Abstract
Midichloria mitochondrii is an intracellular bacterium found in the hard tick Ixodes ricinus. In this arthropod, M. mitochondrii is observed in the oocytes and in other cells of the ovary, where the symbiont is present in the cell cytoplasm and inside the mitochondria. No studies have so far investigated whether M. mitochondrii is present in the salivary glands of the tick and whether it is transmitted to vertebrates during the tick blood meal. To address the above issues, we developed a recombinant antigen of M. mitochondrii (to screen human sera) and antibodies against this antigen (for the staining of the symbiont). Using these reagents we show that (i) M. mitochondrii is present in the salivary glands of I. ricinus and that (ii) seropositivity against M. mitochondrii is highly prevalent in humans parasitized by I. ricinus (58%), while it is very low in healthy individuals (1·2%). These results provide evidence that M. mitochondrii is released with the tick saliva and raise the possibility that M. mitochondrii is infectious to vertebrates. Besides this, our study indicates that M. mitochondrii should be regarded as a package of antigens inoculated into the human host during the tick bite. This implies that the immunology of the response toward the saliva of I. ricinus is to be reconsidered on the basis of potential effects of M. mitochondrii and poses the basis for the development of novel markers for investigating the exposure of humans and animals to this tick species.
Introduction
Midichloria mitochondrii is an intracellular bacterium found in the hard tick Ixodes ricinus.Citation1 In this arthropod, M. mitochondrii is abundant in diverse cell types of the ovary, including oocytes.Citation2 No evidence has so far been published on the presence of this bacterium in the salivary glands of ticks. M. mitochondrii is peculiar in that it is observed not only in the cell cytoplasm, but also inside the mitochondria, within the intermembrane space of these organelles.Citation1,Citation2 In I. ricinus, M. mitochondrii is vertically transmitted to the progeny, as indicated by PCR evidence on eggs and newly-emerged larvae and by the presence of these bacteria in the oocytes of this tick.Citation1,Citation2 Bacteria closely related to M. mitochondrii have also been detected in other tick species;Citation3,Citation4 in addition, 16S rRNA gene sequences that cluster with M. mitochondrii have been amplified from a variety of sources, including fleas, bed bugs, tabanid flies and cnidarians.Citation5–Citation9 Since M. mitochondrii is the first organism formally described for this bacterial cluster,Citation1 and since taxonomic revision for this cluster has not yet been published, we will refer to this group of bacteria as Midichloria and like organisms (hereafter: MALOs). There is circumstantial evidence that MALOs could be transmitted to terrestrial vertebrates during the tick bite:
| i. | 16S rRNA gene sequences related with M. mitochondrii have been amplified from roe deer during a screening for tick-borne bacteria;Citation10 | ||||
| ii. | phylogeny of ticks and their respective MALOs are not congruent, with distantly related ticks harboring MALOs that are identical at the 16S rRNA gene level;Citation3 this implies that MALOs could be transmitted horizontally among ticks, and a simple mechanism that could be hypothesized to explain horizontal transmission is the infection of an host parasitized by different tick species (or co-feeding with bacterial transmission without true infection); | ||||
| iii. | MALO 16S rRNA gene sequences have been amplified from human patients parasitized by ticks.Citation11 | ||||
Based on the above information we designed a study to investigate whether M. mitochondrii is present in the salivary glands of the host tick I. ricinus and whether humans parasitized by I. ricinus develop antibodies against M. mitochondrii. To address the above questions, we used a recombinant antigen from M. mitochondrii and a polyclonal antibody raised against this antigen (described in Mariconti et al.).Citation12 We first used the antibody for immunostaining on tick salivary glands and then we used the recombinant antigen for the analysis of human sera, from healthy blood donors and from persons parasitized by ticks. We also used PCR for searching of M. mitochondrii DNA in tick salivary glands and rostra.
Materials and Methods
Tick samples
Three semi-engorged adult females of I. ricinus and two of Ixodes hexagonus were collected from naturally infected animals (sheep and hedgehog), in the counties of Varese and Bergamo (Northern Italy). Ticks were identified morphologically with standard taxonomic keys.Citation13 Salivary glands and rostra were dissected in sterile conditions and prepared for indirect immunofluorescence assay (salivary glands) or for PCR analysis (salivary glands and rostra).
DNA extraction and PCR analysis
DNA from salivary glands and rostra was extracted using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany), eluted in 50 μl of sterile water, quantified by a spectrophotometer and stored at −80°C before use. PCR was affected using a 16S rDNA-targeted semi-nested procedure.Citation3 Amplified bands were sequenced using ABI technology, to confirm the specificity of the amplification.
Sera samples
A total of 249 samples of human sera were screened for the presence of anti-M. mitochondrii antibodies, using an experimental ELISA assay (see below). These sera were from two groups of subjects: 169 healthy blood donors and 80 subjects exposed to tick bite. In particular, the latter group was composed of persons parasitized by ticks, examined at the emergency services of the hospitals IRCCS Policlinico San Matteo (Pavia), Azienda Ospedaliera Universitaria S.Orsola-Malpighi (Bologna) and Spedali Civili di Brescia (Brescia), or with a reliable request for Lyme disease diagnosis, based on clinical signs and anamnesis (of these, 31 had actually been shown to be seropositive for Borrelia burgdorferi sensu lato before the enrollment in this study; in the context of this study, a further subject was then shown to be seropositive for B. burgdorferi – see below). Unfortunately, in most cases the emergency service physicians did not conserve the ticks removed from the patients for identification; when the tick was identified, it was in most cases I. ricinus (13 out of 15 ticks examined). Based on this information, on a previous study showing that 90% of the ticks removed from humans in Northern Italy are I. ricinus,Citation14 and on preliminary results of a parallel study in the same area (Bandi et al., unpublished results), we assume that most of the patients had been parasitized by I. ricinus. All of the above sera were collected after no less than six weeks from the removal of the tick. The screening on human sera was conducted under the regulation of the Ethical Committees of the S. Orsola Malpighi University Hospital (Bologna), Spedali Civili di Brescia and Fondazione IRCCS Policlinico San Matteo (Pavia); all patients provided informed consent and the study protocol was approved by the Ethical Committees of the above hospitals.
Indirect immunofluorescence assay on tick salivary glands
Salivary glands were stained with MitoTracker Red CMXRos (Invitrogen Carlsbad, CA, USA) and with a polyclonal antibody raised against the recombinant flagellar FliD protein of M. mitochondrii (anti-rFliD) as previously described.Citation12 Observation was recorded with a Leica confocal microscope (LeicaTCSNT, Solms, Germany).
Detection of anti-M. mitochondrii antibodies in human sera
The recombinant flagellar protein FliD of M. mitochondrii (rFliD) was produced in Escherichia coli and purified as previously described.Citation12 Anti-rFliD antibody levels in human sera were determined using an enzyme-linked immunosorbent assay (ELISA), using 96-well microtiter plates coated with 0·1 μg/well of rFliD protein. Each sample was diluted 1/100 in phosphate buffered saline supplemented with 1% bovine serum albumin and 100 μl of each diluted sera were tested following the procedure previously described in Gaibani et al.Citation15 Threshold value was established as the mean optical density (OD)450/630 of the sera from the healthy blood donors plus three times the standard deviation (i.e. mean OD450/630+3 standard deviations). Using this method the threshold was set at 0·793. Each sample was considered negative if its OD450/630 was less than the threshold value, and positive if its OD450/630 was higher than or equal to the threshold.
Serological screening for B. burgdorferi
Even though part of the sera used in this study had already been diagnosed for B. burgdorferi infection, we examined all of the sera for the presence of IgG antibodies specific for B. burgdorferi. This screening was performed using a commercial Western blot kit (Borrelia ViraStripe Test Kit IgG; Viramed Biotech, Planegg, Germany). Western blot results were interpreted following the manufacturer’s recommendations.
Experimental Western blot assays for B. burgdorferi and M. mitochondrii
A culture of B. burgdorferi was pelleted with centrifugation at 4000g for 5 minutes, and the pellet was suspended in 150 μl of Tris HCl 25 mM (pH 8); after sonication to promote the release of the proteins, the supernatant was recovered after centrifugation at 16 000g for 10 minutes at 4°C. The soluble proteins in the supernatant of B. burgdorferi or the purified rFliD were then used as antigens for Western blot assays, performed according to standard procedures,Citation16 with dilution of secondary anti-human antibodies at 1∶5000. To test the possible cross-reactivity of rFliD in patients infected by B. burgdorferi s.l., the following sera were examined, at 1:1000 dilution: five sera positive for B. burgdorferi; five sera positive for M. mitochondrii; five sera positive for both B. burgdorferi and M. mitochondrii. Serological positivity to M. mitochondrii and B. burgdorferi were determined using respectively the ELISA method and the Western blot kit described in the above paragraphs.
Results and Discussion
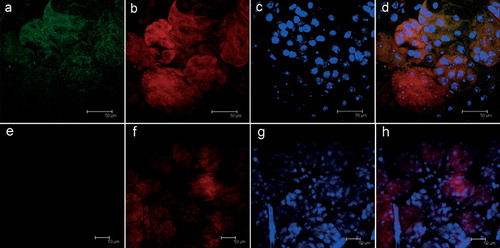
The indirect immunofluorescence assay on salivary glands of adult I. ricinus females using a primary antibody directed against the rFliD of M. mitochondrii revealed the presence of intensely green-stained bodies (); these were generally collected in clusters, and closely associated with mitochondria (as revealed by the staining using MitoTracker Red). No staining was observed using the anti-rFliD antibody on the salivary glands of I. hexagonus (), a tick closely related to I. ricinus that does not harbor M. mitochondrii. No stained bodies were observed in I. ricinus when the primary anti-rFliD antibody was not used (not shown). The above observations were obtained on all the samples examined for I. ricinus (three positive, out of three adult females) and for I. hexagonus (two negative, out of two adult females). Based on the above observations we assume that the bodies recognized by the anti-rFliD antibody in the salivary glands of I. ricinus are M. mitochondrii bacteria, or aggregates of the FliD protein from this bacterium. PCR analysis with primers specific for M. mitochondrii was also congruent with the above results: amplification of M. mitochondrii DNA was obtained from all three salivary gland samples from I. ricinus. In addition, PCR on the rostra was positive for M. mitochondrii in two of the three adult females of I. ricinus examined (all of the PCR products obtained were sequenced, and matched the 16S rRNA sequence of M. mitochondrii). PCRs on the salivary glands and rostra were negative on all of the samples from I. hexagonus. Taken together, the results of immunostaining and PCR on the salivary glands show that M. mitochondrii (or proteins and DNA from M. mitochondrii) is present in the salivary glands of I. ricinus; PCR positivity on the rostra indicates that this bacterium (or DNA from this bacterium) could be released with the saliva.
Figure 1. Indirect immunofluorescence assay (FITC-conjugated secondary antibodies) on salivary glands from Ixodes ricinus (a–d) and Ixodes hexagonus (e–h) semi-engorged adult females; (a, e) staining using polyclonal antibodies raised against the rFliD from Midichloria mitochondrii (green); (b, f) live mitochondria stained with MitoTracker Red CMXROS (red); (c, g) cellular nuclei stained for cell viability with TOTO-3 iodide; (d, h) merging of the images. In d the yellow spots indicate the overlap between M. mitochondrii (green) and mitochondria (red). Colour version available online.
The above results prompted us to investigate whether humans parasitized by I. ricinus are seropositive for M. mitochondrii. To this purpose, we used the flagellar rFliD protein from M. mitochondrii as an antigen, in an ELISA screening on healthy blood donors and on subjects exposed to tick bite (). In tick-exposed subjects, the average OD values for IgG antibodies reacting with rFliD was 0·845 (SD = 0·422); in healthy blood donors, OD values were significantly lower (U-MannWitney test; P<0·001), with an average of 0·373 (SD = 0·140). After setting a threshold at an OD value of 0·793 (see Methods), we could then estimate that the seroprevalence for M. mitochondrii was 58·75% in subjects exposed to tick bite (47 out of 80), and 1·18% in the healthy blood donors (2 out of 169). These prevalence values are significantly different between the two groups (U-MannWitney test; P<0·001). The above results clearly indicate that subjects exposed to tick bite produce antibodies that react with an antigen from M. mitochondrii, and indicate that this bacterium is inoculated into the human host during the tick bite.
Figure 2. Results of the serological screening for Midichloria mitochondrii and Borrelia burgdorferi on sera from 80 tick-exposed subjects (main figure) and from 169 healthy blood donors (inset). M: sera positive to M. mitochondrii; B: sera positive to B. burgdorferi; M+B: sera positive to both M. mitochondrii and B. burgdorferi. Sera negative to both bacteria (NEGATIVE) are also indicated.
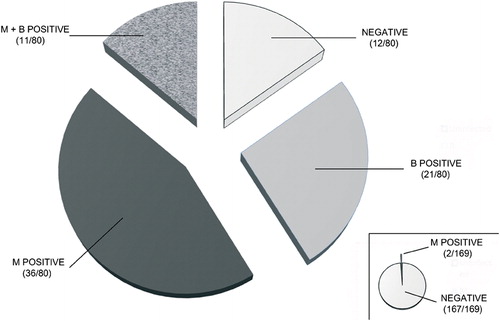
The antigen that we used for the above ELISA screening (rFliD) corresponds to a portion of a flagellar protein of M. mitochondrii. Thus, we did not expect any cross-reactivity towards antibodies generated during an infection caused by any other Rickettsiales. Indeed, M. mitochondrii is the sole member of the order Rickettsiales that has so far been shown to possess flagellar genes. However, I. ricinus is the main vector in Europe of the Lyme disease spirochetes (B. burgdorferi s.l.),Citation17 bacteria that possess flagella, well known for their immunogenicity.Citation18 One could thus argue that production of IgG antibodies in tick-exposed subjects had been induced by a Borrelia infection, and that these antibodies cross-reacted with rFliD from M. mitochondrii. Considering the numerous amino-acid differences between the FliD proteins of the two bacteria, we consider cross-reactivity rather unlikely.Citation12 However, we decided to address this issue by screening all of the sera included in this study with a Western blot diagnostic kit for the detection of antibodies against B. burgdorferi s.l. Using this kit, positivity for B. burgdorferi s.l. was revealed in 32 out of the 80 samples of sera from the subjects exposed to tick bite; none of the healthy blood donors was positive to B. burgdorferi s.l. Among the 32 subjects positive to B. burgdorferi s.l., 11 were concurrently positive to M. mitochondrii; however, a total of 36 subjects positive to M. mitochondrii were negative to B. burgdorferi. Finally, 21 subjects were positive only to B. burgdorferi (). In summary, the above results show that a high proportion of the subjects positive to M. mitochondrii were not positive to B. burgdorferi (and vice-versa), indicating that the positivity to the former bacterium does not derive from the cross-reactivity with the latter.
We further addressed the above issue using an experimental Western blot assay, using as antigens rFliD from M. mitochondrii and proteins from B. burgdorferi s.l. This Western blot assay was carried out on five sera each from the following types of subjects: positive for M. mitochondrii, positive for B. burgdorferi s.l., and positive for both bacteria. The following results were obtained. Sera from the first five patient labeled one band at 38 kDa, corresponding to the molecular weight of rFliD; no band corresponding to the molecular weight of B. burgdorferi FliD (78 kDa) was observed in these subjects. The sera from the patients positive to B. burgdorferi s.l. reacted with a band at 78 kDa (corresponding to B. burgdorferi FliD), while no labeling was observed at 38 kDa. Sera from patients that were positive both for B. burgdorferi s.l. and M. mitochondrii labeled bands at both 78 and 38 kDa (results not shown). These results indicate that the antibodies raised against the FliD protein of B. burgdorferi did not react with the homologous protein of M. mitochondrii, and vice-versa.
Conclusions
Our work provides strong evidence for the transmission of M. mitochondrii to humans during the blood meal of I. ricinus. Based on the results here reported, we cannot conclude that M. mitochondrii replicates in the human host, determining a true infection. However, should we assume that M. mitochondrii does not replicate into the human host, we would have to conclude that the amount of bacteria (or bacterial antigens) inoculated is by itself sufficient for stimulating an antibody production. Overall, we are more prone to hypothesize that live M. mitochondrii bacteria (and not just proteins/DNA) can be inoculated into the vertebrate host, and that some replication can occur therein. At any case, our work shows that M. mitochondrii is to be regarded not only as an important symbiont of I. ricinus, but also as a package of antigens that ticks can inoculate into vertebrate hosts, and as a potential tick-borne microorganism that deserves further investigations. Among the 80 tick-exposed patients that we examined in this study, 47 were seropositive to M. mitochondrii, according to the defined threshold value. We emphasize that we cannot be certain that all of the subjects had been parasitized by I. ricinus; in addition, for some of the subjects, the duration of the blood meal could have been insufficient for an effective inoculation of bacteria. Furthermore, some I. ricinus nymphs present a very low M. mitochondrii load,Citation19 thus possibly resulting in inoculation of a low amount of bacteria during their blood meal. These considerations could explain why not all of the parasitized subjects were seropositive for this bacterium. On the other hand, immunostaining and PCR for M. mitochondrii on the salivary glands of I. ricinus were positive in all three specimens examined.
Since we still do not know whether M. mitochondrii replicates into the human host, it would be premature to discuss whether this bacterium could be responsible for any pathological alteration. For sure, considering the high seroprevalence for M. mitochondrii that we determined in tick-exposed subjects, we would conclude that this bacterium does not cause overt pathology in humans, at least in the vast majority of the cases. On the other hand, the high seroprevalence that we recorded in tick-exposed subjects (associated with the extremely low seroprevalence in healthy blood donors) raises the possibility that M. mitochondrii plays a role in the immune response and immune-modulation determined by the I. ricinus saliva, which is important both for the success of the tick blood meal and for the establishment of the infection by the pathogens vectored by the tick.Citation20,Citation21 We emphasize that in the case of filarial nematodes, the discovery of Wolbachia bacterial endosymbionts in these parasites and of their immunological role led to a profound re-thinking of the immunology of filarial diseases.Citation22–Citation24 Finally, anti-Midichloria antibodies can now be considered as potential serological markers for I. ricinus bite. Such markers could be extremely useful to determine the risk of infection by I. ricinus-borne pathogens in given areas, and for investigating the epidemiological association of a variety of pathological alterations with parasitism by this tick. The present study was focused on M. mitochondrii, but other MALOs could possibly be transmitted by ticks and other arthropods to a variety of vertebrates, including humans (see introduction). Further studies are now urgent, to determine whether MALOs represent a novel class of emerging infectious agents.
We thank Micaela Brandolini, Massimo Pajoro, Dario Pistone and Lina Tomasoni for their help with the collection of sera and ticks. This work was partially supported by a grant by Fondazione IRCCS Policlinico San Matteo, 2012 to PM, and by MIUR Prin (to ChB). Confocal observations have been carried out thanks to the facilities of CIMA, Università degli Studi di Milano.
References
- Sassera D, Beninati T, Bandi C, Bouman EA, Sacchi L, Fabbi M, et al.. ‘Candidatus Midichloria mitochondrii’, an endosymbiont of the tick Ixodes ricinus with a unique intramitochondrial lifestyle. Int J Syst Evol Microbiol. 2006;56:2535–40.
- Sacchi L, Bigliardi E, Corona S, Beninati T, Lo N, Franceschi A. A symbiont of the tick Ixodes ricinus invades and consumes mitochondria in a mode similar to that of the parasitic bacterium Bdellovibrio bacteriovorus. Tissue Cell. 2004;36:43–53.
- Epis S, Sassera D, Beninati T, Lo N, Beati L, Piesman J, et al.. Midichloria mitochondrii is widespread in hard ticks (Ixodidae) and resides in the mitochondria of phylogenetically diverse species. Parasitology. 2008;135:485–94.
- Beninati T, Riegler M, Vilcins IM, Sacchi L, McFadyen R, Krockenberger M, et al.. Absence of the symbiont Candidatus Midichloria mitochondrii in the mitochondria of the tick Ixodes holocyclus. FEMS Microbiol Lett. 2009;299:241–7.
- Erickson DL, Anderson NE, Cromar LM, Jolley A. Bacterial communities associated with flea vectors of plague. J Med Entomol. 2009;46:1532–6.
- Richard S, Seng P, Parola P, Raoult D, Davoust B, Brouqui P. Detection of a new bacterium related to ‘Candidatus Midichloria mitochondrii’ in bed bugs. Clin Microbiol Infect. 2009;15:84–5.
- Hornok S, Földvári G, Elek V, Naranjo V, Farkas R, de la Fuente J. Molecular identification of Anaplasma marginale and rickettsial endosymbionts in blood-sucking flies (Diptera: Tabanidae, Muscidae) and hard ticks (Acari: Ixodidae). Vet Parasitol. 2008;154:354–9.
- Matsuura Y, Kikuchi Y, Meng XY, Koga R, Fukatsu T. Novel clade of alphaproteobacterial endosymbionts associated with stinkbugs and other arthropods. Appl Environ Microbiol. 2012;78:4149–56.
- Sunagawa S, Woodley CM, Medina M. Threatened corals provide underexplored microbial habitats. PLoS One. 2010;5:e9554.
- Skarphédinsson S, Jensen PM, Kristiansen K. Survey of tickborne infections in Denmark. Emerg Infect Dis. 2005;11:1055–61.
- OIu Mediannikov, Ivanov LI, Nishikawa M, Saito R, IuN Sidel'nikov, Zdanovskaia NI, et al.. Microorganism ‘Montezuma’ of the order Rickettsiales: the potential causative agent of tick-borne disease in the Far East of Russia. Zh Mikrobiol Epidemiol Immunobiol. 2004;1:7–13.
- Mariconti M, Epis S, Sacchi L, Biggiogera M, Sassera D, Genchi M, et al.. A study on the presence of flagella in the order Rickettsiales: the case of ‘Candidatus Midichloria mitochondrii’. Microbiology. 2012;158:1677–83.
- Manilla G (editor). Acari Ixodida. Bologna: Ed Calderini; 1998.
- Manfredi MT, Dini V, Piacenza S, Genchi C. Tick species parasitizing people in an area endemic for tick-borne diseases in north-western Italy. Parassitologia. 1999;41:555–60.
- Gaibani P, Pierro A, Alicino R, Rossini G, Cavrini F, Landini MP, et al.. Detection of Usutu-virus-specific IgG in blood donors from northern Italy. Vector Borne Zoonotic Dis. 2012;12:431–3.
- Sambri V, Marangoni A, Eyer C, Reichhuber C, Soutschek E, Negosanti M, et al.. Western immunoblotting with five Treponema pallidum recombinant antigens for serologic diagnosis of syphilis. Clin Diagn Lab Immunol. 2001;8:534–9.
- Stanek G, Strle F. Lyme borreliosis. Lancet. 2003;15:1639–47.
- Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of lyme borreliosis. Clin Microbiol Rev. 2005;18:484–509.
- Sassera D, Lo N, Bouman EA, Epis S, Mortarino M, Bandi C. ‘Candidatus Midichloria’ endosymbionts bloom after the blood meal of the host, the hard tick Ixodes ricinus. Appl Environ Microbiol. 2008;74:6138–40.
- Fontaine A, Diouf I, Bakkali N, Missé D, Pagès F, Fusai T, et al.. Implication of haematophagous arthropod salivary proteins in host-vector interactions. Parasit Vectors. 2011;28:187.
- Lieskovska J, Kopecky J. Effect of tick saliva on signalling pathways activated by TLR-2 ligand and Borrelia afzelii in dendritic cells. Parasite Immunol. 2012;34:421–9.
- Bandi C, Anderson TJ, Genchi C, Blaxter ML. Phylogeny of Wolbachia in filarial nematodes. Proc Biol Sci. 1998;265:2407–13.
- Bazzocchi C, Ceciliani F, McCall JW, Ricci I, Genchi C, Bandi C. Antigenic role of the endosymbionts of filarial nematodes: IgG response against the Wolbachia surface protein in cats infected with Dirofilaria immitis. Proc Biol Sci. 2000;267:2511–6.
- Taylor MJ, Bandi C, Hoerauf A. Wolbachia bacterial endosymbionts of filarial nematodes. Adv Parasitol. 2005;60:245–84.