Abstract
Immunocytochemistry (ICC) is a very important tool in a diverse range of biomedical research as well as in diagnostic cytopathology. Smears prepared from cervical scrapes contain a large amount of overlying mucus that interferes with the standard immunocytochemical staining protocol. A modified ICC protocol is described, which involves pretreatment of these smears with 1 mg/ml solution of Ambroxol hydrochloride in methanol for 1 hour. Source of Ambroxol hydrochloride was a 30 mg Mucolite™ tablet, at a cost of 1.70 rupees (∼3·5 US cents) per tablet. This mucolytic solution effectively clears the mucus, facilitating the accessibility of the antibody to the antigenic determinants. This pretreatment resulted in the increased percentage of positively stained cells as well as staining intensity, leading to improved overall ICC staining and score. This is a novel modification that can be cost-effectively applied in ICC staining protocols for cytology samples characterized by the presence of excess mucus.
Introduction
Immunocytochemistry (ICC) is the localization of antigens in cells by the use of labeled antibodies as specific reagents, through antigen–antibody interactions that are most commonly visualized by using chromogenic substrates like diaminobenzidene (DAB) or amino-ethyl carbazole (AEC). Immunocytochemistry has become a crucial and widely used technique in many medical research laboratories as well as in clinical diagnostics. Using ICC, proteins localized on the cell membrane and/or cytoplasm and/or nucleus are detected using specific antibodies. Hence it is vital to ensure that an antigen is fully exposed or accessible to interact optimally with its specific antibody. Many cytology samples such as buccal scrapes, Papanicolaou smears, brush cytology of various organ linings have mucus secretions in abundance, which pose a major challenge for ICC. This is one such challenge in ICC on cervical cancer brush cytology samples. This study was performed as part of an ongoing research protocol. The study proposed to determine the efficacy of curcumin, along with standard radiotherapy and chemotherapy, in improving outcome in patients with advanced carcinoma of cervix. Molecular assessment for the presence of human papillomanvirus (HPV) and proliferation marker like proliferating cell nuclear antigen (PCNA) in these patients was carried out in pretreatment punch biopsies and in cervical scrapes collected using an endocervical brush as post-treatment follow-up samples. HPV polymerase chain reaction (PCR) was performed using deoxyribonucleic acid (DNA) from the exfoliated cells, and ICC was performed on the cytosmears prepared from the scrapes. Proliferating cell nuclear antigen is a protein that acts as a processivity factor for DNA polymerase delta by forming a ring around the DNA. It thus creates a topological link to the genome. Emerging evidence suggests that PCNA is central to many essential cellular processes such as repair of DNA damage, DNA replication, chromosome segregation, chromatin structure maintenance, and cell cycle progression. It is commonly used as a proliferation marker in several studies.Citation1
The plan was to carry out ICC staining for PCNA in smears prepared from cervical scrapes on the above ongoing project. The cervical scrape/brush specimens contain a large amount of overlying mucus that hinders the antibody accessibility to the antigenic determinants, thus interfering in the immunocytochemical staining. Removal of the overlying mucus from the cervical scrapes was essential for achieving optimal immunocytochemical staining.
This study describes a modified method to overcome mucus hindrance, which resulted in improved staining in cervical smears characterized by the presence of excess mucus.
Materials and Methods
Instruments and equipment used in this study were glass jars and cradles to stain and wash the slides, humid chambers to incubate the slides, hydrophobic marker pens (Dako, Glostrup, Denmark) to encircle the smears, a microwave oven for antigen retrieval, a refrigerator for 4°C incubation, a rocking platform for washing the slides, a timer, and endocervical brushes (Qiagen, Valencia, CA, USA).
Antibodies and reagents
The antibodies and reagents used in this study were silane (Sigma, St. Louis, MO, USA), acetone, methanol, graded alcohol, and xylene (S.D. Fine Chem, Mumbai, India), hydrogen peroxide (Rankem, Faridabad, India), phosphate buffered saline (PBS, pH 7·4) for antibody dilution (prepared in-house), 1X PBS with 0·05% Triton X-100 (Sigma, St. Louis, MO, USA), 10 mM sodium citrate buffer (pH 6·0) for antigen retrieval (prepared in-house), PCNA mouse monoclonal antibody (Santacruz Biotech, Dallas, Texas, USA), Vectastain Elite Kit which contains anti-mouse horse IgG as a secondary antibody and avidin–biotin reagent as a tertiary reagent for signal amplification (Vector lab, Burlingame, CA, USA), chromogenic substrate DAB (Sigma), DPX mountant (S.D. Fine Chem, India), hematoxylin (S.D. Fine Chem), and Mucolite™ tablets, composition: ambroxol hydrochloride (30 mg) (Dr Reddy Laboratories Ltd, Hyderabad, India).
Collection of patient samples and preparation of smears
Recruited patients were screened and were included in the study if they had histologically-proven invasive squamous cell carcinoma of the cervix (stages IIB, IIIA, and IIIB) and fulfilled the inclusion and exclusion criteria. HPV testing was not used for selection in these patients.
Cervical scrapes that contained the exfoliated cells were collected from advanced carcinoma of cervix patients 6 weeks after stoppage of all treatment, as follow-up specimens. Informed consent was obtained from all the patients prior to the study. Cervical scrapes were collected by means of an endocervical brush in the supplied buffer, and smears were prepared from these scrapes on silane-coated slides. These were then fixed with chilled methanol, air dried, and stored at room temperature until further use. The modified ICC protocol was applied to 25 cervical smears. All these were tested by PCR for HPV, prior to ICC, and all were positive for HPV. The study was approved by the Institutional Review Board of Tata Memorial Centre.
Preparation of mucolyte solution
Ambroxol hydrochloride is completely soluble in methanol. A single 30 mg Mucolite™ tablet was crushed into powder form and stirred with 30 ml methanol for 1 hour at room temperature. The resulting turbid solution contains dissolved ambroxol hydrochloride at a concentration of 1 mg/ml and tablet binder material which remains undissolved in methanol. The methanol containing the dissolved ambroxol hydrochloride was then separated by centrifugation at 5000 rpm for 10 minutes. Supernatant was used as the mucolyte solution.
Immunocytochemistry
ICC was performed using two different protocols, namely the standard protocol and the modified protocol, which involved mucolyte pretreatment of the smears.
One set of the cervical cancer smears was treated with mucolytic solution as per the modified protocol and, in a duplicate set, ICC was carried out using the standard protocol. A comparative chart showing both the protocols is given in .
Table 1. Immunocytochemistry protocol
Results
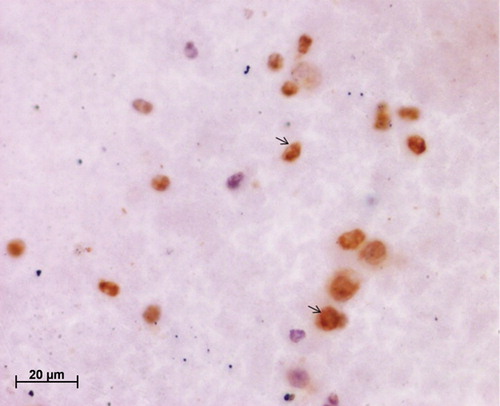
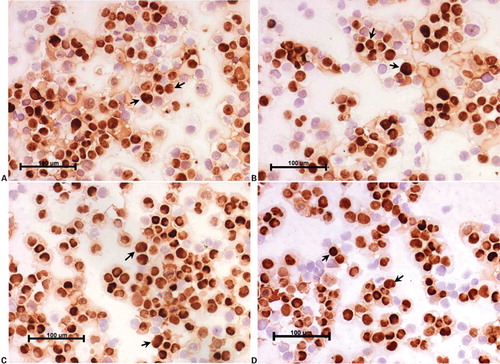
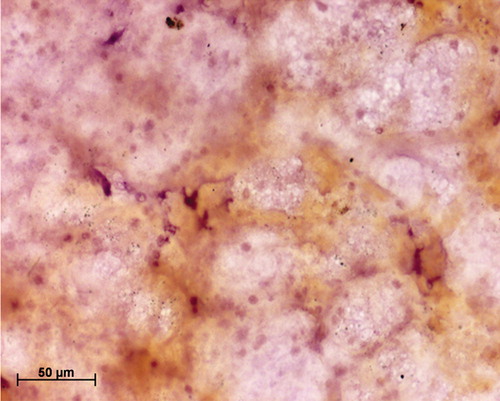
Twenty-five cervical scrapes (follow-up at 6 weeks) included in this study were analyzed in duplicates for the expression of PCNA, as a pilot study to assess impact of mucolyte treatment on ICC staining for cervical smears. indicates a representative cervical smear counterstained with hematoxylin where no mucolyte treatment was given, which shows the presence of mucus in the smear along with the underlying cells. demonstrates a hematoxylin-counterstained cervical smear of the same patient when treated with mucolyte, exhibiting better visibility of cells and lesser mucus as compared to the smear shown in . Further PCNA staining in 25 cervical cancer smears treated with ambroxol hydrochloride revealed an average 30% positivity for PCNA versus 5% in untreated samples. As seen from , a representative cervical smear stained using the modified protocol exhibited higher PCNA positive cells as compared to the cervical smear from the same patient (), where PCNA ICC was performed using the standard protocol and in which lower PCNA positivity was observed. Additionally, a two-fold increase in the staining intensity was also observed ( versus ). Analysis of HeLa and CaSki cytosmears treated with and without ambroxol hydrochloride exhibited comparable PCNA positivity in treated versus untreated smears (), indicating that the treatment with ambroxol hydrochloride may not affect or alter the ICC staining. The non-specific primary antibody binding can give rise to false positive staining, while inappropriate fixation/permeabilization step can give rise to false negativity. A negative control slide (omission of primary antibody, with all other steps remaining unchanged), and a positive control cytosmear (HeLa Cells) were kept with each set. Thus the ICC results, as judged by the quantitative and qualitative observation, appeared to be improved by this simple and cost-effective modified protocol.
Figure 1. Hematoxylin-stained cervical smear without mucolyte treatment; note the presence of overlying mucus.
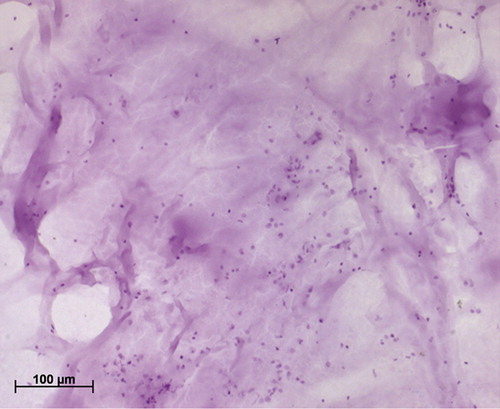
Figure 2. Hematoxylin-stained cervical smear treated with mucolyte; note the clearing of mucus and improved visibility of cells.
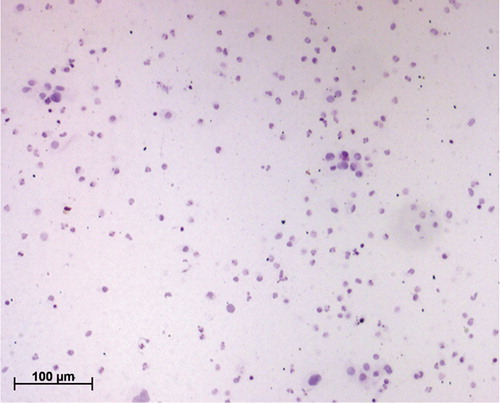
Figure 3. Immunocytochemical staining of cervical smear with proliferating cell nuclear antigen (PCNA) antibody using the standard protocol; note the poor visibility of the PCNA-positive cells due to the presence of mucus.
Discussion
In the present study, the ICC staining quality achieved by using the standard protocol was compared with a modified protocol that involved the use of ambroxol hydrochloride, a mucolytic agent. In the modified protocol, the single pretreatment with mucolytic agent was the only change and as per the experiment, it did not appear to affect the PCNA staining (antigen) and positivity or its expected nuclear localization. In ICC, sensitivity and specificity of the antibody used and the technical procedure are crucial to avoid false positive and false negative results. To reduce or avoid ICC false positive/false negative results, proper controls, a robust and specific detection system, a well characterized antibody clone, an efficient heat-induced antigen retrieval (HIER), and standard cell fixation procedure were used.
Mucus hypersecretion can be relieved by several classes of pharmacological agents.Citation2 Ambroxol hydrochloride is a metabolite of bromhexine and its chemical name is 2,4-dibromo-6-{[cyclohexyl(methyl)amino]methyl}aniline. It is an expectoration enhancer and a systemically active mucolytic agent. It is commonly used in the treatment of chronicbronchitis and neonatal respiratory distress syndrome.Citation3–Citation5 One of the earlier reports suggests that bromhexine, a prodrug of ambroxol hydrochloride, induces enzymes that are released by the lysosomes into the cytoplasm. Thus, bromhexine may be partly involved in the mucolytic action of this agent on the acid glycoproteins contained in the mucus granules of submucosal glands.Citation6 Bromhexine acts by disrupting the structure of acid polysaccharide fibers in the mucus-containing secretions, thus producing mucus with reduced viscosity. A recent report indicates the inhibition of nitric oxide (NO)-dependent activation of soluble guanylate cyclase as one of the molecular mechanisms executing the therapeutic action of ambroxol hydrochloride.Citation7 To date, the mode of action of ambroxol hydrochloride is not fully understood. Mucolytic drugs generally act by decreasing the mucus viscosity by reducing the dicysteine bridges that offer rigidity to mucins.Citation8 Ambroxol hydrochloride is also reported to stimulate the secretion of a surfactant,Citation2 which decreases mucus adhesion to the bronchial lining, producing an expectorant effect.Citation9 A recent review also suggests that ambroxol exhibits antioxidant, anti-inflammatory, and anesthetic properties.Citation10 The antioxidant effect of ambroxol hydrochloride is well demonstrated. Lipid peroxidation initiated by t-butyl hydroperoxide or doxorubicin was shown to be suppressed in the presence of ambroxol hydrochloride via scavenging of hydroxyl radicals and cellular superoxide radical anions.Citation11 Another report also demonstrated the antioxidant effect of ambroxol in mononuclear and polymorphonuclear cells in vitro.Citation12 Ambroxol hydrochloride produces an anti-inflammatory effect by blocking the expression of pro-inflammatory messengers. This inhibits the release of histamine from mast cells and the generation of cytokines and interleukins.Citation13
A recent study investigated the effect of mucoactive agents in vitro using primary human airway epithelial cell cultures. The cultures are complex organotypic human airway model containing major cell types such as basal, ciliated, non-ciliated, and goblet cells.Citation14 A study that used similar cultures showed that ambroxol increased the mucociliary transport rate and reduced elasticity and viscosity of the secretions. Also it was seen that there was no effect of ambroxol on cell viability, indicating that it is non-toxic to cells at the concentration used.Citation15
Conclusion
In the present study, it was concluded that a similar mechanism(s) of action of ambroxol hydrochloride may aid in clearing the mucus on the smears, thus facilitating the ICC staining. Ambroxol hydrochloride is commercially available for research purposes from biotechnology companies, but at a high cost.
In this study, a tablet of 30 mg Ambroxol hydrochloride marketed as a drug by Dr. Reddy Laboratories LLC, India, was used; this drug is available at a very low cost of 1·70 rupees for a 10 mg tablet (∼3·5 US cents). There are so far no reports claiming ambroxol hydrochloride improves the quality of cytology-based staining or immunocytochemical staining, in particular. Thus, this is a novel pretreatment with a very simple and cost-effective modification, which can be widely applied in a cytology laboratory. This modification can be readily applied to samples characterized by the presence of thick mucus to unmask various underlying cells/antigenic determinants to improve staining quality in general and immunocytochemical staining in particular by improving antibody accessibility to the antigenic determinants. There are so far no reports illustrating the use of ambroxol hydrochloride as a mucolyte to overcome excess mucus for better ICC staining. Use of mucolyte pretreatment in the ICC staining protocol yields better results in cervical scrape cytology smears with mucus. The use of ambroxol hydrochloride may aid in improving ICC staining in samples such as endoscopic upper/lower respiratory tract aspirates, gastric lavage, or frozen sections which are generally characterized by the presence of excess mucus. This method thus needs to be evaluated for other mucus-containing smears.
Acknowledgments
The authors would like to thank Amruta Naik, Alok Dalvi, Shivner Sawant, and Premalatha Salian for their technical assistance and Department of Biotechnology (DBT), Government of India for the funding.
References
- Stiomenov I, Helleday T. PCNA on the cross road of cancer. Biochem Soc Trans. 2009;37:605–13.
- Rogers DF. Mucoactive agents for airway mucus hypersecretory diseases. Respir Care. 2007;52:1176–93.
- Germouty J, Jirou-Najou JL. Clinical efficacy of ambroxol in the treatment of bronchial stasis. Clinical trial in 120 patients at two different doses. Respiration. 1987;51(Suppl 1):37–41.
- Schmalisch G, Wauer RR, Bohme B. Effect of early ambroxol treatment on lung functions in mechanically ventilated preterm newborns who subsequently developed a bronchopulmonary dysplasia (BPD). Respir Med. 2000;94:378–84.
- Wauer RR, Schmalisch G, Bohme B, Arand J, Lehmann D. Randomized double blind trial of ambroxol for the treatment of respiratory distress syndrome. Eur J Pediatr. 1992;151:357–63.
- Takeda H, Misawa M, Yanaura S. A role of lysozomal enzymes in the mechanism of mucolytic action of bromhexine. Jpn J Pharmacol. 1983;33:455–61.
- Severina IS, Bussygine OG, Pyatakova NV, Khropov YV, Khennoperol RA. Ambroxol as inhibitor of nitric oxide dependent activation of soluble guanylate cyclase. Eur J Pharmacol. 2000;407:61–4.
- Rubin BK. The pharmacologic approach to airway clearance: mucoactive agents. Respir Care. 2002;47:818–22.
- Wirtz HR. Effect of ambroxol on surfactant secretion and synthesis in isolated type II alveolar cells. Pneumologie. 2000;54:278–83.
- Malerba M, Ragnoli B. Ambroxol in the 21st century: pharmacological and clinical update. Expert Opin Drug Metab Toxicol. 2008;4:1119–29.
- Nowak D, Pierscinski G, Drzevoski J. Ambroxol inhibits doxorubicin-induced lipid peroxidation in heart of mice. Free Radic Biol Med. 1995;19:659–63.
- Gillissen A, Bartling A, Schoen S, Schultze-Werninghaus G. Antioxidant function of ambroxol in mononuclear and polymorphonuclear cells in vitro. Lung. 1997;175:235–42.
- Gibbs BF, Schmutzlar W, Vollrath IB, Brosthardt P, Braam U, Wolff HH, et al.. Ambroxol inhibits the release of histamine, leukotrienes and cytokines from human leukocytes and mast cells. Inflamm Res. 1999;48:86–93.
- Fulcher LM, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2004;107:183–206.
- Seagrave J, Albrecht HH, Hill DB, Rogers DF, Solomom G. Effects of guaifenesin, N-acetylcysteine, and ambroxol on MUC5AC and mucociliary transport in primary differentiated human tracheal-bronchial cells. Respir Res. 2012;13:98.