOL and, to optimize resource allocation, migrated to a concurrent project established to develop a measure of environmental factors that impede or enhance social participation in individuals with disabilities.Citation21
Several of the finalized item banks were unique and new and based upon the SCI qualitative feedback and literature. The domains that they measured had not been covered by existing Neuro-QOL or PROMIS item banks. However, there were also issues and domains that were more universal, experienced by the general population as well as the SCI population. In these instances, SCI-QOL item banks cover subdomains that had already been measured by existing PROMIS or Neuro-QOL item banks (e.g. Depression, Pain Interference, Ability to Participate in Social Roles). In these cases, the SCI-QOL ‘version’ of each item bank is based on the original PROMIS or Neuro-QOL bank through the use of common, verbatim items. However, the new samples of individuals with SCI collected as part of this study were used to re-calibrate these item banks in an SCI sample so that the items banks would be optimized for use in individuals with SCI. To ensure comparability and interpretability with the PROMIS and Neuro-QOL scales, the item calibration parameters were transformed to the PROMIS (or Neuro-QOL) metric. To ensure that sufficient common items were available to link, 182 items from Neuro-QOL and 56 items from PROMIS (22 of those items were common to Neuro-QOL and PROMIS) were merged back into the pool along with the 510 new items. The final set of SCI-QOL items totaled 726 items across 24 pools, covering the various domains and sub-domains of Physical Medical, Emotional, Social Health, and Sexual Functioning. Additionally, 324 items in a fifth domain area (physical function) were developed and tested in a companion project, the Spinal Cord Injury – Functional Index (SCI-FI).Citation14,Citation18
Phase III: Field testing
We calibrated the item pools using an IRT Graded Response Model (GRM).Citation22 Estimation of the GRM requires a participant sample that is heterogeneous with regard to functioning (i.e. representative of the population) and large enough (e.g. n ≥ 500) to produce stable parameter estimates.Citation23
Phase 3: field testing - Participants
A sample of 877 individuals with SCI was recruited from five SCI Model Systems (SCIMS) centers and SCI Center of Excellence in the Department of Veterans Affairs: University of Michigan, Kessler Foundation/Kessler Institute for Rehabilitation, Rehabilitation Institute of Chicago, University of Washington, Craig Hospital, and the James J. Peters/Bronx VA Medical Center. The study protocol was reviewed and approved by each site's Institutional Review Board. Persons with a documented traumatic SCI who were 18 ys. or older and could read, speak, and understand English fluently were eligible to participate. Recruitment goals were stratified by diagnosis (paraplegia vs. tetraplegia), completeness of injury (complete vs. incomplete), and time since injury (<1 year, 1–3 years, and >3 years) to ensure that the final sample was heterogeneous with regard to SCI-specific characteristics. Each participant's diagnosis was confirmed by medical records, and each participant's neurologic level was documented by their most recent American Spinal Injury Association Impairment Scale (AIS) rating.Citation24
Phase 3: field testing - Data collection procedures
All items were presented in a structured interview to participants either in person or over the phone. Each interviewer received in-person training in interviewing techniques and used a semi-structured script to ensure standardization of the interview format. A detailed Manual of Procedures was prepared and distributed to all sites. Throughout the data collection for this calibration study, interviewers from all sites participated in biweekly conference calls with the study coordinator to discuss progress and goals, specifically with regard to meeting sampling stratification goals.
Due to the large number of items in the calibration version of the SCI-QOL study (k = 726), data collection was divided into three sessions. An additional interview session was held to administer the physical functioning item pools, and many of the same participants elected to participate in that session. All items within an individual item pool (e.g. Pain Interference) were administered during the same session. All responses were entered into a customized web-based data collection software system that allowed data to be automatically uploaded and stored on a secure server immediately. Because the response options differed somewhat from one set of items to the next, participants were shown a response card to facilitate their responses.
Phase 3: field testing - Participant demographic characteristics
Of the total sample of 877 individuals, 757 completed Session 1 (containing items related to Physical Medical issues), 717 completed Session 2 (Emotional Well-Being items), and 641 completed Session 3 (Social Participation, Stigma, and Sexual Functioning items). While each participant was encouraged to complete all of the sessions, this was not required and therefore different sample sizes were obtained for each interview session.
Among the 877 total participants, mean age was 42.9 years (SD 15.4) and 79% of participants were male. Of the sample, 70% self-identified as Caucasian, 18% as African-American, 2% as more than one race, 2% as Asian, and 8% as Other. Additionally, 11% of participants were of Hispanic or Latino origin or descent. In terms of level and completeness of injury, 24% of participants were diagnosed with complete paraplegia, 18% with incomplete paraplegia, 23% with complete tetraplegia, and 34% with incomplete tetraplegia. The average time since injury was 6.7 years (SD = 9.9): 28% of participants had been injured for less than 1 year at the time of study participation, 27% between 1 and 3 years, and 45% for more than three years. More detailed demographic information for the overall sample, as well as sample demographics for the individual sessions, may be found in .
Table 4. Calibration Sample Demographics
Phase IV: Psychometric analysis
The analysis steps used in this project followed closely in line with those outlined by Reeve et al.,Citation25 including evaluation of dimensionality and estimation of IRT-based item parameters. We examined the dimensional structure of each item pool, ensuring that each represented an essentially unidimensional construct which is a prerequisite for conducting IRT analysis. We used the results to evaluate the appropriateness of the SCI-QOL items in each pool, and to inform the removal of biased or misfitting items from each final item bank. The final goal of this phase was to obtain IRT parameters of the items to develop a computer adaptive test (CAT) and provide data for use in short form item selection.
We evaluated internal consistency (Cronbach's alpha), corrected item-total correlations, data completeness, and underlying dimensionality of responses. Since unidimensionality is a prerequisite for conventional IRT analysis and CAT, dimensionality of each bank was assessed using confirmatory and exploratory factor analyses (CFA/EFA). We tested all items in the bank on their fit to a unidimensional construct. Several indices of goodness-of-fit served as criteria for acceptable unidimensionality, including Bentler's Comparative Fit Index (CFI),Citation26 the Tucker-Lewis Index (TLI)Citation27 (where values of 0.90 or above indicate acceptable fit to the model and values of 0.95 or above indicate good fit),Citation28 and the root mean square error of approximation (RMSEA)Citation29 (where values below 0.08 indicate acceptable fit, and values below 0.06 are considered good fit). We assessed local item dependence (LID), which occurs when a pair of items violates the underlying assumption that responses to individual items should be uncorrelated with each other at any given level of the construct being measured. A criterion of residual correlation >0.2 was used to identify item pairs with potentially problematic LID. Unidimensional models were tested in separate CFA analyses for each of the 20 item pools. When poor fit to a unidimensional model was indicated by model fit statistics (e.g. CFI, TLI, RMSEA), we examined the entire item bank and selected items for removal based on low factor loadings or the presence of LID. Analyses were then iteratively re-run after each wave of item removal until CFA results supported a unidimensional model, all items exhibited satisfactory factor loadings (i.e. ≥0.30; ideally ≥0.40), and LID was minimized.
IRT parameters and IRT-based model fit were subsequently estimated using the Graded Response Model. Within each item bank, each item was evaluated for misfit (S-X2 index)Citation30 and differential item functioning (DIF)Citation31 for age, sex, education level, diagnosis (paraplegia vs. tetraplegia), completeness of injury (complete vs. incomplete), and time since injury (<1 year vs. >1 year). If any additional items were removed from the item pool at this time, the IRT and DIF analyses were re-run following their removal.
Next, item banks containing a substantial number of items from PROMIS (e.g. Anxiety, Depression, Pain Interference) or Neuro-QOL (e.g. Positive Affect and Well-Being, Ability to Participate in Social Roles and Activities, Satisfaction with Social Roles and Activities) were transformed onto the PROMIS or Neuro-QOL metric as appropriate, using IRT-linking techniques. This linking procedure utilized common items as ‘anchors,’ using Stocking-LordCitation32 linking techniquesCitation33 to identify slope and intercept transformation constants, and performing a linear transformation of each item calibration so that SCI-QOL item parameters were placed on the respective PROMIS or Neuro-QOL metric. In other words, the item parameters were estimated based on SCI samples in order to obtain most optimal (reliable and valid) estimates and then transformed to the PROMIS/Neuro-QOL metric to facilitate comparisons across populations. This procedure provides the dual advantage of having a SCI-specific sample inform the optimal CAT item selection algorithm, thereby ensuring the administration of the most informative item at each level of the underlying trait, while still allowing direct comparison with the general populationCitation34 and/or other studies via the respective PROMIS or Neuro-QOL metric. For subdomains that did not have a comparable PROMIS or Neuro-QOL item bank (i.e. an item bank that was unique to SCI), the calibrations based upon the SCI sample were used to develop the IRT parameters.
Phase 4: Psychometric analysis - CAT programming
CAT is a dynamic way to present a select subset of items from a calibrated item bank that are specifically relevant to the individual being assessed. For example, on the Depression CAT, someone who indicates that they ‘Never’ feel sad will not see items about suicidal ideation. As described by Cella et al., IRT-calibrated items are considered ‘pre-validated’ (pg. 134),Citation35 wherein the score on any subset of these items, whether administered by CAT or as a static short form (SF), is directly comparable to the full-bank score.
For this project, once all analyses were completed and all parameters transformed (where applicable), our final step was to develop CATs and static short forms (SF) for each item bank. The CATs were developed using final (transformed) item calibration parameters obtained from the last iteration of the IRT analyses. The final IRT parameters were programmed into the Assessment CenterSM platform,Citation36 available at http://www.assessmentcenter.net. SCI-QOL uses the default PROMIS CAT settings so that a minimum of 4 items are administered to each person. The CAT then continues to administer items until the standard error of measurement falls below 0.3 or until a maximum of 12 items is administered. This typically results in a CAT length of 6–8 items For most item banks, Assessment Center may also be set to administer any item on the SCI-QOL short form that has not been selected by the CAT so that both Short Form and CAT scores can be obtained.
Phase 4: Psychometric analysis - Short form selection
A short form (SF) is a brief, fixed-length version of an item bank that is developed using a balance of psychometric and clinical considerations. Since all items in each bank are calibrated on a single underlying metric, the score on a given SF is directly comparable to the CAT or full bank administration of the same bank. For each item bank, a small group of co-investigators, including at least one individual with clinical/topic-area expertise and two measurement experts, reviewed the difficulty (location) and slope (discrimination) parameters for each item. As a starting place, items were divided into quintiles based on location (i.e. the mean of category threshold parameters for each item), and at each quintile, the 1–2 item(s) with the highest slope were chosen. Clinical relevance, item wording, and, in an effort to include a diverse set of items on each form, similarity to other included items were also considered. All SFs contain between 7 and 10 items and are available through the Assessment Center or from the corresponding author. Bivariate correlations (Pearson's r) between CAT and SF scores on each item bank were computed using FirestarCitation37 CAT simulations with calibration data. For our a priori hypothesis, we expected high correlations (i.e. approaching 1.0) between different modes of administration (i.e. CAT versus short form) of the same SCI-QOL item bank.
Phase 4: Psychometric analysis - Scoring metric for SCI-QOL item banks
All SCI-QOL scores have been transformed to a T-metric, with a mean of 50 and standard deviation of 10. For all banks that have been linked and placed on the PROMIS or Neuro-QOL metric (see ), the population used to calibrate the extant item bank serves as the reference group.
Table 5. Linkages with PROMIS and Neuro-QOL
All PROMIS v1.0 item banks and the majority of Neuro-QOL banks were developed using general population samples. In these cases, the person's score on the SCI-QOL reflects their standing in the general population. An exception to this is Stigma, where scores on this bank reflect individuals' standing in reference to a mixed neurological sample (i.e. stroke, epilepsy, Parkinson's disease, amyotrophic lateral sclerosis). For all banks that are ‘new’ to SCI-QOL (e.g. Bladder Management, Bowel Management, Resilience, Grief/Loss), an individual's score represents their standing among individuals with traumatic SCI.
Phase 4: Psychometric analysis - Scoring direction
In keeping with PROMIS convention, higher scores on an instrument indicate more of the trait being measured. As seen in , this means that SCI-QOL item banks/scales that measure positive constructs (e.g. Resilience, Positive Affect and Well-Being) are scored in a positive direction, with higher scores indicating better functioning. Item banks/scales that measure a negative construct (e.g. Depression, Bladder Management Difficulties) are scored in a negative direction, with higher scores indicating greater symptom severity.
Table 6. SCI-QOL Item Bank Statistics
Phase IV: Psychometric analysis - RESULTS
Psychometric analyses were conducted on 17 of the 24 tested item pools (see for a list of analyzed pools). Of the 17 analyzed item pools, 14 calibrated item banks and 3 brief, fixed-length scales were developed. See for a brief domain definition for each final bank/scale. Additionally, expanded definitions may be found in Tulsky et al.Citation10 in this issue for the domain definitions for final banks/scales.
Table 7. SCI-QOL Domain Definitions
Thirteen SCI-QOL item banks exhibited excellent fit statistics and have been developed as an IRT-calibrated item bank which may be administered as a full item bank, CAT, or short form (SF). Independence has a limited number of items (8 items) and a high RMSEA indicating possible multidimensionality but was also developed as an IRT-calibrated item bank. For three item pools, decreased sample sizes due to sparse cells in conjunction with a small number of acceptable items limited our ability to develop calibrated item banks which may be administered as CATs. Pressure Ulcers, Bladder Complications, and Pain Behavior are available as IRT-calibrated fixed-length scales.
All calibrated item banks demonstrated excellent internal consistency reliability. Coefficient alpha values ranged from 0.89 (Independence) to 0.97 (Positive Affect and Well Being). The three fixed-length scales demonstrated a high degree of internal consistency with coefficient alpha values ranging from 0.79 (Bladder Complications) to 0.92 (Pressure Ulcers).
Detail on the number of items tested, the number of items retained, and final CFA results are located in . Details on the individual iterations and reasons for item removal may be found in the individual domain-specific manuscripts throughout this special issue (e.g. Kisala et al.,Citation38 Victorson et al.,Citation39 Kalpakjian et al.).Citation40 Bivariate correlations between Firestar-simulated CAT scores and SF scores on each domain can be found in .
Table 8. Correlations between Short Form and simulated CAT scores
Seven of the developed item pools were not analyzed. Due to the low number of individuals in our calibration sample who had experienced respiratory complications (i.e. only 34% of the sample had ever experienced respiratory complications since their SCI, and the Respiratory items are in the context of the past 7 days), we lacked an adequate distribution of responses across cells to move forward with analysis of this subdomain. Further analysis and finalization of the Respiratory item pool will require an additional wave of data collection in a sample of individuals who have recently experienced respiratory complications as a result of their SCI. Similarly, many of the Sexual Functioning items required that the participant had a sexual partner (many of whom did not), and therefore, were not completed by a sufficient number of individuals to conduct IRT analyses. Finally, additional Control over Participation and Involvement in Life Situations item pools consisted primarily of items from the Community Participation IndicatorsCitation41 measure and were not calibrated as part of this study.
Phase V: Psychometric evaluation of final SCI-QOL CATs/SFs in a new sample
Phase 5: Psychometric evaluation in new sample - METHODS
The overall purpose of this phase of research was to test the psychometric properties of the SCI-QOL measurement system in a new, independent sample. The SCI-QOL CATs and Short Forms were administered at an initial baseline interview (at study enrollment). A retest assessment was conducted between 7–14 days post baseline. All items were administered in interview format by trained examiners; this methodology helped ensure that the same individual with completing the assessment at both time points. Bivariate correlations (Pearson's r) and intraclass correlation coefficients (ICC) were computed to compare SCI-QOL CAT test-retest reliabilities. In general, the performance on the two administrations should be highly related with correlations coefficients >0.80. Test-retest reliability coefficients of a slightly lower magnitude (e.g. between 0.70 and 0.80) would be considered acceptable. Pearson's r correlation coefficients were also computed between the CAT and SF versions of each bank at the baseline assessment, to empirically test the assumption that SF and CAT scores will be nearly equal given the underlying IRT parameters.
Phase 5: Psychometric evaluation in new sample - Participants
A sample of 245 individuals with SCI was recruited from the following four SCI Model Systems (SCIMS) centers: University of Michigan, Kessler Foundation/Kessler Institute for Rehabilitation, Rehabilitation Institute of Chicago, and Craig Hospital. The reliability study was part of a larger research project wherein SCI-QOL CATs and static short forms were assessed serially at multiple intervals over a longer study period. The study protocol was reviewed and approved by each site's Institutional Review Board. Persons with a traumatic SCI that had been documented in their medical chart, who were 18 years or older, and who could read, speak, and understand English fluently were eligible to participate. The sample was stratified by level (paraplegia versus tetraplegia) and completeness of injury (complete vs. incomplete) to ensure that the final sample was a heterogeneous sample of individuals with SCI. All participants were community-dwelling individuals who were injured more than four months before the assessment and were stratified by diagnosis (paraplegia vs. tetraplegia), severity (complete vs. incomplete), and time since injury (≤2 years, >2 years). Each participant's diagnosis was confirmed by medical records and each participant's neurologic level was documented by their most recent American Spinal Injury Association Impairment Scale (AIS) rating.Citation24
Phase 5: Psychometric evaluation in new sample - Data collection procedures
The CAT administration and all data collection were performed through a web-interface connected to the Assessment CenterSM. All data points were obtained in a structured interview with a trained research assistant reading the questions from a computer screen and entering responses directly into the Assessment Center data platform. A detailed Manual of Procedures was also prepared and distributed to all sites. Throughout the data collection for validation study, data collectors from all sites participated in biweekly conference calls with the study coordinator to discuss progress and goals, specifically with regard to meeting sampling stratification goals.
Phase 5: Psychometric evaluation in new sample - RESULTS
Values of Pearson's r coefficients indicate that scores on the baseline and retest assessments are highly related for each of the SCI-QOL banks and scales. As seen in , Pearson's r values for calibrated SCI-QOL item banks range from 0.74 (Bowel Management Difficulties) to 0.96 (Self Care). Pearson r values for SCI-QOL fixed length scales range from 0.70 (Bladder Complications) to 0.79 (Pressure Ulcers). Furthermore, ICC (2,1) values for item banks range from 0.74 (Ability to Participate; 95% CI: 0.67 to 0.79) to 0.96 (Self Care; 95% CI: 0.94 to 0.97). ICC (2,1) values for the fixed-length scales range from 0.69 (Bladder Complications; 95% CI: 0.61 to 0.76) and 0.79 (Pressure Ulcers; 95% CI: 0.74 to 0.84).
Table 9. SCI-QOL Test-Retest Reliability
The simulated and empirically tested correlation coefficients between the CAT and SF administrations of the same bank are located in . CAT and SF scores were consistently very highly related. For CAT simulations from calibration data were compared to SF scores from the same dataset, Pearson's r values ranged from 0.92 (Satisfaction with SRA and Self Esteem) to 0.99 (Independence). When reliability study participants completed CATs and then subsequently completed any remaining items in the short form (i.e. ‘No Duplicates’ option in Assessment Center) with Pearson's r values ranging from 0.91 (Satisfaction with SRA, Self Esteem, Stigma, and Power Wheelchair Mobility) to 0.99 (Independence).
Discussion
The SCI-QOL has been developed specifically with and for individuals with SCI. The SCI-QOL is innovative in its use of cutting edge qualitative and quantitative methods throughout its development and calibration. The SCI-QOL project team has consistently adhered to the scientific standards laid out by the PROMIS network,Citation1 and has incorporated innovative psychometric techniques by transforming SCI-calibrated item banks back to the PROMIS or Neuro-QOL metrics.
There are PROMIS and Neuro-QOL scales that are intended to be appropriate across all health conditions, and the SCI-QOL has included many of these items verbatim. As such, this project marks the largest test of those PROMIS and Neuro-QOL banks in SCI to date. As described in later papers in this issue, some areas of these scales, such as anxiety and depression, are universally applicable; in these areas, with the SCI-QOL banks consisting of primarily of PROMIS items, but with updated IRT parameters to optimize measurement for individuals with SCI. In other areas, however, existing items were not as relevant in an SCI population. Within the Ability to Participate in Social Roles and Activities (SRA) and Satisfaction with SRA item banks, for example, there were many work-related items from PROMIS and Neuro-QOL that were psychometrically problematic due to their inapplicability (i.e. there were bimodal distributions due to the large number of individuals with SCI who are unable to return to work due to physical or financial reasons) due to their inapplicability. The SCI-QOL versions of these banks, therefore, are optimized not only in terms of administration order but also in the selection of items included in the final banks. One of the most important goals in developing the SCI-QOL system was to ensure that individuals with SCI are no longer faced with forms containing irrelevant (and potentially offensive) items when researchers or clinicians attempt to assess HRQOL.
Notably, though the SCI-QOL versions of existing banks have been optimized for SCI, the scores have been transformed to reflect the original metric (i.e. PROMIS or Neuro-QOL), thereby using SCI-specific development work and item calibrations, but yielding scores referencing the general population in order to ensure comparability across different studies and even across populations. In addition to the expanding these existing measurement systems, SCI-QOL has broken new ground by developing item banks in areas that are broadly relevant but deemed especially important in individuals with SCI (such as Resilience and Grief/Loss), as well as those that tend to be very specific to SCI, such as Bladder Management Difficulties, Bowel Management Difficulties, and Pressure Ulcers. Future directions include testing the Respiratory and Sexual Functioning items in larger samples of individuals for whom these items are directly relevant to ensure adequate distributions of responses to perform IRT analysis.
Study limitations
Further work is needed on the responsiveness of this scale, in light of how individuals with SCI evolve and change following their injury. Results from ongoing intervention studies that administer SCI-QOL item banks both pre- and post-intervention will provide additional evidence for responsiveness of SCI-QOL measures to observed change in individuals with SCI. Establishment of clinically relevant scoring classifications and minimal clinically important differences will be important in facilitating use of SCI-QOL in clinical trials. Further, the research team must continue to marshal data in the process of gathering additional validation data on the SCI-QOL measurement system. Finally, stakeholder participation in all phases of the study has been limited to those individuals with traumatic SCI. It remains to be seen if results are generalizable to individuals with SCI of non-traumatic etiology.
Conclusions
The SCI-QOL measurement system has been developed through multiple phases of research using advanced qualitative and quantitative methods, advanced computer technology and modern psychometric theory. Nineteen SCI-QOL item banks and 3 fixed scales, including those described in the 11 topic-specific manuscripts in this special issue as well as the physical functioning banks which have been described previously,Citation14,Citation18,Citation42 have utilized the methodology described in this manuscript. All SCI-QOL instruments are available for SCI research or clinical practice through Assessment Center or via the lead author.
Disclaimer statements
Contributors All authors have contributed significantly to the design, analysis and writing of this manuscript. The contents represent original work and have not been published elsewhere. No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated.
Funding This study was supported by grant #5R01HD054659 from the National Institutes of Health – Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Center on Medical Rehabilitation Research and the National Institute on Neurological Disorders and Stroke. The SCI-FI development work was supported by the US Department of Education, National Institute of Disability and Rehabilitation Research Grant Numbers: H133N120002, H133N060014, H133N110006, H133N110020, H133N110002, H133N110021, H133N110011, H133N110007, H133N110009.
Conflicts of interest All SCI-QOL Items are copyright © 2015 David Tulsky and Kessler Foundation. All rights reserved. All SCI-QOL items originally from Neuro-QOL are copyright © 2008-2013 David Cella on behalf of the National Institute for Neurological Disorders and Stroke (NINDS). All items are freely available to the public via the Assessment Center platform (http:\\www.assessmentcenter.net) and, at present, there are currently no plans for Dr. Tulsky, Kessler Foundation, or the NINDS to profit from the use of the copyrighted material.
Ethics approval Institutional Review Board approval was received at each participating site.
References
- PROMIS. PROMIS Instrument Development and Psychometric Evaluation Scientific Standards 2012; http://www.nihpromis.org/Documents/PROMIS_Standards_050212.pdf. Accessed 12/27/2012.
- Quatrano LA, Cruz TH. Future of outcomes measurement: impact on research in medical rehabilitation and neurologic populations. Arch Phys Med Rehabil 2011;92(10 Suppl):S7–11.
- Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010;63(11):1179–94.
- Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care 2007;45(5 Suppl 1):S3–S11.
- DeWalt DA, Rothrock N, Yount S, Stone AA, Grp PC. Evaluation of item candidates – The PROMIS qualitative item review. Med Care 2007;45(5):S12–S21.
- Amtmann D, Cook KF, Johnson KL, Cella D. The PROMIS Initiative: involvement of rehabilitation stakeholders in development and examples of applications in rehabilitation research. Arch Phys Med Rehabil 2011;92(10):S12–S19.
- Reeve BB. Special issues for building computerized-adaptive tests for measuring patient-reported outcomes: the National Institute of Health's investment in new technology. Med Care 2006;44(11 Suppl 3):S198–204.
- Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, et al. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care 2007;45(5 Suppl 1):S22–31.
- Cella D, Nowinski C, Peterman A, Victorson D, Miller D, Lai JS, et al. The Neurology Quality-of-Life Measurement Initiative. Arch Phys Med Rehabil 2011;92(10, Supplement):S28–S36.
- Tulsky DS, Kisala PA, Victorson D, Tate DG, Heinemann AW, Charlifue S, et al. Overview of the Spinal Cord Injury – Quality of Life (SCI-QOL) measurement system. J Spinal Cord Med 2015;38(3):257–69.
- Center NSCIS. Spinal Cord Injury Model Systems: 2013 Annual Report, Complete Public Version. Birmingham, Alabama 2013.
- Tulsky DS, Kisala PA, Victorson D, Tate D, Heinemann AW, Amtmann D, et al. Developing a Contemporary Patient-Reported Outcomes Measure for Spinal Cord Injury. Arch Phys Med Rehabil 2011;92(10):S44–S51.
- Kisala PA, Tulsky DS. Opportunities for CAT applications in medical rehabilitation: development of targeted item banks. J Appl Meas 2010;11(3):315–30.
- Tulsky DS, Jette AM, Kisala PA, Kalpakjian D, Dijkers MP, Whiteneck G, et al. Spinal cord injury-functional index: item banks to measure physical functioning in individuals with spinal cord injury. Arch Phys Med Rehabil 2012;93(10):1722–32.
- Slavin MD, Kisala PA, Jette AM, Tulsky DS. Developing a contemporary functional outcome measure for spinal cord injury research. Spinal Cord 2010;48(3):262–7.
- Glaser BGS, A.L. The discovery of grounded theory: Strategies for qualitative research. New Brunswick, NJ: Aldine Transaction; 1967.
- Strauss AC, J. Basics of qualitative research: Techniques and procedures for developing grounded theory. 2nd ed. Thousand Oaks, CA: SAGE Publications; 1998.
- Jette AM, Tulsky DS, Ni P, Kisala PA, Slavin MD, Dijkers MP, et al. Development and initial evaluation of the spinal cord injury-functional index. Arch Phys Med Rehabil 2012;93(10):1733–50.
- The LEXILE framework for reading [computer program]. Durham, NC1995.
- Eremenco SL, Cella D, Arnold BJ. A comprehensive method for the translation and cross-cultural validation of health status questionnaires. Eval Health Prof. 2005;28(2):212–32.
- Heinemann AW, Magasi S, Hammel J, Carlozzi NE, Garcia SF, Hahn EA, et al. Environmental factors item development for persons with stroke, traumatic brain injury and spinal cord injury. Arch Phys Med Rehabil. 2015;96(4):589–95.
- Samejima F, van der Liden W, Hambleton R. The graded response model. Handbook of modern item response theory. New York: Springer; 1996:85–100.
- Reise SP, Yu J. Parameter recovery in the Graded Response Model using MULTILOG. J Educ Meas 1990;27(2):133–44.
- American Spinal Injury Association. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association, International Spinal Cord Society; 2002.
- Reeve BB, Hays RD, Chang CH, Perfetto EM. Applying item response theory to enhance health outcomes assessment. Qual Life Res 2007;16:1–3.
- Bentler PM. Comparative fit indexes in structural models. Psychol Bull 1990;107(2):238–46.
- Tucker L, Lewis C. A reliability coefficient for maximum likelihood factor analysis. Psychometrika 1973;38(1):1–10.
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equation Model 1999;6(1):1–55.
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, (eds.) Testing structural equation models. Newbury Park, CA: Sage; 1993:136–62.
- Orlando M, Thissen D. Further investigation of the performance of S-X2: An item fit index for use with dichotomous item response theory models. Appl Psychol Meas 2003;27:289–98.
- Choi SW, Gibbons LE, Crane PK. Lordif: An R Package for detecting differential item functioning using iterative hybrid ordinal logistic regression/item response theory and monte carlo simulations. J Stat Softw 2011;39(8):1–30.
- Stocking ML, FM L. Developing a common metric in item response theory. Appl Psychol Meas. 2983;7(2):201–10.
- Choi SW, Schalet B, Cook KF, Cella D. Establishing a common metric for depressive symptoms: linking the BDI-II, CES-D, and PHQ-9 to PROMIS depression. Psychol Assess 2014.
- Liu H, Cella D, Gershon R, Shen J, Morales LS, Riley W, et al. Representativeness of the patient-reported outcomes measurement information system internet panel. J Clin Epidemiol 2010;63(11):1169–78.
- Cella D, Gershon R, Lai JS, Choi S. The future of outcomes measurement: item banking, tailored short-forms, and computerized adaptive assessment. Qual Life Res 2007;16(Suppl 1):133–41.
- Gershon R, Rothrock NE, Hanrahan RT, Jansky LJ, Harniss M, Riley W. The development of a clinical outcomes survey research application: Assessment Center. Qual Life Res 2010;19(5):677–85.
- Choi SW. Firestar: Computerized adaptive testing simulation program for polytomous item response theory models. Appl Psychol Meas 2009;33(8):644–5.
- Kisala PA, Tulsky DS, Choi SW, Kirshblum SC. Development and psychometric characteristics of the SCI-QOL Pressure Ulcers scale and short form. J Spinal Cord Med 2015;38(3):303–14.
- Victorson D, Tulsky DS, Kisala PA, Kalpakjian CZ, Weiland B, Choi SW. Measuring resilience after spinal cord injury: Development, validation and psychometric characteristics of the SCI-QOL Resilience item bank and short form. J Spinal Cord Med 2015;38(3):366–76.
- Kalpakjian CZ, Tate DG, Kisala PA, Tulsky DS. Measuring self-esteem after spinal cord injury: Development, validation and psychometric characteristics of the SCI-QOL Self-esteem item bank and short form. J Spinal Cord Med 2015;38(3):377–85.
- Heinemann AW, Magasi S, Bode RK, Hammel J, Whiteneck GG, Bogner J, et al. Measuring enfranchisement: importance of and control over participation by people with disabilities. Arch Phys Med Rehabil 2013;94(11):2157–65.
- Heinemann AW, Dijkers MP, Ni P, Ni P, Tulsky DS, Jette A. Measurement properties of the Spinal Cord Injury-Functional Index (SCI-FI) short forms. Arch Phys Med Rehabil 2014;95(7):1289–97.