Abstract
A new approach leading to poly(lactic acid) (PLA) nanocomposites designed with improved nucleating/crystallization ability has been developed. As proof of concept, nanofillers of different morphology (organo-modified layered silicates, halloysite nanotubes and silica) were surface-treated with ethylene bis-stearamide (EBS), a selected fatty amide able to promote chain mobility during PLA crystallization from the melt and nucleation. The fine dispersion of the nucleating additive via nanoparticles (NPs) as ‘nano-template’ is leading to nanocomposites showing unexpected improvements in PLA crystallization rate. This was evidenced by differential scanning calorimetry (DSC) from the high values of the degree of crystallinity (20–40%) with respect to neat PLA (4.3%) and the sharp decrease in crystallization half-time under isothermal conditions (at 110°C), even below one minute. Furthermore, after injection molding the outstanding crystallization properties of PLA were again confirmed. Accordingly, the PLA-nanofiller/EBS nanocomposites revealed remarkable degree of crystallinity (in the range of 30–40%). Surprisingly, the presence of EBS can significantly increase the impact resistance of PLA and PLA based nanocomposites. By considering the remarkable increasing in crystallinity, a key parameter to allow PLA utilization in durable applications, the development of the new approach is expected to lead to significant improvements in the processing and performances of PLA products.
Introduction
The extraordinary interest for biopolymers encompasses various factors including consumer demand for more environmentally sustainable products, the development of bio-based feed stocks, the increasing of restrictions for the use of petro-polymers with high ‘carbon footprint’ and others.
Poly(lactic acid) or polylactide (PLA), is industrially obtained respectively, through the polymerization of lactic acid or by ring opening polymerization (ROP) of lactide (the cyclic dimer of lactic acid, as an intermediate). PLA is an environmentally friendly and commercially available aliphatic polyester produced from renewable resources and that has a key position on the market of biopolymers. Characterized by very interesting properties, it is presently considered between the most promising biomaterials with the brightest development prospect.Citation1 Currently, PLA is receiving a considerable attention not only for traditional utilization in packaging and textile products, but more recently it has shown an increasing interest for technical applications.Citation1–Citation7 The last tendencies show clearly that the growth of PLA production indeed comes from the demand of long lasting bioplastics in industry sectors such as electronics and automotive, end-user markets requiring similar performances and processing characteristics that match those of existing polymers, traditionally derived from petroleum or other fossil resources.Citation8,Citation9
However, although PLA shows interesting physical and mechanical properties (high tensile strength and stiffness, transparency, biodegradability…), the poor impact resistance and low elongation at break, sensitivity to hydrolysis, permeability to gas, as well as the low rate of crystallization, impede its utilization in technical applications.Citation1,Citation10–Citation12 In this respect, the profile of PLA properties [rigidity, dimensional stability, heat deflection temperature (HDT), etc.] was improved by combining this polyester matrix with micro- and nano-fillers, impact modifiers, flame retardants, plasticizers, other polymers, etc.Citation1,Citation13–Citation21 The increase in HDT (i.e. the temperature at which the polymer deforms under a specified load) is a main goal and an important property required for the design, engineering and manufacture of PLA products considered for durable applications. However, significant improvements regarding this parameter are reported by production of PLA nanocomposites and by increasing PLA crystallinity.Citation22,Citation23
Unfortunately, the use of PLA in technical applications is actually not so high because PLA has a slow crystallization rate when compared with many other thermoplastics. In fact, this is a kind of ‘Achilles' heel’, limiting PLA use in a high performance application. Therefore, it is believed that new PLA grades with improved properties are needed. The control and increase in crystallinity will extend the use of PLA to electrical appliances and automotive parts (as substitution of petroleum based polymers). This parameter is particularly essential to control PLA's degradation rate, thermal resistance, as well as optical, mechanical and barrier properties.Citation23,Citation24 It is important to remind that under the real injection molding conditions, because of the high cooling rate, only almost amorphous items from PLA can be obtained, which results in a lower HDT and mechanical properties.Citation25 Therefore, to produce PLA of high crystallinity many efforts are made in this direction by both academia and industry. Furthermore, this subject has generated a wide interest and an impressive number of studies and reviews are focusing specifically on the current understanding of PLA crystallization properties.Citation19,Citation24–Citation28
To increase the crystallization rate of PLA, different methodsCitation24 have been considered such as: addition of organic nucleating agents,Citation29,Citation30 microfillers,Citation31–Citation33 nanofillers,Citation34–Citation37 stereocomplexes,Citation27,Citation38 derivatives of carboxylic and fatty acids (low molecular weight aliphatic amides),Citation23,Citation39 layered metal phosphonates,Citation40,Citation41 etc. The nucleating additives can increase the number of primary nucleation sites reducing the nucleation induction period, and therefore initiate PLA crystallization at higher temperature on cooling. An alternative is to add plasticizers, which increase the polymer chain mobility, and therefore enhance the crystallization rate by reducing the energy required during crystallization for the chain folding process.Citation32 For this reason, various nucleating agents and plasticizers (in combination or not) have been used in order to increase the crystallization rate of PLA.Citation13,Citation24,Citation42 However, a decrease in thermal stability and mechanical properties (tensile and flexural strength, rigidity, etc.) after the addition of plasticizers is noticed.
A challenge to improve PLA nucleation and crystallization kinetics is appealing, and accordingly tremendous efforts are made in this direction. Following this objective, the main goal of this study is to propose a new approach allowing to tune-up the crystallization properties of PLA, whereas the performances and specific end-use properties of PLA nanocomposites are maintained or even improved. In this regard, the experimental study was directed to the production (as proof of concept, PoC) of PLA nanocomposites characterized by improved features, going from crystallization to mechanical properties. Commercially available nanofillers (OMLS, halloysite, silica) of different morphology and high surface area, which potentially could act as nucleation sites for PLA, were used as ‘nano-template’ for a selected fatty amide, i.e. ethylene bis-stearamide (EBS), an organic additive claimed to promote lubrication, chain mobility and nucleating ability of PLA during the crystallization from the melt. Compared to the anterior studiesCitation23,Citation24 the nanofillers will play a key role allowing not only obtaining a PLA with specific properties, but also the finer dispersion of EBS.
For the illustration of the concept, the nanofillers were previously surface-treated with EBS and used for melt-compounding with PLA. Using adapted techniques, the resulting polymer nanocomposites (PNC) were characterized to highlight the properties of nucleation and overall crystallization rate of PLA. Furthermore, the tuning of loading and nanofiller/EBS ratio was presented as additional tool for tailoring the mechanical properties of PLA nanocomposites.
Experimental
Materials
Poly(L,L-lactide) – hereafter called PLA, was the 4032D grade (supplier NatureWorks LLC) with Mn = 133 000, dispersity, Mw/Mn = 1.94 (Mw and Mn, being respectively, weight- and number-average molar mass, determined by size exclusion chromatography (SEC) using polystyrene standards for column calibration), whereas upon the supplier the other characteristics are as follows: D isomer = 1.4%; relative viscosity = 3.94; residual monomer = 0.14%.
Nanofillers having respectively, one, two and three dimensions of the particulates in the order of few nanometers (according to ISO/TS 27687 (2008), i.e. Terminology and definitions for nano-objects – nanoparticle, nanofibre and nanoplate) were considered: organo-modified layered silicates (with one dimension in nanometer scale, thus will be hereinafter mentioned as ‘1D’), halloysite naotubes (2D) and equi-axed (isodimensional) silica (3D).
As 1D nanofiller, the selected organo-modified layered silicate (OMLS) used in this study was Cloisite 25A (C25A) as supplied by Southern Clay Products. Upon the supplier, C25A consists of nanometric platelets of layered magnesium aluminum silicate organically modified with dimethyl 2-ethylhexyl (hydrogenated tallow alkyl) quaternary ammonium at a modifier concentration of 95 meq/100 g clay. Based on thermogravimetric analysis (TGA), C25A contains about 70 wt-% inorganic silicate.
As 2D-nanofiller, halloysite nanotubes (HNT) were supplied by Aldrich with the following characteristics: 30–70 nm in diameter, 1–3 μm the length of nanotubes, surface area of 64 m2 g− 1 and 1.26–1.34 mL g− 1 pore volume.
A high surface fumed silica (SiO2) supplied by Cabot as CAB-O-SIL H5 was used as 3D nanofiller. The principal characteristics are as follows: BET surface area = 300 m2 g− 1; specific gravity = 2.2 g cm− 3; purity: >99.8% SiO2; average particle (aggregate) length = 0.2–0.3 μm; whereas the primary particle size is given to be about 8 nm. N,N′- Ethylenebis(stearamide), a fatty amide with linear formula [CH3(CH2)16CONHCH2–]2 and traditionally known as ‘EBS’, was supplied by Sigma–Aldrich.
Treatment of nanofillers by EBS and realization of PLA blends using internal kneaders
After the dry-mixing of nanofillers and EBS, at a weight ratio of 80/20 (for this study, vide infra) using a laboratory Rondol turbo-mixer, this first stage was followed by the ‘dry-coating’ at temperature (160°C) in an internal mixer (5 min at 30 rev min− 1 for adequate feeding, then 15 min at 100 rev min− 1) to allow the melting of EBS on the surface of nanofiller used as nano-template for the nucleating agent.
To minimize the water content before processing, PLA, nanofillers and EBS have been dried overnight at 80°C under vacuum. To produce various PLA formulations (), 3 wt-% of nanofiller were firstly dry mixed with PLA pellets (Rondol turbo-mixer, 2 min, 2000 rev min− 1), step followed by the moderate mixing (cam blades) at 200°C by using a Brabender bench scale kneader (model 50 EHT) following a specific procedure: 3 min premixing at 30 rev min− 1, speed in order to avoid an excessive increase in the torque during melting of PLA, followed by 7 min mixing at 70 rev min− 1. For the sake of comparison, the neat PLA matrix was processed by melt compounding under similar conditions.
Table 1. Composition and codification of main PLA samples selected for this study
In fact, the experimental program was more exhaustive, involving different nanofiller/EBS ratios and various loadings of nanofiller. For simplicity and to focus on the PoC, only the compositions from are mainly discussed hereafter.
To get first information about the mechanical properties of the nanocomposites produced at laboratory scale, plates (∼3.1 mm thickness) were produced by compression molding at 190°C by using an Agila PE20 hydraulic press. More specifically, the material was first pressed at low pressure for 200 s (three degassing cycles), followed by a high pressure cycle at 150 bars for 150 s. The resulting samples were then cooled under pressure (50 bars) for 300 s using water as cooling agent (temperature slightly >10°C, allowing a rapid cooling) and used in the next step to produce by gridding (Ray-Ran CNC Test Sample Profile Cutter) the specimens for mechanical characterization. Specimens (discs of 25 mm diameter and 1.5 mm thickness) required for both, WAXS and additional DSC analyses, were performed with a DSM micro injection molding machine using the following conditions: temperature of injection = 200°C, temperature of the mold = 110°C, residence time in the mold of 30 s.
Characterization
Differential scanning calorimetry (DSC)
DSC measurements were performed by using a DSC Q200 (TA Instruments) under nitrogen flow. The procedure was as follows: first heating scan at 10°C/min from 0°C up to 200°C, isotherm at this temperature for 2 min, then scan at different cooling rates (typically at 10°C min− 1) down to − 20°C and finally, second heating scan from − 20 to 200°C at 10°C min− 1. Additional cooling rates (from 2.5°C min− 1 up to 40°C min− 1) have been used to evidence the PLA crystallization from the melt.
The first scan was realized to erase the prior thermal history of the samples. For all samples only the amount of PLA was considered. In order to evidence and quantify the crystallization of PLA during non-isothermal crystallization experiments, the peak of crystallization temperature upon cooling (Tcc) and the crystallization enthalpy upon cooling (ΔHcc) were measured and the correct integration was attested systematically in the subsequently heating run. Then, the events of interest upon heating (h), i.e. the glass transition temperature (Tgh), cold crystallization temperature (Tch), enthalpy of cold crystallization (ΔHch), temperature and enthalpy of polymer chain rearrangement known also as pre-melt crystallization (Trh and ΔHrh respectively), melting temperature (Tmh) and melting enthalpy (ΔHmh) were determined from the second scan. The degree of crystallinity was determined by subtracting ΔHch and ΔHrh (if available) from ΔHm and by considering a melting enthalpy of 93 J g− 1 for 100% crystalline PLA.Citation43,Citation44 It is also important to mention that the specimens performed by injection molding for WAXS investigations were used for additional DSC analyses to assess the values of the degree of crystallinity following the first scanning.
Screening tests to evaluate the crystallization kinetics of PLA were performed through the determination of crystallization half-time (t1/2) during isothermal crystallization. Using DSC technique, the PLA samples were heated to 200°C at a rate of 10°C min− 1, held 2 min at this temperature to erase their thermal history, step followed by high speed cooling (40°C min− 1) to the iso-crystallization temperature of interest, typically 110°C, and maintained under isothermal conditions for up to 120 min. The relative crystallinity was calculated by integrating the total area under the curve for each crystallization exotherm. The t1/2 was taken to be the time at which the relative crystallinity (area) was equal to 50%.
Mechanical testing measurements
Tensile testing measurements were performed using a Lloyd LR 10K tensile bench in accordance to the ASTM D 638-02a norm at a tensile rate of 1 mm min− 1 using specimens type V and a distance of 25.4 mm between grips. Notched impact resistance (Izod) measurements were performed using a Ray-Ran 2500 pendulum impact tester and a Ray-Ran 1900 notching apparatus, according to the ASTM D 256 norm (method A, 3.46 m s− 1 impact speed, 0.668 kg hammer). All mechanical tests were carried out using specimens previously conditioned for at least 48 h at 20( ± 2)°C under a relative humidity of 50( ± 3)% and the values were averaged out over five measurements.
Scanning electron microscopy (SEM)
SEM was performed using a scanning electronic microscope Philips XL at an accelerated voltage up to 30 kV and various magnitudes. SEM was equipped for both secondary electron (SE) and back scattered electron (BSE) imaging.
Transmission electron microscopy (TEM)
Transmission electron micrographs were obtained with a Philips CM200 apparatus using an accelerator voltage of 120 kV. The nanocomposite samples (70–80 nm thick) were prepared with a Leica UCT ultracryomicrotome by cutting at − 100°C. Reported microphotographs represent typical morphologies as observed at, at least, three various places.
Wide angle X-ray scattering (WAXS) characterizations
The specimens for WAXS characterization (discs of 25 mm diameter and 1.5 mm thickness) were produced by injection molding. The WAXS analysis was performed on a Siemens D5000 diffractometer using Cu Ka radiation (wavelength, 1.5406 Å) at room temperature with a scanning rate of 2° min− 1.
Results and discussion
Preliminary considerations in relation to nanofiller treatment
Following the interest in the utilization of bionanocomposites, nanofillers of different morphologies and with at least one dimension ( < 100 nm) within the nanoscale range (three-dimensional ‘isotropic’ nanofillers, two-dimensional such as nanotubes or nanofibers, or one-dimensional sheet-like geometry of nanoparticlesCitation45) were used with satisfactory achievements in the design of PLA nanocomposites.Citation46 It has been also reported that the effectiveness of nanofiller addition on PLA properties can be evidenced even at very low loadings, i.e. 1–3%.Citation47,Citation48,Citation49 To qualitatively assess the versatility of our new experimental approach, nanofillers of different morphology were selected for melt-mixing with PLA, knowing that previously they have been tested with positive results in the production of PLA nanocomposites.Citation44,Citation50–Citation53
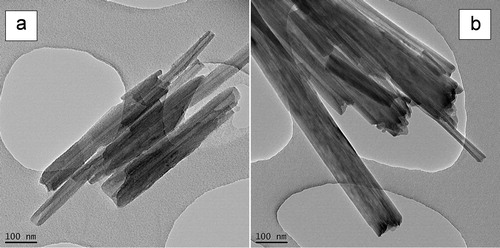
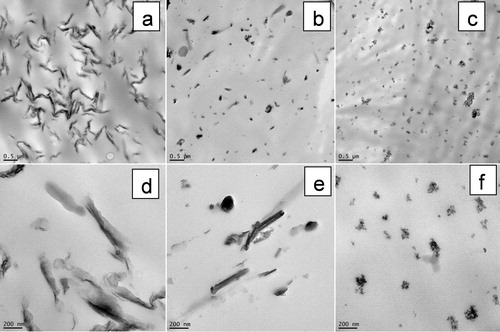
shows the typical TEM images at high magnification of the NPs considered in the work, attesting for their nanoscale morphological features (1D, 2D or 3D). As mentioned before (see experimental part) these NPs have one (C25A), two (HNT) or three dimensions of nanometer scale (silica). Silica (SiO2) is also classified in the category of isodimensional nanofillers.Citation45 As illustrated by the representative SEM images shown in , it is noteworthy that the raw NPs behave as aggregates and agglomerates before dispersion into PLA. The specific surface of NPs is different, going from the highest specific surface for C25A (1D) (about 725 m2 g− 1 for exfoliated montmorillonite), the intermediate one for silica (3D) (about 300 m2 g− 1), to the lowest one for HNT (2D) (above 60 m2 g− 1).
Figure 1. a–c TEM images to illustrate morphology at nano-scale of designated fillers: 1D = OMLS (C25A), 2D = HNT, 3D = SiO2 (scale bar is of 100 nm)
Figure 2. a–c scanning electron micrographs (SEM) to illustrate initial aggregate structure of as received fillers: 1D = OMLS, 2D = HNT, 3D = SiO2 (NB: for better evidencing of NPs different morphology, dissimilar magnifications were used)

In the first experimental step, the fillers of different morphology and surface area were mixed at 160°C with EBS to enable its complete melting (the melting point as determined by DSC is at 145°C) following a ‘dry-coating’ process. EBS is currently used as wax, dispersing agent or as lubricant additive to facilitate and to improve the dispersion of solid materials, to enhance the processability and to decrease the friction in polymer applications (PLA is included).Citation54 Furthermore, Harris et al., have found that this additive can act as nucleating additive for PLA, leading to the increase of crystallinity and crystallization rate through an optimized injection molding process. Accordingly, they have reported significant improvements in PLA mechanical performances (HDT, flexural strength, flexural modulus, etc.).Citation23
Regarding the treatment with EBS, as illustrated in for HNT and silica in the presence of water, it is very clear that the dry coating with this additive modifies the surface properties of the fillers, therefore these nanofillers turn from hydrophilic to show hydrophobic behaviour. The treatment with EBS yields a hydrocarbon-like surface for the nanofillers, making them much less polar than the starting nanofillers. This process also shows some similarities with the ‘dry-coating’ of fillers with fatty acids, such as stearic acid (SA), or stearate salts.Citation55,Citation56
Figure 3. a–d illustration of ‘hydrophobicity’ in the presence of water of HNT and SiO2 treated with b, d EBS with respect to a, c respectively untreated nanofillers
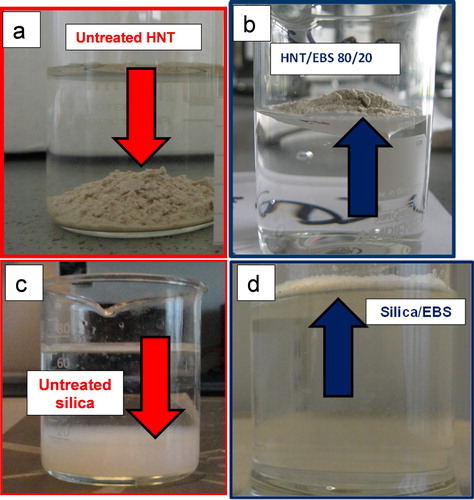
C25A is already hydrophobic due to the intercalation of hydrogenated tallow alkyl quaternary ammonium ions between montmorillonite (MMT) layers. In this respect no comparative images are shown here. It is important also to mention that the molecular characterizations by SEC (size exclusion chromatography) of the samples considered in this study attest that the addition of NP/EBS is leading to the same molecular parameters for the PLA matrix (for sake of clarity, these results are not shown here). Therefore, these nanofillers are considered to be less sensitive to moisture, while it is reasonable to ascribe the outstanding increase of crystallinity of PLA nanocomposites to the effective role of EBS as nucleating agent (see sections on ‘Non-isothermal crystallization’ and ‘Isothermal crystallization and injection molding experiments’).
Regarding the ‘coating’ of the considered nanofillers, it is important to mention that other techniques (not reported here) have also been tested with promising results such as the ‘wet’ method, i.e. the surface treatment and intensive pre-mixing of NPs with solutions of EBS in selected solvents (ethanol, isopropanol, etc.). Nevertheless, it is believed that the ‘dry-coating’ is more interesting for extrapolation at larger scale than the techniques based on the utilization of organic solvents. Furthermore, it is noteworthy that TGA can be successfully used as rapid method to quantify and assess the presence of EBS in the desired nanofiller/EBS ratio, whereas the SEM and TEM techniques were considered as additional tools, giving information about the morphology of the nanofillers. Unfortunately, as revealed in the case of treated or untreated HNT (), TEM observations cannot give a clear conclusion about the main differences related with the presence of layers of EBS onto the surface of nanofiller. However, it is assumed that these fillers of different morphology and specific surface area can be considered as templates acting as ‘nano-support’ of high surface for EBS. As a result of this treatment, the amount of hydrophobic molecules (EBS) might overload the nanofiller surface, whereas the slight disaggregation of particle agglomerates can be also assumed. An overloading with EBS can be deliberately aimed by considering that the additive will be finally found at the polyester/nanofiller interface and finely dispersed throughout the PLA matrix. On the other hand, here was preferred the nanofiller/EBS ratio of 80/20 based on a previous experimental investigation, especially to induce the crystallization from the melt at higher temperature. In fact, by decreasing the content of EBS the crystallization peak was recorded somewhat at lower temperatures. Therefore, it was preferred to remain in the optimum temperature range traditionally indicated for PLA crystallization,Citation27 but additional parameters (e.g. mechanical properties) were also considered (vide infra).
Finally, it is assumed that various factors can affect, more or less, the crystallization kinetics of PLA, going from the nature of PLA (molecular weight, dispersity, isomeric purity, etc.), nanofiller loading, morphology and treatment, quality of distribution and dispersion through the polyester matrix, etc.
For simplicity and to focus on the PoC, only selected results are discussed hereafter at nanofiller-to-EBS weight ratio of 80/20. Furthermore, as it will be disclosed in the section on ‘Morphology of nanocomposites and mechanical properties’, it is possible to modulate the performances of PLA nanocomposites following different objectives: medium to high crystallinity, tensile strength and stiffness, toughness, etc., optimizing the loading of nanofiller and nanofiller/EBS ratio.
Non-isothermal crystallization
To evidence the crystallization of polymers such as PLA, various techniques can be successfully used, going from the differential scanning calorimetry (DSC), wide-angle X-ray scattering (WAXS), infrared spectroscopy (IR), nuclear magnetic resonance (NMR), dynamic mechanical analysis (DMA), to atomic force microscopy (AFM) and polarized optical microscopy (POM).Citation28
Primarily, we have considered that DSC is probably the simplest technique which could give relevant and fast information about the crystallization properties of PLA nanocomposites. For an easier understanding of the key-points, hereafter are commented mainly the results obtained by studying the non-isothermal crystallization from the molten state.
shows the representative DSC curves of different PLA samples as they were recorded respectively, during cooling from the melt (rate of 10°C min− 1) after erasing the prior thermal history, followed by the second DSC heating (the quantification of data is reported in ). By considering the crystallization from the molten state of PLA samples () it is seen that, in all cases addition of surface treated NPs (1D/EBS, 2D/EBS, 3D/EBS) leads to the clear PLA crystallization during cooling (i.e. the presence of well evidenced exothermic crystallization) in contrast to the pristine PLA and to PLA nanocomposites without any EBS-based treatment. For instance, the nanocomposites containing EBS display a crystallization process with a maximum peak (Tcc) recorded at 105–107°C. From the quantification of crystallization enthalpy recorded during cooling (ΔHcc), addition of 1D/EBS filler shows the highest effectiveness (a ΔHcc of 24 J g− 1, assigned to a gain of crystallinity higher than 26%), compared to the addition of 2D/EBS and 3D/EBS, that are giving lower values (i.e. ΔHcc of about 17 J g− 1, a gain in crystallinity higher than 18%). Furthermore, neat PLA does not show any detectible crystallization, whereas the PLA-EBS sample shows only poor and broad exotherms of crystallization (ΔHcc of about 4 J g− 1) with a Tcc at 99°C. Similar results were reported by Harris et al., following the evaluation of EBS and talc as nucleating agents for PLA.Citation23 Additionally, from the DSC traces recorded during cooling of PLA containing EBS, it can be seen very small exothermic peaks within the temperature range 125–135°C (indicated by arrows in ) that can be reasonably ascribed to the crystallization of EBS itself. Furthermore, it is believed that the additive (EBS) is either largely localized at the interface between PLA-nanofiller or finely dispersed through PLA matrix, especially in the case of an overloading with EBS of nanofillers of lower specific surface. The additive plays a complex role in the crystallization of PLA (from the melt) promoting nucleation as well as chains mobility. Besides, dramatic improvements in crystallization can be obtained by combining as nucleating agents EBS and NPs, both having ability to endorse the crystallization of PLA, first of all, due to formation of large fraction of nuclei. Concerning the nanocomposites without EBS, it is obvious from the traces recorded during cooling that they do not reveal any noticeable crystallization peak. Nevertheless, the data reported in the literature on this subject are very different, showing that the nanofillers could promote, more or less, the PLA crystallization.
Figure 5. a, b comparative DSC traces of PLA (with and without EBS) and those of PLA nanocomposites containing nanofillers (with/without EBS) as they were recorded a during cooling and b during second heating (rate of 10°C min− 1 was used in both DSC scans)
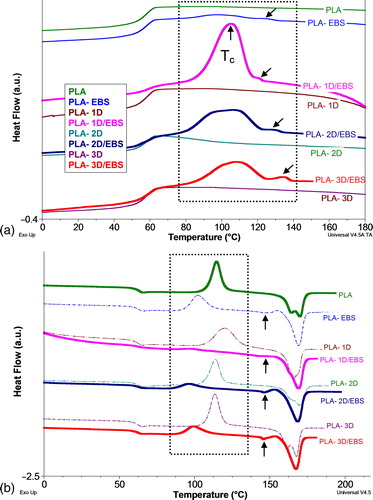
Table 2. Comparative DSC data of PLA and PLA nanocomposites containing nanofillers with/without EBS (second DSC heating, 10°C min− 1)
The data derived from DSC curves shown in (recorded during second DSC heating) are reported in . Because the addition of nanofillers does not modify significantly the Tg values, we will focus our comments mainly on the differences of crystallinity for different PLA based materials as obtained during second heating step. The multiple melting peaks (Tm) or the presence of shoulders on DSC curves, are usually ascribed to the melting of crystalline regions of various size and perfection formed during cooling and crystallization processes. It is worth noting that the influence of additional factors may further affect the melting of PLA (different crystalline structures, presence of fractions of low molecular weight, the effect of thermo-mechanical processing, etc.). Furthermore, the lower temperature peak is generally associated to the melting of the small crystals produced by secondary crystallization, whereas the peak recorded at higher temperature corresponds to the melting of the major crystals formed in the primary crystallization process.Citation57
Interestingly in , the addition of EBS and NPs/EBS into PLA leads to a single Tm (not multiple melting peaks or additional shoulders), assuming a higher uniformity for the crystals formed during cooling from the melt. Some statements for this hypothesis were obtained via POM analyses (see Supplementary Material, Fig. S1: www.maneyonline.com/doi/suppl/10.1179/2055033214Y.0000000008), that have proved for PLA-(1D-3D)/EBS nanocomposites compared to the neat PLA not only higher kinetics of crystallization, but the formation of a microcrystalline/microspherulitic structure as well. This appears to be another main characteristic supporting the improvements in the mechanical properties of these nanocomposites. The presence of EBS is attested from the DSC traces obtained during second heating by the small melting peaks at above 145°C (evidenced by arrows on ). It is also important to highlight that some nanocomposites have shown pre-melting crystallization of relatively low enthalpy, which has been considered in the calculation of the degree of crystallization. The second DSC scanning also reveals that neat PLA and its nanocomposites in absence of EBS undergo evident cold crystallization as it is attested by the high cold crystallization enthalpies (ΔHch). Moreover, the values of Tch are in the range 110–120°C, temperatures significantly higher than those attributed to PLA samples containing EBS (Tch from 97 to 102°C). Therefore, the presence of EBS onto nanofiller surface can facilitate the cold crystallization of the polymer matrix during heating as well.
Furthermore, linked to the behaviour recorded during cooling scan, the degree of crystallinity (χc) of PLA in the nanocomposites without EBS remains very low (i.e. in the range 4–5.3%), meaning that the considered nanofillers in absence of EBS do not behave as efficient nucleating agent for PLA under the specific experimental conditions. However, the χc of PLA in the nanocomposites (without EBS) is comparable to those recorded for the pristine PLA (4.3%), proving the low crystallization ability of PLA. In contrast, the nanocomposites containing NP/EBS show remarkable values of χc (going from 20 to 38%) and the following order of effectiveness for the treated nanofillers can be suggested: 1D/EBS>3D/EBS ≥ 2D/EBS. This could be explained by the surface area of these nanofillers. For instance, the 1D-nanofiller has the highest surface area among them and leads to the better results in terms of PLA crystallization rate. However we cannot exclude that other factors could influence PLA crystallization such as the nature of nanofiller, existence or not of any previous treatment, etc.
Last, in relation to the non-isothermal crystallization from the melt, it is necessary to mention that comparative investigations using the conventional DSC technique were also realized using different rates of cooling to attest the versatility of the new approach (please consider the Supplementary Material, Fig. S2 www.maneyonline.com/doi/suppl/10.1179/2055033214Y.0000000008).
Isothermal crystallization and injection molding experiments
Another confirmation of the effectiveness of NPs/EBS as nucleating agents comes from the determination of the crystallization half-time (t1/2) using the isothermal crystallization experiments. Commonly, the data reported in the literature attest that the crystallization rate of PLA is the highest at temperatures between 100 and 120°C.Citation26 It is worth mentioning that various nanofillers, talc, multi-amide products, etc. were already tested to nucleate PLA matrices, determining the remarkable decreasing of t1/2 and spectacular changes in the crystallization rate, polymer morphology and properties of the resultant PLA compounds.Citation23,Citation58
The effects of NP/EBS addition on the crystallization kinetics of PLA were here compared through t1/2 obtained during isothermal crystallization experiments. Crystallization exotherms were measured as a function of time as reported elsewhere.Citation23,Citation35 Selected data obtained from the isothermal crystallization at 110°C of neat PLA and those of nanocomposites with or without EBS are reported in . These results fully confirm that NP/EBS combinations behave as remarkable nucleating agents for PLA, their addition into the polyester matrix leading to lower t1/2 with respect to the nanocomposites containing untreated NPs and neat PLA. All nanocomposites containing EBS show intense and narrow exotherms on the recorded DSC diagrams, while as received or processed PLA displayed much broader traces. PLA before processing show a huge t1/2 (about 52 min), which attests for the low crystallization ability of this polyester. These results are in good agreement with the values currently reported in literature using a similar method.Citation23 Again, it is worth reminding that the t1/2 of the PLA depends on crystallization temperature, optical purity, molecular weight, processing parameters, etc. For instance Battegazzore et al. have reported a t1/2 of 82 min for a PLA containing 4% D-isomer following its isothermal crystallization at 110°C.Citation32 However, Pantani et al.Citation28 have assumed that following the mechanical degradation induced by processing, a limited number of smaller chains can induce a significant rise in the nucleation rate, leading to a significant increase of PLA crystallization kinetics.
Table 3. Half-time of crystallization of neat PLA and PLA nanocomposites containing nanofillers with/without EBS
From , the most interesting results are obtained by addition of NP/EBS that shows a very high effectiveness in the reduction of t1/2, a parameter of real interest in the frame of applications related with injection-molding processes. It (t1/2) was found to be less than 1.5 min using 3D/EBS treated nanofiller, and equal or even less than 1 min (using 1D/EBS filler) respectively, in the case of PLA- 2D/EBS and PLA- 1D/EBS samples.
It has also been reported in the literature that very interesting and useful information regarding the crystallization properties can be obtained via injection molding experiments.Citation23,Citation27 Thus, the injection molding technique was used to practically prove the increase of crystallinity, results confirmed by DSC analyses and comparative WAXS measurements (see hereafter). Following DSC data (), neat PLA is again characterized by a low degree of crystallinity (χc above 6%) using this particular technique. The degree of crystallinity is higher in the case of nanocomposites containing untreated NPs and was found to be in the range 11–18%. Interestingly, all nanocomposites containing both, nanofiller (as template) and EBS, show unconventional crystallization ability, and consequently an increased χc (in the range 28–41%) is evidenced under similar conditions of processing (i.e. 30 s at 110°C). In other terms, under our experimental procedure, the considered NPs can also develop significant crystallization ability, but not as high as surface treated NPs. It may be assumed that this parameter (χc) can be also influenced by the composition of the nanocomposites (e.g. loading in EBS and nanofiller) and additional other factors (e.g. processing conditions).Citation42
Table 4. Comparative DSC data on specimens performed by injection molding of PLA and PLA nanocomposites containing nanofillers with/without EBS (first DSC heating, 10°C min− 1)
shows the WAXS patterns of the specimens performed by injection molding using an annealing time in the mold (at 110°C) of only 30 s. By comparing the effects of untreated and treated NPs, it comes out that the nanocomposites containing NPs/EBS show in all cases stronger diffraction peaks at 2θ of about 16.5° with respect to those of nanocomposites obtained using untreated NPs. These peaks are ascribed to the presence of the crystalline phase of PLA attributed to (200) and/or (110) planes crystal facets, which are characteristic to the α-crystal form.Citation59 Additional small peaks, e.g. at 2θ of about 19° that are traditionally attributed to (203) planes, are also observed for nanocomposites.Citation59,Citation60 Furthermore, in good agreement with the DSC results, the neat PLA shows only a small crystallization peak with a maximum at 2θ = 16.5°, indicating that the sample is almost amorphous.
Figure 6. Comparative WAXS patterns on specimens performed by injection molding of PLA and PLA nanocomposites containing nanofillers with/without EBS
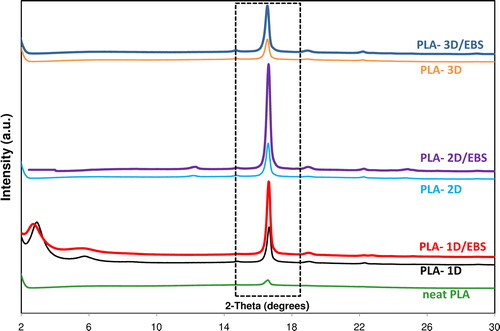
Additionally, by analyzing the comparative WAXS spectra of PLA-1D and PLA-1D/EBS samples (shown in ), it can be concluded that the treatment of nanofiller (C25A) by EBS may lead to some improvement in the morphology of nanocomposites, mainly in the quality of intercalation of PLA chains between the silicate layers. As shown in , for instance the d001 value slightly increased (above 2 Å) in the nanocomposites containing 1D/EBS filler with respect to the nanocomposite containing non-treated fillers (d001 of about 30 Å). It is also interesting to underline that the intensities of the peaks (at 16.5°) were found almost correlated to the degree of crystallinity, as determined by DSC on the samples obtained by injection molding (results shown in ).
As preliminary conclusion coming from these characterizations, it is clear that the PLA- (1D-3D)/EBS nanocomposites lead to a noteworthy degree of crystallinity (e.g. 30–40%), at shorter injection molding cycle time. The increase in the crystallinity and substantial reduction of t1/2 through co-addition of NPs and EBS as (co)nucleating agents is seen as a versatile way that allows obtaining direct and significant improvements in the processing of commercial PLA grades.
Morphology of nanocomposites and mechanical properties
It is well known that the NPs have a strong tendency to form agglomerates and aggregates (see also the section on ‘Preliminary considerations in relation to nanofiller treatment’). Therefore, even non-reactive surface treatments can lead to important changes not only at the interface with the polymer matrix, as well as in the interactions between particles, by reducing their surface energy and/or allowing a finer dispersion or overcoming their re-agglomeration.Citation55 It is worth pointing out that PLA nanocomposites containing the considered nanofillers (OMLS, HNT, SiO2) already did the subject of previous studies published in the literature.Citation46,Citation52,Citation53,Citation61–Citation63 We will discuss succinctly hereinafter some aspects related only to the morphology of PLA-(1D- 3D)/EBS nanocomposites.
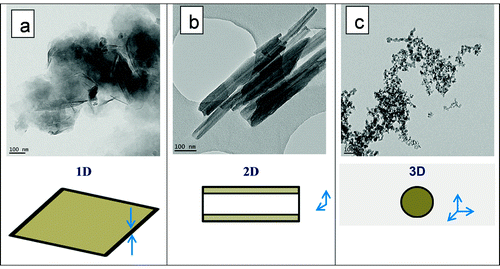
shows selected TEM pictures of the nanocomposites produced by addition into PLA of 3% nanofiller (previously treated with EBS), at low and high magnification respectively. It is obvious from the TEM images that a quite good NPs distribution/dispersion was reached within the polyester matrix, whereas the melt mixing was performed under moderate shear using internal kneaders. Besides, the TEM images of PLA-3D/EBS samples containing amorphous silica () attest for the presence of small associations of NPs of nanometric dimension (lower than 100 nm), that are not totally separated. Following the results reported elsewhere,Citation53 the dimension of aggregates can be even of micron size, being quite problematic to separate the individual silica nanoparticles. This may be explained by the very low dimension of silica NPs (about 8 nm) and the presence of strong interactions endorsed by the high concentration of silanol groups. The NPs thereby show strong self-networking ability and therefore it is more difficult to break the big aggregates during the melt-blending process.Citation64
Figure 7. a–f selected TEM images at low and high magnification of nanocomposites loaded with 3% NPs (coated by EBS): a, d PLA-1D/EBS; b, e PLA-2D/EBS; c, f PLA-3D/EBS
By considering also the results obtained in the frame of other experimental programs, it is assumed that the presence of EBS onto nanofiller surface (used as ‘nano-template’ for the nucleating additive) has no negative effects in relation to the morphology of nanocomposites.
summarizes the results of the mechanical characterization of PLA nanocomposites loaded with 3% nanofiller to evidence the main effects of EBS addition. The stress-strain and impact tests were carried out at room temperature after the previous conditioning of specimens produced from plates obtained by compression molding (see the experimental part), whereas comparison to neat PLA and PLA-EBS samples is also given. First, due to non-reactive interaction with the polymer matrix, it comes out that the presence of EBS into PLA or onto the surface of NPs slightly affects the values of tensile strength (values in range 58–64 MPa compared to 64–67 MPa respectively, for nanocomposites with and without EBS).
Table 5. Comparative mechanical properties of PLA and PLA nanocomposites (with/without EBS)
Surprisingly, the rigidity (Young's modulus) of PLA nanocomposites increases in all cases in presence of EBS, while preserving the low nominal strain at break. Following the data reported in , addition of EBS has a key role in the improvement of the impact resistance of PLA, i.e., two-fold (5.5 kJ m− 2) in the case of PLA/EBS sample with respect to the neat PLA (2.7 kJ m− 2). To the best of our knowledge, this is one of the first papers dealing with the possibility to tune PLA toughness by the addition of tiny amount of EBS as additive. The nanocomposites containing NPs/EBS show in all cases improved impact resistance, with a special mention for PLA-3D/EBS sample. These results shall need rigorous and comprehensive interpretation connected to the morphology of nanofiller and nanocomposites.Citation64 However, different hypotheses are concerned: the importance of microcrystalline structure knowing that formation of spherulites of lower dimension could positively affect the impact resistance, the nanofillers or fine dispersed EBS (via nanofiller-template) may impart enhanced impact resistance, the specific effect of nanofiller morphology and stiffness, and so on.Citation44,Citation65
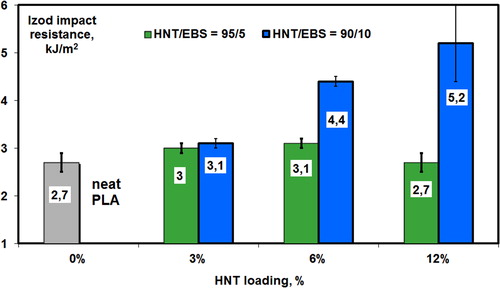
On the other hand, it is assumed that additional improvements in properties can be expected following the industrial processing by injection molding. The level of tensile strength, rigidity or impact resistance can be tuned up by modifying the loading of nanofiller and by optimizing the NPs/EBS ratio, while maintaining advanced crystallization properties. In the frame of additional studies was experimentally demonstrated that by finely varying the NPs/EBS ratio it is possible to obtain, respectively, a higher tensile strength or to induce both, the crystallization from the melt at higher temperature, while improving simultaneously the impact resistance. As exemplified in in the case of HNT (‘2D’), the nanofiller/EBS ratio can play a key role in relation to the mechanical properties of the nanocomposites, e.g. impact resistance. Thus, following the requirements of the application, different parameters can be optimized, including the nanofiller/EBS ratio and nanofiller loading.
Figure 8. Effects of HNT/EBS ratio and nanofiller loading on impact resistance (Izod) of PLA–(3–12)%HNT/EBS nanocomposites: HNT/EBS ratio of 95/5 and 90/10
Finally, the improvements of mechanical properties and increases in crystallinity (other characteristics can be also concerned: HDT, flexural strength and modulus, water vapour barrier, etc.), suggest that the nanocomposites loaded with NP/EBS are potentially interesting for engineering applications.
Conclusions
The use of PLA in technical applications is currently limited because PLA shows a slow crystallization rate under typical processing conditions. A new approach leading to PLA nanocomposites designed with improved nucleating/crystallization ability and accordingly, better processing and enhanced properties has been developed. As PoC and to attest the flexibility of the method, various nanocomposites were produced by melt blending PLA with nanofillers of different morphology (one to three nano-dimensions). The nanofillers used as nano-templates for the nucleating agent, were surface treated with ethylene bis-stearamide (EBS), a selected fatty amide having the role to promote both chain mobility in the melt and nucleating ability. This treatment leads to changes of interfacial properties in nanocomposites as revealed by mechanical testing, whereas the fillers become hydrophobic and less sensitive to moisture.
The properties of crystallization were evidenced using DSC as a main tool of investigation. In all cases addition of treated NPs into PLA leads to surprising properties of crystallization evidenced during cooling (crystallization from the melt), a higher effectiveness being associated to the use of 1D/EBS as nanofiller. The degree of crystallinity of the nanocomposites containing EBS was found in the range 20–40%, values much higher than those of pristine PLA and of PNCs without EBS (crystallinity limited to ∼5%). Another confirmation of the effectiveness of the NPs/EBS as nucleating agents was obtained from the determination of the crystallization half-time (t1/2) in isothermal crystallization experiments. These results fully confirmed that NP/EBS combinations show very important increases in PLA crystallization rate leading to much lower t1/2 (even in the range 0.5–1 min). Furthermore, it was evaluated the crystallinity development in molding conditions. WAXS in full agreement with DSC measurements confirmed the unconventional crystallization ability (χc of 30–40%) of PLA containing NPs/EBS. By analyzing the morphology of PLA–(1-3D)/EBS nanocomposites, good nanofiller distribution/dispersion was observed (via TEM images) whereas by considering their mechanical properties, some increase in rigidity and impact resistance was noticed. Interestingly, it is assumed that the new approach, due to its versatility, can be regarded as a remarkable possibility to design new PLA products with enhanced processing and properties.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Acknowledgements
Authors thank the Wallonia Region, Nord-Pas de Calais Region and European Commission for financial supports in the frame of INTERREG IV: NANOLAC project. They also thank their partners in the frame of NANOLAC project for helpful discussions. Authors also thank the Wallonia Region and their partners in the frame of BEETPACK project. We are grateful to Professor Mirosław Pluta (Center of Molecular and Macromolecular Studies Lodz, Poland), Alice Belfiore and Lisa Dangreau (Materia Nova, Belgium) for assistance in analyses. LPCM thanks the Belgian Federal Government Office of Science Policy (SSTC- PAI 6/27) for general support and is much indebted to both ‘Région Wallonne’ and the European Commission ‘FSE and FEDER’ for financial support in the frame of Phasing-out Hainaut: Materia Nova. J.-M. Raquez is FRS-FNRS research associate.
ynan_a_11668508_sm0001.doc
Download MS Word (4.1 MB)References
- Madhavan Nampoothiri K., Nair N. R. and John R. P.: Bioresour. Technol., 2010, 101, (22), 8493–8501.
- Babu R., O'Connor K. and Seeram R.: Prog. Biomater., 2013, 2, (1), 1–16.
- Auras R., Harte B. and Selke S.: Macromol. Biosci., 2004, 4, (9), 835–864.
- Gupta B., Revagade N. and Hilborn J.: Progr. Polym. Sci., 2007, 32, (4), 455–482.
- Drumright R. E., Gruber P. R. and Henton D. E.: Adv. Mater., 2000, 12, (23), 1841–1846.
- Jamshidian M., Tehrany E. A., Imran M., Jacquot M. and Desobry S.: Comprehens. Rev. Food Sci. Food Safety, 2010, 9, (5), 552–571.
- Dubois P. and Murariu M.: JEC Compos. Magaz., 2008, 45, 66–69.
- Smith C.: Compound. World, Jun. 2012, 45–51.
- Smith C.: Compound. World, Sep. 2013, 31–36.
- Bitinis N., Verdejo R., Maya E. M., Espuche E., Cassagnau P. and Lopez-Manchado M. A.: Compos. Sci. Technol., 2012, 72, (2), 305–313.
- Rasal R. M., Janorkar A. V. and Hirt D. E.: Progr. Polym. Sci., 2010, 35, (3), 338–356.
- Bordes P., Pollet E. and Avérous L.: Progr. Polym. Sci., 2009, 34, (2), 125–155.
- Murariu M., Da Silva Ferreira A., Alexandre M. and Dubois P.: Polym. Adv. Technol., 2008, 19, (6), 636–646.
- Murariu M., Bonnaud L., Yoann P., Fontaine G., Bourbigot S. and Dubois P.: Polym. Degrad. Stabil., 2010, 95, (3), 374–381.
- Murariu M., Ferreira A. D. S., Duquesne E., Bonnaud L. and Dubois P.: Macromol. Sympos., 2008, 272, (1), 1–12.
- Fukushima K., Murariu M., Camino G. and Dubois P.: Polym. Degrad. Stabil., 2010, 95, (6), 1063–1076.
- Kfoury G., Raquez J.-M., Hassouna F., Odent J., Toniazzo V., Ruch D. and Dubois P.: Front. Chem., 2013, 1, (32), 1–46 (Published online 2013 December 17, doi:10.3389/fchem.2013.00032).
- Bourbigot S. and Fontaine G.: Polym. Chem., 2010, 1, (9), 1413–1422.
- Anderson K. S., Schreck K. M. and Hillmyer M. A.: Polym. Rev., 2008, 48, (1), 85–108.
- Wang Y., Chiao S. M., Hung T. F. and Yang S. Y.: J. Appl. Polym. Sci., 2012, 125, (S2), E402–E412.
- Murariu M., Da Silva Ferreira A., Degée P., Alexandre M. and Dubois P.: Polymer, 2007, 48, (9), 2613–2618.
- Sinha Ray S., Yamada K., Okamoto M. and Ueda K.: Polymer, 2003, 44, (3), 857–866.
- Harris A. M. and Lee E. C.: J. Appl. Polym. Sci., 2008, 107, (4), 2246–2255.
- Saeidlou S., Huneault M. A., Li H. and Park C. B.: Progr. Polym. Sci., 2012, 37, (12), 1657–1677.
- Gui Z., Lu C. and Cheng S.: Polym. Test., 2013, 32, (1), 15–21.
- Di Lorenzo M. L.: Eur. Polym. J., 2005, 41, (3), 569–575.
- Anderson K. S. and Hillmyer M. A.: Polymer, 2006, 47, (6), 2030–2035.
- Pantani R., De Santis F., Sorrentino A., De Maio F. and Titomanlio G.: Polym. Degrad. Stabil., 2010, 95, (7), 1148–1159.
- Cai Y., Yan S., Fan Y., Yu Z., Chen X. and Yin J.: Iran Polym. J., 2012, 21, (7), 435–444.
- Cai Y., Yan S., Yin J., Fan Y. and Chen X.: J. Appl. Polym. Sci., 2011, 121, (3), 1408–1416.
- Yu F., Liu T., Zhao X., Yu X., Lu A. and Wang J.: J. Appl. Polym. Sci., 2012, 125, (S2), E99–E109.
- Battegazzore D., Bocchini S. and Frache A.: Expr. Polym. Lett., 2011, 5, (10), 849–858.
- Xiao H. W., Li P., Ren X., Jiang T. and Yeh J.-T.: J. Appl. Polym. Sci., 2010, 118, (6), 3558–3569.
- Park S. H., Lee S. G. and Kim S. H.: Compos. A: Appl. Sci. Manuf., 2013, 46, (0), 11–18.
- Murariu M., Dechief A. L., Bonnaud L., Paint Y., Gallos A., Fontaine G., Bourbigot S. and Dubois P.: Polym. Degrad. Stabil., 2010, 95, (5), 889–900.
- Krikorian V. and Pochan D. J.: Macromolecules, 2004, 37, (17), 6480–6491.
- Lee J. and Jeong Y.: Fibers Polym., 2011, 12, (2), 180–189.
- Tsuji H., Takai H. and Saha S. K.: Polymer, 2006, 47, (11), 3826–3837.
- Nam J. Y., Okamoto M., Okamoto H., Nakano M., Usuki A. and Matsuda M.: Polymer, 2006, 47, (4), 1340–1347.
- Han Q., Wang Y., Shao C., Zheng G., Li Q. and Shen C.: J. Compos. Mater., 2014, 48, 2737–2746.
- Suryanegara L., Okumura H., Nakagaito A. and Yano H.: Cellulose, 2011, 18, (3), 689–698.
- Li H. and Huneault M. A.: Polymer, 2007, 48, (23), 6855–6866.
- Fischer E. W., Sterzel H. and Wegner G.: Kolloid-Z.u.Z. Polym., 1973, 251, (11), 980–990.
- Murariu M., Dechief A.-L., Paint Y., Peeterbroeck S., Bonnaud L. and Dubois P.: J. Polym. Environ., 2012, 20, (4), 932–943.
- Kumar A. P. and Depan D.: Progr. Polym. Sci., 2009, 34, (6), 479–515.
- Raquez J.-M., Habibi Y., Murariu M. and Dubois P.: Progr. Polym. Sci., 2013, 38, (10–11), 1504–1542.
- Zaidi L., Bruzaud S., Bourmaud A., Médéric P., Kaci M. and Grohens Y.: J. Appl. Polym. Sci., 2010, 116, (3), 1357–1365.
- Shieh Y.-T., Twu Y.-K., Su C.-C., Lin R.-H. and Liu G.-L.: J. Polym. Sci. B: Polym. Phys., 2010, 48, (9), 983–989.
- Murariu M., Doumbia A., Bonnaud L., Dechief A. L., Paint Y., Ferreira M., Campagne C., Devaux E. and Dubois P.: Biomacromolecules, 2011, 12, (5), 1762–1771.
- Pluta M., Paul M.-A., Alexandre M. and Dubois P.: J. Polym. Sci. Part B: Polym. Phys., 2006, 44, (2), 299–311.
- Gorrasi G., Pantani R., Murariu M. and Dubois P.: Macromol. Mater. Eng., 2014, 299, (1), 104–115.
- Krikorian V. and Pochan D. J.: Chem. Mater., 2003, 15, (22), 4317–4324.
- Fukushima K., Tabuani D., Abbate C., Arena M. and Rizzarelli P.: Eur. Polym. J., 2011, 47, (2), 139–152.
- McDaniel J. B.: ‘Polylactic acid shrink films and methods of manufacturing same’, US7846517 B2, published 7 December 2010, Google Patents: 2010.
- Rothon R. N.: ‘Functional polymers and other modifiers’, in ‘Functional fillers for plastics’, 105–128; 2005, New York, Wiley-VCH.
- Osman M. A., Atallah A. and Suter U. W.: Polymer, 2004, 45, (4), 1177–1183.
- Su Z., Li Q., Liu Y., Hu G.-H. and Wu C.: J. Polym. Sci. B: Polym. Phys., 2009, 47, (20), 1971–1980.
- Song P., Wei Z., Liang J., Chen G. and Zhang W.: Polym. Eng. Sci., 2012, 52, (5), 1058–1068.
- Nam J. Y., Sinha Ray S. and Okamoto M.: Macromolecules, 2003, 36, (19), 7126–7131.
- Wang H. and Qiu Z.: Thermochim. Acta, 2012, 527, 40–46.
- Li Y., Han C., Bian J., Han L., Dong L. and Gao G.: Polym. Compos., 2012, 33, (10), 1719–1727.
- Ojijo V. and Sinha Ray S.: Progr. Polym. Sci., 2013, 38, (10–11), 1543–1589.
- Solarski S., Ferreira M., Devaux E., Fontaine G., Bachelet P., Bourbigot S., Delobel R., Coszach P., Murariu M., Da Silva Ferreira A., Alexandre M., Degee P. and Dubois P.: J. Appl. Polym. Sci., 2008, 109, (2), 841–851.
- Xiu H., Huang C., Bai H., Jiang J., Chen F., Deng H., Wang K., Zhang Q. and Fu Q.: Polymer, 2014, 55, (6), 1593–1600.
- Du M., Guo B. and Jia D.: Polym. Int., 2010, 59, (5), 574–582.