Abstract
We report on an improvement of the open circuit voltage over 0.8 V, together with decent fill factor and large short circuit current, through the incorporation of ester-functionalized single wall carbon nanotubes in a poly(3-hexylthiophene): 1-(3-methoxycarbonyl) propyl-1-phenyl[6,6]C61 bulk heterojunction solar cell. The technique may open new margins for an extra efficiency enhancement through increase of the open circuit voltage using state of the art highly crystalline low band gap bulk heterojunction polymer solar cells.
Introduction
Several attempts to incorporate carbon nanotubes in the active layer of bulk heterojunction (BHJ) solar cells have been reported.Citation1 Kymakis et al. very early reported a solar cell in which single walled carbon nanotubes (SWCNTs) replaced the fullerene in the polymer BHJ.Citation2 Interestingly, such cells resulted in a remarkable open circuit voltage of 0.75 V,Citation3 whereas the open circuit voltage of fullerene BHJ cells is currently in the range of 0.6 V.Citation4 Carbon nanotubes (CNTs) should be present as few wt-% quantities in the polymer:fullerene BHJ to yield acceptable photovoltaic (PV) efficiencies and promising applications.Citation5–Citation7 Pradhan et al. have introduced functionalized multi walled carbon nanotubes (MWCNTs) into the BHJ,Citation5 while Berson et al. have reproduced state of the art results by incorporating pristine SWCNTs or MWCNTs in the BHJ without annealing procedure.Citation6 More recently, Stylianakis et al. also obtained results close to state of the art (1.78%) with large fill factor by insertion of thiophene functionalized SWCNTs in the BHJ.Citation7 We previously achieved significant efficiency using functionalized SWCNTs in a polymer BHJ, although after annealing, the pristine cells still remained the most efficient.Citation8 Using a different strategy based on the alignment of the tubes, Radbeh et al. also reported a significant improvement of BHJ solar cells efficiency (4.53% reported).Citation9 However, despite many attempts, recent systematic studies on acid shortened tubes tend to show that SWCNTs degrade the performances of BHJ solar cells.Citation10 In the present paper, we report on the achievement of large open circuit voltage V oc over 0.8 V, together with decent fill factor and large short circuit current, through the incorporation of high pressure CO conversion (HiPCO) ester functionalized SWCNT into a poly(3-hexylthiophene) (P3HT): 1-(3-methoxycarbonyl) propyl-1-phenyl[6,6]C61 (PCBM) BHJ. The resulting photovoltaic efficiency is improved with respect to our reference cells.
Materials and methods
Thermogravimetric analysis was performed with a TA INSTRUMENTS TGAQ500 apparatus under a nitrogen flow (60 mL min− 1), and with a TGA/DSC 111 instrument from SETARAM under an argon flow (1 L h− 1), at a scan rate of 10° min− 1. The IR spectra were recorded on a BRÜKER interferometer model Vertex 70, with an ATR Diamond simple reflection equipment. Sonication was performed with a BANDELIN HD3100 ultrasonic homogenizer (20 KHz) operating at 15 W.
SWCNTs were purchased from Unidym, Inc. (HiPCO, lot R0539 and lot R0559, raw material, 0.8–1.2 nm average diameter). Chemicals were purchased from Sigma-Aldrich or Acros Organics and were used as received. Dry solvents were distilled over appropriate drying agents before use.
Regioregular P3HT (M w = 37680 g/mol, PD = 1.48) was purchased from Rieke,Citation11 PEDOT:PSS (Baytron P) from H.C. Starck, PCBM (99.5%) from SES Research, LiF from Aldrich, Al pellets (99.999%) from Goodfellow and ITO (Ip = 4.8 eV, roughness < r> = 6 nm, thickness e = 200 nm, sheet resistance R < 10 Ω sq− 1 and transmittance T = 70–90% between 350 and 800 nm) from Merck.
Preparation of SWCNT-CO2H 2
SWCNTs 1 of 100 mg were stirred in 100 mL aq. nitric acid 2.6 M at 125°C for 48 h. The reaction mixture was cooled to room temperature, then the reaction medium was filtered through a PVDF (0.22 μm) Millipore membrane, washed with deionized water up to pH = 7, then with 2 mL 0.001M NaOH solution, and finally with water up to neutrality. The aqueous NaOH treatment is used in order to remove carboxylated carbonaceous fragments.Citation12 The remaining solid was dried at 60°C under vacuum for 2 days, thus leading to 70 mg purified SWCNTs, which were then added to 70 mL freshly prepared “piranha solution” (H2SO4 96%/H2O2 30% 4:1 v/v) previously cooled between 30–35°C in an ice bath. This resulting mixture was heated at 45°C under vigorous stirring for 1.5 h. Then the reaction medium was transferred into an Erlenmeyer flask containing 75 g ice. After cooling for 10 min, the mixture was filtered through a PVDF (0.22 μm) Millipore membrane, and washed with deionized water up to pH = 7. The remaining solid was dried overnight under vacuum giving SWCNT-CO2H 2 (55 mg).
Preparation of SWCNT-COCl 3
SWNT-CO2H 2 (130 mg) were stirred in 22 mL SOCl2 added with 1 mL DMF at 70°C for 24 h under nitrogen.Citation16 After cooling down to room temperature, the reaction mixture was filtered through a PTFE (0.1 μm) Millipore membrane. The remaining solid was washed with anhydrous THF, then dried overnight under vacuum giving SWNT-COCl 3 (110 mg).
Preparation of SWCNT ester derivatives 4
CH3-(OCH2CH2)3-OH (7 mL) was added to SWNT-COCl 3 (170 mg), and this reaction medium was vigorously stirred at 95°C for 96 h. After cooling to room temperature, the reaction mixture was filtered through a PTFE (0.1 μm) Millipore membrane and washed with ethanol. The remaining solid was dried under vacuum at 60°C during 1 week, giving ester derivative SWNT-CO2(CH2-CH2O)3-CH3 4 (160 mg).
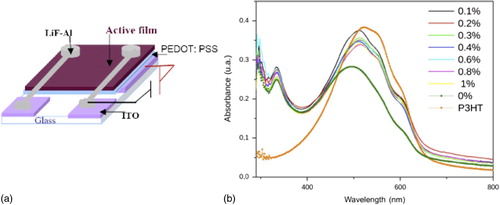
Solar cells were fabricated on patterned ITO-coated glass substrates, with the layout shown in , according to the same method as previously described.Citation13 A 40 nm thin layer of PEDOT: PSS was spin coated on the ITO and baked at 110°C for 1 h inside a vacuum hot plate (Prolabo). The active mixture solution was deposited as a 50 nm thin film using a WS-400-6NPP-LITE (Laurell) spin coater. Dried substrates were then transferred inside an Edwards evaporation plant attached to an argon filled MBRAUN 200B glove box with oxygen and moisture levels < 0.1 ppm. A 0.7 nm thin film of LiF was evaporated on top under a base pressure lower than 10− 4 Pa. It was followed by evaporation of 60 nm of aluminum, without breaking the vacuum. Thermal treatments were performed using a hot plate inside the glove box. All characterizations were achieved keeping the samples inside the glove box. The absorption spectrum of the thin films under study is shown in .
Figure 1. a layout of solar cells prepared on 20 × 25 mm substrates with 1 mm thickness (diameter of two Al circular electrodes was 6 mm) and b UV-Vis absorption spectra of P3HT, pristine and SWCNT doped active layers under study (before annealing)
Current density–voltage (J–V) characteristics of the PV cells were recorded in the dark and under white light illumination respectively using a Keithley 236 source measurement unit. For measuring the PV response, devices were illuminated with simulated light from a power calibrated AM 1.5 solar simulator (Steuernagel Solar constant 575) through the transparent ITO anode. Power calibration was achieved using a Newport Si photodetector. The power conversion efficiency (PCE) was corrected for solar mismatch on the basis of the incident photon to current efficiency (IPCE) of the reference cell (see Supplementary Material, Figure S1, www.maneyonline.com/doi/suppl/10.1179/2055031615Y.0000000001).Citation4 J–V characteristics of the cells kept inside the glove box were stable for several days.
Functionalized SWCNT properties
The pristine HiPCO SWCNTs 1 were transformed into corresponding ester derivatives 4 (), in order to obtain CNTs easy to disperse in organic media. This overall transformation is achieved in three main steps depicted in : (1) purification oxidation in order to eliminate metallic catalysts and amorphous carbon, and to create carboxylic acid groups;Citation14–Citation16 (2) formation of corresponding acid chloride group;Citation14,Citation15,Citation17 and (3) esterification through reaction with the alcohol CH3–(OCH2–CH2)3–OH.
Figure 2. a synthesis scheme of ester derivative 4, starting from as purified SWCNTs, b TGA profiles, under nitrogen, of SWCNT-CO2H 2, issued from purification oxidation in two steps (HNO3 2.6M+“piranha” solution) and of SWCNT-ester 4 and c ATR-IR spectra of SWCNT 1, 2 and 4
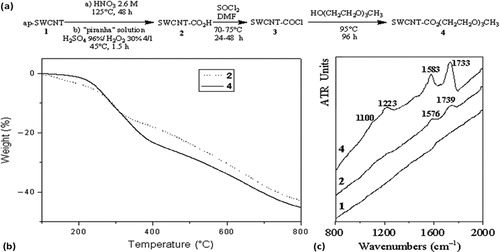
Characterization of the functionalized SWCNT 2 and 4 was achieved by thermogravimetric analyses (TGA) and infra-red spectroscopy. TGA profiles issued from samples 2 and 4 show a clear weight loss in the 180–350 and 200–430°C temperature intervals, respectively (). The ∼13% weight loss in the 180–350°C interval was ascribed to a CO2 release from the carboxylic acid sitesCitation18 present in sample 2.
The ester group content in the functionalized SWCNT 4 was evaluated as ∼24 wt-% by measuring the weight loss observed from the pyrolysis of these groups between 200 and 430°C.Citation19 On this basis, the average degree of functionalization in SWCNT derivatives 4 was estimated as one ester group for each ∼45 carbon atoms. Furthermore, the covalent functionalization of pristine SWCNTs 1 was unambiguously corroborated with the ATR-IR spectra run in the solid state from samples 1, 2 and 4 involved in the different steps of the chemical process schematized in . The spectra obtained in the 800–2000 cm− 1 range are presented in . As observed in previous studies, the spectrum of pristine sample 1 did not exhibit any band,Citation20 whereas the presence of the carbon structure was evidenced after oxidation by a band at 1576 cm− 1 in the spectrum of 2, shifted at 1583 cm− 1 in the spectrum of SWCNT 4. These bands are ascribed to the activated C = C stretching mode.Citation19–Citation22 The functionalization was further shown by the presence of bands at 1739 cm− 1 (C = O carboxylic acid groups) from 2, and 1733 cm− 1 (C = O ester groups)Citation20,Citation21 1223 and 1100 cm− 1 (C–O bonds in ester and ether groups)Citation21,Citation23 from the SWCNT-ester 4.
Proper interaction between materials in the BHJ depends critically on the insertion of the SWCNT 4 within the active layer, which will be composed of a SWCNT 4–P3HT–PCBM blend. P3HT–PCBM blends are cast as efficient PV cell materials from a chlorobenzene solution, which was recognized to provide an appropriate morphology to the active layer.Citation24,Citation25 Hence, the first step in our work towards the optimisation of the BHJ consisted in dispersing SWCNT 4 by sonication in chlorobenzene, which noteworthy was reported as being stable under sonication.Citation26 It yielded an homogeneous and stable medium containing 0.1 mg mL− 1 SWCNT 4, as determined by the method of Dyke and Tour.Citation27
Solar cell properties
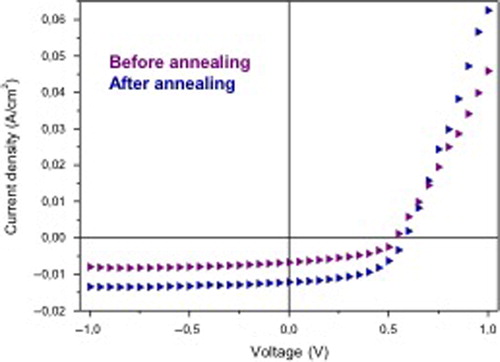
In order to evaluate the influence of the SWCNT 4 on the PV characteristics of SWCNT 4–P3HT–PCBM blend cells, the blends were prepared as follows (). A SWCNT 4–P3HT mixture was first prepared, starting from the previous SWCNT 4 dispersion in chlorobenzene: a volume v (mL) of this dispersion was added to P3HT (10 mg), dissolved in a chlorobenzene volume v′ = (1 − v) mL, so that the obtained mixture contained 0.1–0.4 wt-% SWCNT 4 related to P3HT in a final 1 mL volume. PCBM (8 mg) was then added to the solution. The final blend is thus characterized by three values: the amounts of P3HT (10 mg) and PCBM (8 mg), and the chlorobenzene volume (1 mL). As a consequence, the thickness of the active layer of the corresponding BHJ cells is expected to be invariant whatever the SWCNT 4–P3HT–PCBM blend studied. Current–voltage characteristics of the pristine ITO/PEDOT:PSS/P3HT:PCBM/LiF-Al devices, before and after annealing, are given in .
Table 1. Relative amounts of SWCNT 4 in mixtures with P3HT
Figure 3. J–V characteristics under illumination with 100 mW cm− 2 of pristine P3HT–PCBM organic solar cells, before and after annealing at 100°C for 10 min
Current–voltage characteristics of the various devices, ITO/PEDOT:PSS/SWCNT 4:P3HT:PCBM/LiF-Al, before and after annealing, are given in . The same characteristics were obtained under increasing and decreasing voltage swipes. Characteristics in the dark are given in (see Supplementary Material, Figure S2, www.maneyonline.com/doi/suppl/10.1179/2055031615Y.0000000001). PV parameters extracted from the J–V characteristics in are given in . It confirms that the presence of the SWCNT 4 in the active layer improves the PV characteristics of the solar cells before annealing.Citation6 Best improvement is obtained in cells incorporating 0.1 and 0.2 wt-% SWCNT 4. State of the art efficiencies are obtained after annealing of the BJH.Citation4 Most importantly, a large open circuit voltage of 0.83 V is obtained with 0.1% SWCNT 4, whereas the open circuit voltage of the pristine annealed solar cells was 0.58 V. Interestingly, the large open circuit voltage is a common feature to all the SWCNT doped cells studied in this work.
Figure 4. J–V characteristics under illumination with 100 mW cm− 2 of organic solar cells based on composites SWCNT 4–P3HT–PCBM, before a and after annealing b at 100°C for 10 min
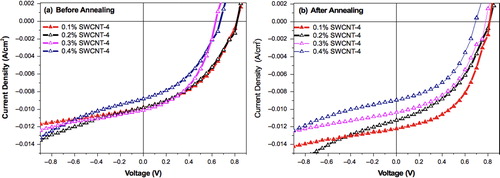
Table 2. PV parameters under 100 mW cm− 2 illumination, before and after annealing at 100°C during 10 min: PCE was corrected for solar mismatch on basis of IPCE of reference cell
The influence of the SWCNT 4 on the active layer was studied by absorption spectroscopy. shows the spectra of pristine and SWCNT 4 doped BHJ materials before annealing. Clear shoulders are visible at 550 and 610 nm in the absorbance of the SWCNT 4 doped BHJ materials; they correlate with the increase of the π stacking of the conjugated polymer in the BHJ.Citation4 This shows that SWCNTs promote polymer crystallization before annealing, as reported previously by Berson et al.Citation6 This behavior is strongly correlated to the X-ray diffraction data (see Supplementary Material, Figure S3, www.maneyonline.com/doi/suppl/10.1179/2055031615Y.0000000001) that show evidence of π stacking as diffraction peaks at 2θ ≈ 32° (d = 2.8 ).
Discussion
Improvement of solar cell's characteristics is possible owing to the previous observation that covalent functionalization of pristine SWCNTs strongly diminishes, if not suppresses, the metallic character of carbon nanotubes, without affecting the semiconducting ones.Citation28,Citation29 Metallic SWCNTs short circuit the cells, thus favoring charge recombination and reducing the V oc, which is not the case in our cells. The fact that the previously used Carbolex SWNTs did not work as well at increasing V ocCitation8 than the present HiPCO ones may originate from intrinsic differences between the Carbolex and the HiPCO SWCNTs. Raman Spectroscopy studies evidenced that the purity of pristine HiPCO SWCNTs is superior the Carbolex one.Citation30 Moreover, it was reported that the smaller diameter HiPCO SWCNTs (0.8–1.2 versus 1.4 nm) exhibit improved conductivity,Citation31 owing to the larger number of tubes per unit mass of product. BHJ solar cells are spatially inhomogeneous, as Rappaport et al. evidenced by carrying spatially resolved mobility measurements.Citation32 One can argue that the CNTs improve the order and crystallinity of the BHJ, as Berson et al. evidenced,Citation6 but that does not justify an increase in the V oc of the solar cells. Unlike the short circuit current, the measured V oc is not affected by an eventual solar mismatch or calibration issue with our solar simulator as V oc increases at best as the logarithm of the illumination.Citation33 We attribute the large V oc to the P3HT–PCBM junction. We disregard a possible P3HT–SWCNT junction which, owing to the low band gap of semiconducting CNTs (∼1 eV),Citation34 would deliver V oc as low as 0.3 V,Citation35 in opposition with experimental evidence. We should also see some net absorbance in the near infrared, which is not the case. An open circuit voltage of 0.83 V is about the maximum theoretical voltage than could be extracted from a P3HT–PCBM BHJ, if the system would behave as a metal–insulator–metal (MIM) junction.Citation36 The effect of the SWCNTs may differ basically from the effect obtained with the use of an indene C60 bisadduct which boosted the efficiency of P3HT based solar cells to 6.5%.Citation37 We propose a different interpretation. According to Scharber et al.,Citation38 the HOMO energy of P3HT is − 5.1 eV, whereas the LUMO energy of PCBM is − 4.3 eV and the difference is the maximum theoretical potential under which charges can ideally be extracted from a MIM type cell.Citation39 Therefore, we would not pay in our SWCNT cells the 0.3 eV penalty that Scharber et al. considered as necessary in state of the art high efficiency solar cells owing to a field dependent photogenerated current.Citation31 A Schottky contact at the aluminum interface would produce the same effect of V oc reduction.Citation40 There appears as a consequence of our experiments that introduction of functionalized SWNTs in our cells makes them behave unexpectedly as ideal MIM junctions.Citation34 Along the modelling of Koster et al.,Citation31 that means that charge recombination in the cells is reduced by the introduction of functionalized SWCNT. That means also that unbalanced charge mobility (∼10− 3 cm2 V− 1 s− 1 for the electrons and ∼10− 4 cm2 V− 1 s− 1 for the holes in P3HT–PCBM blends) which also reduces V oc,Citation41 is suppressed. We attribute the suspected improvement of the mobility balance in our devices to the wrapping of the polymer chains around the SWCNTs,Citation42,Citation43 which may increase the hole mobility. Two orders of magnitude hole mobility improvement in a blend of aligned SWCNT has recently been evaluated in field effect transistors.Citation44 A possible mechanism which leads to a mobility increase was suggested recently by Nikitenko et al.Citation45 It is the enhanced hopping mobility at donor/acceptor interfaces (i.e. P3HT–SWCNT) promoted by interface polarization. The process is related to the exponential field dependence of the Pole–Frenkel mobility which increases significantly at a polarizable interface. That is the case at a SWCNT interface owing the low effective mass for electrons of SWCNTs.Citation46
Conclusion
We have obtained a significant improvement in the photovoltaic properties of P3HT–PCBM based PV cells through the incorporation of SWCNT ester derivative 4 into the active layer. The maximum open circuit voltage that is theoretically accessible to the BHJ is attained experimentally. Our findings shed a new light on the origin of V oc in polymer PV cells which is still a matter of debate. Lower band-gap photovoltaic polymers in which at constant open circuit voltage, the short circuit current was increased significantly, were reported to improve the PV conversion efficiency.Citation47–Citation49 We expect that the technique demonstrated herein will open new margins for additional efficiency enhancement using state of the art low bandgap BHJ solar cell materials through the increase of the open circuit voltage, provided that the polymers show high crystallinity like the ones reaching efficiencies in excess of 10%.Citation50 Work is currently in progress to validate our claims.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
We acknowledge the Région des Pays de la Loire (UNIVALOIRE) and the Conseil Général de Maine-et-Loire for supporting Dr Hassina Derbal and Dr Céline Bergeret respectively. Research at Queen's University was supported by the Natural Sciences and Engineering Research Council (NSERC # 327116), the NSERC Photovoltaic Innovation Network, the Canada Research Chairs program and the Canada Foundation for Innovation.
yadv_a_11723811_sm0001.pdf
Download PDF (158.9 KB)References
- Ratier B., Nunzi J. M., Aldissi M., Kraft T. M. and Buncel E.: Polym. Int., 2012, 61, 342.
- Kymakis E. and Amaratunga G. A. J.: Appl. Phys. Lett., 2002, 80, 112.
- Kymakis E., Alexandrou I. and Amaratunga G. A. J.: J. Appl. Phys., 2003, 93, 1764.
- Dennler G., Scharber M. C. and Brabec C. J.: Adv. Mater., 2009, 21, 1323.
- Pradhan B., Batabyal S. K. and Pal A. J.: Appl. Phys. Lett., 2006, 88, 093106.
- Berson S., de Bettignies R., Bailly S., Guillerez S. and Jousselme B.: Adv. Funct. Mater., 2007, 17, 3363.
- Stylianakis M. M., Mikroyannidis J. A. and Kymakis E.: Sol. Energ. Mater. Sol. Cells, 2010, 94, 267.
- Derbal-Habak H., Bergeret C., Cousseau J. and Nunzi J. M.: Sol. Energ. Mater. Sol. Cells, 2011, 95, S53.
- Radbeh R., Parbaile E., Chakaroun M., Ratier B., Aldissi M. and Moliton A.: Polym. Int., 2010, 59, 1514.
- Skupov K. and Adronov A.: Can. J. Chem., 2014, 92, 68.
- Chen T. A., Wu X. and Rieke R. D.: J. Am. Chem. Soc., 1995, 117, 233.
- Shao L., Tobias G., Salzmann C. G., Ballesteros B., Hong S. Y., Crossley A., Davis B. G. and Green M. L. H.: Chem. Commun., 2007, 2007, 5090.
- Alem S., de Bettignies R., Nunzi J. M. and Cariou M.: Appl. Phys. Lett., 2004, 84, 2178.
- Liu J., Rinzler A. G., Dai H., Hafner J. H., Bradley R. K., Boul P. J., Lu A., Iverson T., Shelimov K., Huffman C. B., Rodriguez-Macias F., Shon Y.-S., Lee T. R., Colbert D. T. and Smalley R. E.: Science, 1998, 280, 1253.
- Rinzler A. G., Liu J., Dai H., Nikolaiev P., Huffman C. B., Rodriguez-Macias F. J., Boul P. J., Lu A. H., Heymann D., Colbert D. T., Lee R. S., Fischer J. E., Rao A. M., Eklund P. C. and Smalley R. E.: Appl. Phys. A, 1998, 67A, 29.
- Chen J., Hamon M. A., Hu H., Chen Y., Rao A. M., Eklund P. C. and Haddon R. C.: Science, 1998, 282, 95.
- Bonifazi D., Nacci C., Marega R., Campidelli S., Ceballos G., Modesti S., Meneghetti M. and Prato M.: Nano Lett., 2006, 6, 1408.
- Figueiredo J. L., Pereira M. F. R., Freitas M. M. A. and Órfão J. J. M.: Carbon, 1999, 37, 1379.
- Tchoul M. N., Ford W. T., Lolli G., Resasco D. E. and Arepalli S.: Chem. Mater., 2007, 19, 5765.
- Kobashi K., Chen Z., Lomeda J., Rauwald U., Hwang W. F. and Tour J. M.: Chem. Mater., 2007, 19, 291.
- Mawhinney D. B., Naumenko V., Kuznetsova A., J. T. Yates Jr, Liu J. and Smalley R. E.: J. Am. Chem. Soc., 2000, 122, 2383.
- Qin Y., Shi J., Wu W., Li X., Guo Z.-H. and Zhu D.: J. Phys. Chem. B, 2003, 107B, 12899.
- Zhang L., Kiny V. U., Peng H., Zhu J., Lobo R. F. M., Margrave J. L. and Khabashesku V. N.: Chem. Mater., 2004, 16, 2055.
- Shaheen S. E., Brabec C. J. and Sariciftci N. S.: Appl. Phys. Lett., 2001, 78, 841.
- Thompson B. C. and Fréchet J. M. J.: Angew. Chem. Int. Ed., 2008, 47, 58.
- Moonoosawmy K. R. and Kruse P.: J. Am. Chem. Soc., 2008, 130, 13417.
- Dyke C. A. and Tour J. M.: Chem. Eur. J., 2004, 10, 812.
- Bergeret C., Cousseau J., Fernandez V., Mevellec J. Y. and Lefrant S.: J. Phys. Chem. C, 2008, 112C, 16411.
- Kanungo M., Lu H., Malliaras G. G. and Blanchet G. B.: Science, 2009, 323, 234.
- Miyata Y., Mizuno K. and Kataura H.: J. Nanomater. (Hindawi), 2011, 2011, 786763.
- Mustonen K., Susi T., Kaskela A., Laiho P., Tian Y., Nasibulin A. G. and Kauppinen E. I.: Beilstein J. Nanotechnol., 2012, 2012, 692.
- Rappaport N., Solomesch O. and Tessler N.: J. Appl. Phys., 2006, 99, 064507.
- Koster L. J. A., Mihailetchi V. D., Ramaker R. and Blom P. W. M.: Appl. Phys. Lett., 2005, 86, 123509.
- O'Connell M. J., Bachilo S. M., Huffman C. B., Moore V. C., Strano M. S., Haroz E. H., Rialon K. L., Boul P. J., Noon W. H., Kittrell C., Ma J., Hauge R. H., Weisman R. B. and Smalley R. E.: Science, 2002, 297, 593.
- Kanai Y. and Grossman J. C.: Nano Lett., 2008, 8, 908.
- Brabec C. J., Cravino A., Meissner D., Sariciftci N. S., Rispens M. T., Sanchez L., Hummelen J. C. and Fromherz T.: Thin Solid Films, 2002, 403–404, 368.
- Zhao G., He Y. and Li Y.: Adv. Mater., 2010, 22, 4355.
- Scharber M. C., Mühlbacher D., Koppe M., Denk P., Waldauf C., Heeger A. J. and Brabec C. J.: Adv. Mater., 2006, 18, 789.
- Moliton A. and Nunzi J. M.: Polym. Int., 2006, 55, 583.
- Dennler G., Lungenschmied C., Sariciftci N. S., Schwoediauer R., Bauer S. and Reiss H.: Appl. Phys. Lett., 2005, 87, 163501.
- Mandoc M. M., Koster L. J. A. and Blom P. W. M.: Appl. Phys. Lett., 2007, 90, 133504.
- Dalton A. B., Coleman J. N., in het Panhuis M., McCarthy B., Drury A., Blau W. J., Paci B., Nunzi J. M. and Byrne H. J.: J. Photochem. Photobiol. A, 2001, 144A, 31.
- Ikeda A., Nobusawa K., Hamano T. and Kikuchi J. I.: Org. Lett., 2006, 8, 5489.
- Marzouk J., Lucas B., Trigaud T., Pothier A., Boucle J. and Ratier B.: Polym. Int., 2014, 63, 1378.
- Nikitenko V. R., Tameev A. R. and Vannikov A. V.: Org. Electr., 2011, 12, 589.
- Marulanda J. M. and Srivastava A.: Phys. Status Solidi B, 2008, 245B, 2558.
- Park S. H., Roy A., Beaupre S., Cho S., Coates N., Moon J. S., Moses D., Leclerc M., Lee K. and Heeger A. J.: Nat. Photonics, 2009, 3, 297.
- Chen H. Y., Hou J., Zhang S., Liang Y., Yang G., Yang Y., Yu L., Wu Y. and Li G.: Nat. Photonics, 2009, 3, 649.
- Liang Y., Xu Z., Xia J., Tsai S. T., Wu Y., Li G., Ray C. and Yu L.: Adv. Mater., 2010, 22, 1.
- Liu Y., Zhao J., Li Z., Mu C., Ma W., Hu H., Jiang K., Lin H., Ade H. and Yan H.: Nat. Commun., 2014, 5, 5293.
