Abstract
Objective:
A recent pharmacokinetic study with buprenorphine transdermal patches showed similar systemic exposures of buprenorphine in subjects aged ≥75 and 50–60 years. The current prospective, open-label study aimed to verify this in a clinical setting by evaluating efficacy and safety of buprenorphine patches in patients with chronic osteoarthritis (OA) pain.
Methods:
Patients with chronic, moderate to severe osteoarthritic pain (hip and/or knee) were enrolled: 50–60 years (younger group, N = 65) and ≥75 years (elderly group, N = 57). After 2 weeks on paracetamol only, patients received buprenorphine patches (5–40 µg/h) for 12 weeks. Paracetamol rescue was provided. Primary endpoint was the Box-Scale-11 (BS-11) score for pain on average over the last week. WOMAC OA Index, EQ-5D, Patients’ and Investigators’ Global Assessment of Pain Relief, rescue medication use, sleep disturbance and quality of sleep were secondary efficacy endpoints.
Results:
Both groups showed a statistically significant (p < 0.0001) and clinically relevant change from baseline to last visit in BS-11 score, with no significant difference between groups. The least squares (LS) mean change from baseline was 2.20 in elderly and 1.87 in younger patients, with an age group difference of 0.33 (95% CI: −0.42, 1.07). Non-inferiority of the elderly versus the younger group was shown. Both age groups showed a significant improvement in WOMAC total score, patients’ overall health state (EQ-5D visual analogue scale) and sleep quality, and a significant reduction in rescue use and nights woken due to pain, with no significant differences between groups. Elderly patients tolerated buprenorphine patches at least as well as younger patients.
Conclusions:
Efficacy and tolerability of buprenorphine patches was demonstrated in chronic pain patients, regardless of age, supporting the conclusion that no age-related dose adjustment of transdermal buprenorphine is needed. A study limitation is lack of active control but no other opioid was appropriate in elderly patients or this indication.
Introduction
Buprenorphine is a low molecular weight, highly lipophilic, semi-synthetic opioid analgesicCitation1–3. Buprenorphine is available in parenteral, sublingual and transdermal formulations. It has been used in the clinical setting for approximately 30 years and has been shown to be effective for the relief of severe pain in a variety of therapeutic settings. There are two buprenorphine patches available, a 4 day patch and a 7 day patch. The 4 day (96 hour) patch is not available in Sweden where this study was carried out but, where it is available, it is indicated for moderate to severe cancer pain and severe pain which does not respond to non-opioid analgesics. The 7 day patch, however, is indicated for non-malignant pain of moderate intensity when an opioid is necessary for obtaining adequate analgesia. Buprenorphine transdermal patches used in the study described here contain buprenorphine embedded in an acylated benzyl acetate polymer matrix that allows for the delivery of a consistent and steady dose of buprenorphine with limited fluctuation over a 7 day periodCitation4. Clinical studies have shown buprenorphine transdermal patches to be effective in the treatment of moderate to severe osteoarthritis (OA) painCitation5–8.
Osteoarthritis is a degenerative disease with breakdown of the cartilage in the joints that leads to pain and loss of function. Most commonly affected joints are hips, knees, spine and fingers. It is a common disease in the elderly; World Health Organization figures estimate that, worldwide, 9.6% of men and 18.0% of women aged ≥60 years will have symptomatic OACitation9, with radiographic studies of US and European populations showing even higher ratesCitation10. It is reported that 81% of people with OA suffer from constant pain or are limited in their scope to perform everyday tasksCitation11.
Swedish guidelinesCitation12, in common with other countries such as the UKCitation13, have recommended paracetamol and/or non-steroidal anti-inflammatory drugs (NSAIDs) for the initial treatment of OA pain. However, published reports suggest that NSAID treatment among the elderly should be avoided due to the risk of gastric ulcers/bleeding, kidney dysfunction, increased blood pressure and heart failure in this populationCitation14–16.
If the initial treatment regimen does not provide optimal treatment of pain, the guidelines suggest second-line treatment with a high-potency opioid analgesic in a low doseCitation12,Citation17–20. Buprenorphine transdermal patches, which are available in low doses, fit the guideline recommendations in this respect. Buprenorphine patches are considered to be a suitable pain relief among elderly patients compared with other low/high potency opioids due to the pharmacokinetic profile and pharmaceutical form of administration, since no dose adjustment is needed for elderly patientsCitation21,Citation22 or patients with impaired renal functionCitation23–25, and because buprenorphine has been shown to have a favorable safety profile compared with other opioid analgesicsCitation26. Buprenorphine pharmacokinetics are also stable in patients with mild to moderate hepatic impairmentCitation27. To date, however, few studies have investigated the efficacy and safety of buprenorphine transdermal patches in this patient population.
A recently conducted pharmacokinetic study with buprenorphine transdermal patches compared two age groups: 50–60 years versus ≥75 yearsCitation28. The results revealed a similar systemic exposure of buprenorphine in both age groups with a reasonably high level of variability seen in the individual plasma profiles. This outcome confirmed that there is no need to adjust the dose due to the individual’s age from a pharmacokinetic point of view. The aim of the present study was to verify the results of the previous pharmacokinetic study in a clinical setting by evaluating the efficacy and safety of buprenorphine transdermal patches in patients with chronic, moderate to severe OA pain in the same two age groups (50–60 years and ≥75 years). Non-inferiority of buprenorphine transdermal patches in elderly patients aged ≥75 years compared with younger patients aged 50–60 years with regard to the primary endpoint, BS-11 pain scores, was to be inferred if the lower 95% confidence interval (CI) for the age group difference was less than −1.5.
Patients and methods
Study design
This was a prospective, multi-center, open-label, multiple-dose, age-group controlled study conducted at six study sites in Sweden (EudraCT number: 2010-020748-37). The protocol for this study was approved by the local Independent Ethics Committee in Gothenburg (registration number 548-10). The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelineCitation29, the Declaration of HelsinkiCitation30, and applicable regulatory requirements. Written informed consent was obtained from all participating patients.
The study comprised a screening visit to assess patient eligibility, followed by a 2 week screening period during which patients received paracetamol (≥2 g/day but ≤4 g/day), followed by a 12 week treatment period during which patients received buprenorphine transdermal patch 5–40 µg/h, and a 1 week follow-up period following the end of study treatment.
Participants
A total of 122 patients with chronic OA pain of the hip and/or knee were enrolled between February 2011 and August 2012. In accordance with the aim of the study, to investigate the safety and efficacy of buprenorphine patches in elderly patients compared with younger patients, male and female patients were enrolled in two age-groups: 50–60 years and ≥75 years.
To be eligible for inclusion in the study, patients had to have a clinical diagnosis of OA in a knee and/or hip fulfilling American College of Rheumatology (ACR) criteria with radiographic evidence not older than 1 year, and should have been willing to stop their previous OA pain treatment and replace it with paracetamol (maximum tolerated daily dose ≥2 g but ≤ 4 g) during the 2 week screening period. Patients with primary and secondary OA were eligible for the study. At the end of the screening period, patients had to be experiencing sub-optimal analgesia with moderate to severe pain (confirmed by a Box-Scale-11 [BS-11] score ≥4 for their pain on average at their primary OA site during the last 7 days prior to the baseline visit). Patients were excluded from the study if their average BS-11 pain score prior to baseline was <4, if they had previously been treated with high potency opioid analgesics (including buprenorphine) for their OA pain, if they had received a regular dose of tramadol, codeine or dextropropoxiphene for >1 week within 1 month before the screening visit. Patients were also excluded if they required NSAIDs (except aspirin for cardiovascular indications) or COX-2 inhibitors during the study, or if they had a history of/ongoing chronic condition(s) other than OA (e.g. frequent migraine) that required frequent analgesic therapy.
Study treatment
At the screening visit patients discontinued any current analgesic treatment and received study paracetamol (maximum tolerated dose ≥2 g/day but ≤4 g/day) during the 2 week screening period. Patients with a BS-11 score ≥4 for their pain on average during the last 7 days of the screening period in their primary OA joint started open-label treatment with buprenorphine transdermal patches at the baseline visit. All patients received buprenorphine transdermal patches since no comparative treatment was considered suitable or ethical for the age groups to be studied due to the pharmacokinetic profiles of NSAIDs and other low/high potency opioids. Patients received study treatment for 12 weeks, and new patches were applied every 7 days. The 12 week treatment period was in accordance with the EMEA guideline for the conduct of clinical trials in patients with chronic painCitation31.
Patients started on a buprenorphine patch strength of 5 µg/h and were titrated, if necessary, to a maximum of 40 µg/h to achieve stable pain control. Dose levels were 5, 10, 15, 20, 25, 30 and 40 µg/h; each dose comprising one or two patches of strength 5, 10 or 20 µg/h. An effective and tolerated dose was determined based on BS-11 pain scores, amount of rescue medication used and adverse events. Patients were to be treated for between 7 and 14 days before up-titration to the next dose was considered; however, the dose could be increased earlier if the patient was not receiving adequate analgesia and had experienced no moderate to severe opioid-related adverse events for 4 days. Dose titration (up-titration or down-titration) was allowed throughout the treatment period. Patches were applied for 7 days of continuous wear on one of the following areas (right or left): upper arm, anterior thorax, lower anterior axillary line and upper back. Once the patch had been applied to a particular site, the patient was instructed not to use the same site again for 3–4 weeks.
Paracetamol (as 500 mg tablets) was available as rescue analgesic therapy throughout the study. Patients were advised to take 1 g for breakthrough pain up to a daily maximum dose of 4 g. Aspirin for cardiovascular indications (up to 320 mg/day), a stable dose of glucosamine and a stable physiotherapy regimen were also permitted, but any other analgesic medication and steroids were prohibited during the study.
Efficacy assessments
Study visits were at screening (14 days before the baseline visit), baseline (day of first patch application) and weeks 1, 2, 4, 6, 8 and 12. A follow-up visit was performed 1 week after the last patch was removed.
The primary endpoint was the BS-11 pain score for pain on average during the last week. Patients recorded their BS-11 score for pain on average during the day in a patient diary each evening on a scale of 0 (no pain) to 10 (pain as bad as you can imagine) in response to the following question: ‘Overall, what has your pain been like in the primary OA joint today?’ The BS-11 score is a commonly used and accepted pain measure in clinical studiesCitation32–34 and has previously been used to measure pain intensity in patients with OACitation5,Citation6.
The following secondary efficacy variables were also investigated: Western Ontario and McMaster Universities OA Index (WOMAC OA Index, Version NRS 3.1), European Quality of Life Health Questionnaire (EQ-5D), sleep disturbance and quality of sleep questions, Patients’ Global Assessment of Pain Relief, Investigators’ Global Assessment of Pain Relief, and rescue medication use.
The WOMAC OA Index is a standard measure for clinical studies in patients with OA of the hip and knee that measures symptoms and physical functioning using 24 specific items relating to pain, stiffness and physical function. The EQ-5D questionnaire includes the EQ-5D-3L descriptive system, comprising the following dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has three levels: no problems, some problems and extreme problems. The EQ-5D also includes the EQ visual analogue scale (EQVAS), which records the respondent’s self-rated health on a vertical, visual analogue scale from 0 (‘the worst health you can imagine’) to 100 (‘the best health you can imagine’)Citation35. The WOMAC OA Index and EQ-5D were completed at screening, baseline and the week 12/completion visit.
Sleep disturbance and quality of sleep were assessed at all visits using the questions ‘How many nights have you woken due to pain during the past 7 nights?’ and ‘Please rate the quality of your sleep over the past 7 nights’ (very poor, poor, fair, good, very good). Patients’ Global Assessment of Pain Relief was recorded at the week 12/completion visit using the questions ‘How would you rate the study medication at relieving your pain?’ (very poor, poor, fair, good, very good), ‘How would you rate the study medication at relieving your pain compared to your regular (pre-study) medication?’ (much worse, worse, same, better, much better) and ‘How would you rate the study medication overall as a treatment for your pain (taking into account the quality of pain relief achieved and any adverse events you may have encountered)?’ (very poor, poor, fair, good, very good). The Investigator’s Global Assessment of Pain Relief was also completed by investigators at the week 12/completion visit using the same questions with regards to their patient’s pain.
Patients recorded their daily intake of paracetamol (rescue medication) in the patient diary.
Safety assessments
Vital sign measurements (supine respiratory rate, blood pressure and pulse rate), blood chemistry tests and a physical examination were performed at screening and the week 12/completion visit; vital signs were also collected at the follow-up visit. Adverse events were recorded throughout the study, including details of severity and relationship to study medication. The patch application site was assessed for erythema and edema by study site staff after removal of the patch at each visit during the treatment period.
Sample size estimation
The sample size for the study, based on two sample t-tests, was calculated based on a non-inferiority margin for BS-11 score of −1.5, an expected age group difference of 0, a standard deviation (SD) of 2 and 90% power. Using this calculation a total of 78 patients (39 per age group) were required for analysis; based on withdrawal rates in previous studies it was planned to enroll 102 patients (51 per age group).
Statistical methods
The primary objective of the study was to show non-inferiority of buprenorphine transdermal patch in elderly patients aged ≥75 years compared with patients aged 50–60 years with regard to the primary endpoint, BS-11 pain scores for pain on average during the last week. An analysis of covariance (ANCOVA) model, including age group and study site as factors and baseline BS-11 pain score and gender as covariates, was used to compare the change from baseline to last visit (i.e. the week 12 or completion visit) in BS-11 pain scores for the two age groups. The difference in least-square (LS) means for the ≥75 years age group compared with the 50–60 years age group and the two-sided 95% CI for the difference were estimated. Non-inferiority of the elderly age group was to be inferred if the lower 95% CI for the age group difference was less than −1.5. A lower non-inferiority margin of −1.5 for the difference between the age groups in BS-11 score was considered to be clinically acceptable, based on previous studies with buprenorphine transdermal patchesCitation5,Citation6 and in line with the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) recommendationsCitation36. The test of non-inferiority was performed at a one-sided 0.025 level.
The differences between the age groups in the change from baseline to last visit for the WOMAC OA Index (pain, stiffness and physical function subscales and total score), EQ-VAS, EQ-5D Index, the sleep disturbance question and the sleep quality question were analyzed using the Wilcoxon rank sum test. Within-age group differences for these endpoints were tested using a Wilcoxon signed-rank test. The differences between the age groups in the EQ-5D-3L, Patients’ Global Assessment of Pain Relief and Investigator’s Global Assessment of Pain Relief at the last visit were analyzed using chi-square tests without continuity correction. Rescue medication use was summarized using descriptive statistics. All statistical tests performed on the secondary efficacy endpoints were interpreted at the 5% significance level (two-tailed).
Efficacy analyses were performed on the full analysis set (FAS; patients who received buprenorphine patch or study paracetamol and had at least one post-baseline efficacy assessment) and the per-protocol population (PP population; patients in the FAS population without major protocol violations). Both the FAS and the PP population were required to show non-inferiority in the primary efficacy analysis for non-inferiority to be concluded between the age groups. The baseline visit was visit 2 (end of screening period/start of treatment period). Last observation carried forward (LOCF) was used to replace missing data for all efficacy analyses. All analyses performed and presented here were planned prior to database lock, with the exception of the within-age group analyses performed on the secondary efficacy endpoints.
Safety results were descriptively summarized for the safety population (patients who received buprenorphine patch or study paracetamol and had any post-baseline adverse event information). Statistical programming and analyses were performed using SAS Version 9.2.
Results
Study subjects
Out of 199 patients screening for eligibility, 122 were enrolled into the study. Sixty-five patients were aged 50–60 years (hereafter referred to as the younger group) and 57 patients were aged ≥75 years (hereafter referred to as the elderly group). In the younger group, 40 patients completed the study and 25 were withdrawn; 16 due to adverse events, 7 withdrew their consent and 2 due to lack of therapeutic effect. In the elderly group, 39 patients completed the study and 18 were withdrawn; 12 due to adverse events, 3 withdrew their consent, 2 due to protocol violation and 1 due to lack of therapeutic effect.
The mean age of patients was 55 years (range: 50–60 years) in the younger group and 80 years (range: 75–93 years) in the elderly group. Approximately 60% of all patients enrolled were female, and all patients were Caucasian. Comorbidities were reported for more patients in the elderly group (95%) compared with the younger group (79%).
The primary OA joint was the knee for 69% of patients in the younger group and 74% of patients in the elderly group; the OA radiographic grade was II or III for the majority of these patients (91% in the younger group and 74% of patients in the elderly group). The primary OA joint was the hip for 31% of younger patients and 26% of elderly patients; the OA radiographic grade was II or III for the majority of these patients (approximately 85% in both age groups). As may be expected, overall concomitant medication use was higher in the elderly group than the younger group. A total of 84% of patients had used analgesic treatment prior to entering the study; these were mainly anilides (used by 62% of younger patients and 79% of elderly patients), topical NSAIDs (used by approximately 25% of patients in both age groups) and acetic acid derivatives (taken by 35% of younger patients and 21% of elderly patients).
All patients were included in the FAS and the safety population; results for these populations are presented below (FAS for efficacy results and safety population for safety results). A total of 42 patients (24 in the younger group and 18 in the elderly group) were excluded from the PP population due to protocol violations. Results for the PP population are not presented here but supported results for the FAS for all efficacy endpoints, except the WOMAC pain subscale.
Exposure
Percentage compliance was calculated as (number of days on study patch/84) × 100. Mean (SD) compliance was 78% (37.5%) in the younger group and 79% (32.6%) in the elderly group, i.e. 66 days in both age groups. Approximately 64% of patients in both age groups received study treatment for ≥70 days. The maximum buprenorphine patch dose was between 5 and 20 µg/h for the majority of patients, with only 7 patients (11%) in the younger group and 3 patients (5%) in the elderly group receiving a dose of more than 20 µg/h. The median optimal dose (defined as the last dose the patient received) for patients in the PP population who completed the study was 15 µg/h in the younger group and 10 µg/h in the elderly group and the mean optimal dose was 14.5 µg/h in the younger group and 11.3 µg/h in the elderly group (post hoc analysis). These values are representative of use of buprenorphine patches in the clinical setting.
Primary outcome measure
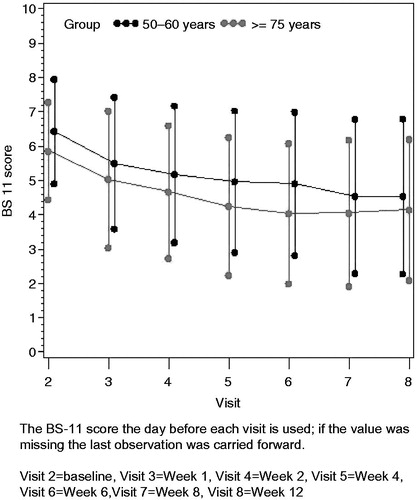
All data presented here represent the average BS-11 scores over the 7 days before each visit. The BS-11 score at baseline was significantly higher in the younger group compared with the elderly group (6.49 vs. 5.91, p = 0.008) (). Both age groups showed a statistically significant change from baseline to last visit in BS-11 score, with a LS mean change (standard error) of 1.87 (0.305) for the younger group and 2.20 (0.307) for the elderly group (). The difference in LS means for the elderly group compared with the younger group was 0.33 (95% CI: −0.42, 1.07; p = 0.383, ), therefore the elderly group was non-inferior to the younger group since the lower limit of the 95% CI was greater than the pre-defined non-inferiority limit of −1.5. This conclusion was supported by results for the PP population (difference in LS means 0.44; 95% CI: −0.55, 1.42; p = 0.380). Although baseline BS-11 scores were higher for the younger group than the elderly group, shows that the absolute change and the percentage change from baseline were very similar in both age groups (1.99/30.1% in the younger group and 1.87/31.7% in the elderly group). Larger reductions were observed in the PP population (2.37/35.9% in the younger group and 2.09/35.6% in the elderly group). The reduction in BS-11 pain score with buprenorphine patch was sustained over the entire 12 week treatment period, with a gradual reduction in BS-11 scores over time (; note that the figure shows BS-11 score on the day before each visit, and not average scores over 7 days).
Table 1. BS-11 pain scores at baseline and last visit, and change from baseline to the last visit: full analysis population.
Table 2. Results of ANCOVA model for change from baseline to the last visit in BS-11 pain score: full analysis population.
Secondary outcome measures
WOMAC OA Index
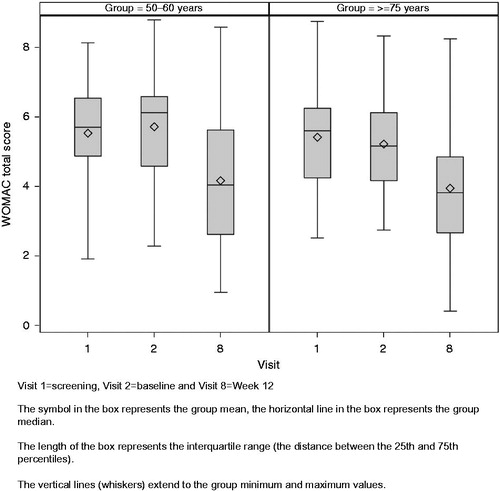
The WOMAC OA Index is a disease-specific instrument used to measure symptoms (pain and stiffness) and physical functioning of patients with OA of the hip and knee. There was a significant improvement in WOMAC total score from baseline to last visit in both the younger group (mean change [SD]: −1.55 [1.696]; 95% CI: −1.97, −1.13; p < 0.0001) and the elderly group (mean change [SD]: −1.28 [1.569]; 95% CI: −1.69, −0.86; p < 0.0001). The improvement is shown as a boxplot in . Marked improvements were also observed for both age groups in terms of the WOMAC pain, stiffness and physical functioning subscales, although the within-age group changes from baseline for the WOMAC subscales were not subjected to statistical analysis (). There were no significant differences between the age groups for the change from baseline to last visit in WOMAC total score (p = 0.446), pain subscale (p = 0.100), stiffness subscale (p = 0.904) or physical function subscale (p = 0.578). Although not significant, the mean (SD) change from baseline to last visit in the pain subscale was larger in the younger group (−1.93 [2.036]) compared with the elderly group (−1.36 [1.777]) for the FAS; the age group difference reached statistical significance for the PP population (mean [SD] change −2.60 [1.884] in the younger group and −1.52 [1.653] in the elderly group, p < 0.003 for age group difference). The smaller change from baseline in the WOMAC pain subscale score for the elderly group for the FAS is probably due to a lower mean baseline score (5.17 [1.568] compared to 5.90 [1.340] for the younger group), meaning the elderly group had less scope for large improvements from baseline. The mean score for the pain subscale at the last visit was similar for each age group (3.97 [2.118] for the younger group and 3.81 [1.932] for the elderly group).
Table 3. Change from baseline to the last visit in WOMAC total score and subscale scores: full analysis population.
EQ-5D
Responses in each of the EQ-5D dimensions at the last visit were broadly comparable across the two age groups, except that the proportion of patients reporting some problems with usual activities and anxiety/depression was higher in the younger group (49% and 39%, respectively) than the elderly group (30% for both dimensions) (). All except two patients in each group reported at least some problems with pain/discomfort. No significant differences between the age groups were identified for any of the EQ-5D dimensions.
Table 4. EQ-5D dimension results at the last visit: full analysis population.
There were significant improvements in patients’ overall health state from baseline to last visit for both age groups indicated by the EQ-5D VAS results (). The EQ-5D VAS score increased by an average of 6.8 in both groups. The age group difference in the change from baseline to last visit in EQ-5D VAS was not statistically significant.
Table 5. Change from baseline to the last visit in EQ-5D VAS: full analysis population.
Sleep disturbance/sleep quality
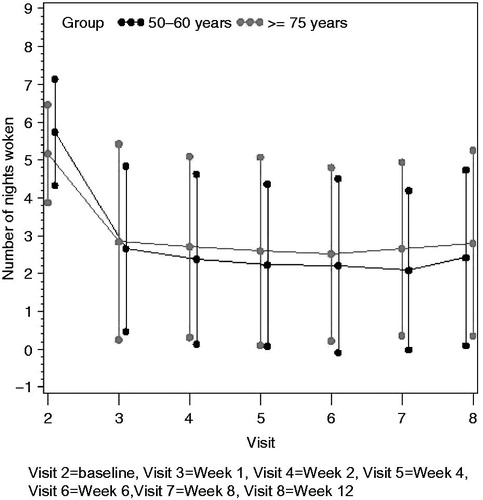
At baseline, the mean number of nights the patient had sleep disturbances due to pain over the past 7 nights was significantly higher in the younger group than the elderly group (mean [SD] 4.11 [2.70] vs. 2.77 [2.92]; p = 0.004). There was a significant decrease in the mean number of nights the patient had sleep disturbances due to pain over the past 7 days from baseline to the last visit in both age groups. The mean (SD) change from baseline was −1.91 (2.62) in the younger group (95% CI: −2.56, −1.26; p < 0.0001) and −1.67 (2.55) in the elderly group (95% CI: −2.34, −0.99; p < 0.0001). There were no significant differences between the age groups regarding the change from baseline to last visit in the number of nights with sleep disturbances due to pain (p = 0.761). The mean number of nights with sleep disturbances due to pain is shown by visit in .
Figure 3. Mean number of nights with sleep disturbances due to pain in last 7 nights by visit: full analysis population.
The proportion of patients rating sleep quality as ‘good’ or ‘very good’ increased from 23% to 49% in the younger group and from 37% to 61% in the elderly group from baseline to last visit. The overall change in sleep quality rating in the elderly group was significant (p = 0.0037) but the change in the younger group did not reach statistical significance (p = 0.057); there was no significant difference in the change from baseline in sleep quality ratings between the age groups (p = 0.394).
Patients’ and Investigators’ Global Assessment of Pain Relief
There were no significant differences between the age groups in responses to the questions of the Patients’ and Investigators’ Global Assessment of Pain Relief (). The majority of patients and investigators rated buprenorphine patches as ‘good’ or ‘very good’ at relieving pain (65% and 68%, respectively, in the younger group and 61% and 59%, respectively, in the elderly group).
Table 6. Patients’ and Investigators’ Global Assessment of Pain Relief at the last visit: full analysis population.
Rescue medication use
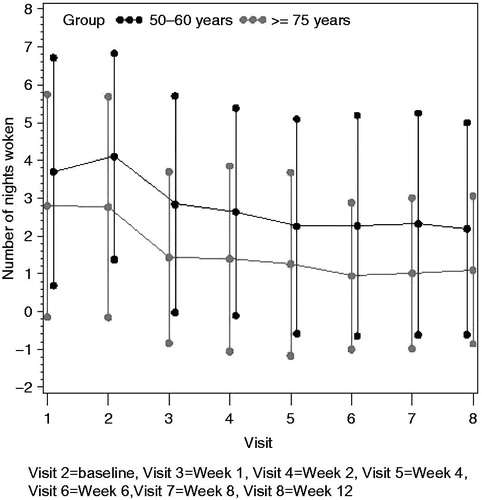
At baseline (end of the screening period), the mean (SD) number of tablets of rescue medication (paracetamol 500 mg) taken each day was 5.7 (1.41) in the younger group and 5.2 (1.29) in the elderly group. This had dropped to 2.7 tablets (2.19) in the younger group and 2.8 tablets (2.59) in the elderly group by the end of week 1 and mean values remained in the range 2.1 to 2.8 tablets at all subsequent visits ().
Safety results
The proportion of patients reporting adverse events was similar for the elderly group (53 patients [93%]) compared with the younger group (61 patients [94%]). Patients in the younger group reported almost twice as many adverse events (338) as patients in the elderly group (175), but the number of unique adverse events was similar for both groups (72 and 65, respectively), indicating that patients in the younger group reported the same type of adverse event on multiple occasions. The most common adverse events reported in both age groups were nausea, dizziness, fatigue, constipation and headache, which are consistent with the expected side-effect profile for opioid analgesics (). The proportion of patients with treatment-related adverse events (those adverse events considered possibly, probably, or definitely related) was higher in the younger group (92%) than the elderly group (77%), as was the proportion of adverse events that were considered treatment-related (279/338, 83% vs. 115/175, 66%). Nausea and dizziness were most frequently considered to be treatment related. Most adverse events (90% in the younger group and 95% in the elderly group) were mild or moderate in severity.
Table 7. Incidence of common adverse events by MedDRA system organ class and preferred term: safety population.
Five patients in the elderly group experienced a total of 10 serious adverse events; pancreatitis, cholelithiasis, foot fracture, joint dislocation, upper limb fracture and pulmonary embolism (all rated as not related or unlikely to be related by the investigators), and increased alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and gamma-glutamyltransferase (all probably related and occurring in the same patient). No serious adverse events were reported in the younger group. Eleven percent of patients in the younger group and 10% of patients in the elderly group required a reduction in study medication dose and 17% of the younger group and 15% of the elderly group were discontinued from study treatment due to adverse events. Adverse events leading to discontinuation of more than two patients in either age group were nausea, dizziness, constipation, vomiting, fatigue and erythema; all of these adverse events led to discontinuation of more patients in the younger group than the elderly group, except constipation, which more frequently led to discontinuation of elderly patients (five patients vs. two patients).
Patch site reactions were assessed by study site staff at each visit. The majority of patients in both age groups did not show any evidence of irritation. Between 11% and 27% of patients in the younger group and between 2% and 9% of patients in the elderly group showed minimal erythema at each visit. The overall proportion of patients with definite/intense erythema was <5% at each visit, except the last visit where it was 8%. All measured clinical parameters (vital signs, body weight, blood chemistry parameters) remained relatively constant between baseline and last visit.
Discussion
The current study demonstrated similar good efficacy and tolerability of buprenorphine patches in elderly patients aged ≥75 years to younger patients aged 50–60 years with chronic OA pain.
Ageing is a source of variability in drug response and, as a result, the usual adult dosage regimen may need to be modified, particularly in the elderly, if optimal therapy is to be achieved. However, a previous pharmacokinetic study with buprenorphine transdermal patch 5 µg/h for 14 days showed only slightly lower systemic exposure of buprenorphine at a steady state in patients aged ≥75 years (mean [SD] AUCtau 9940 pg·h/mL [4827 pg·h/mL]) than in patients aged 50–60 years (mean [SD] AUCtau 11,309 pg·h/mL [3670 pg·h/mL]), suggesting no need to adjust the dose due to the patient’s age, at least from a pharmacokinetic point of viewCitation28.
The current study aimed to verify the results of the pharmacokinetic study in a clinical setting. An open study design was chosen to evaluate the buprenorphine transdermal patches in a clinically relevant manner. A total of 122 patients were enrolled (65 aged 50–60 years and 57 aged ≥75 years) and 40 (62%) and 39 (68%), respectively, completed the study. Patients in both age groups showed treatment compliance for opioid analgesic treatment, receiving 66 of the planned 84 days (78%) of treatment with buprenorphine transdermal patches on average.
In terms of the primary endpoint, both age groups showed a statistically significant (p < 0.0001) change from baseline to last visit in BS-11 score, with no difference between the age groups (p = 0.383). The LS mean change from baseline from the ANCOVA model (adjusting for baseline BS-11 score) was 2.20 in elderly patients and 1.87 in younger patients, with an age group difference of 0.33 (95% CI: −0.42, 1.07). Non-inferiority of the elderly group compared with the younger group was shown since the lower limit of the 95% CI was greater than the pre-defined non-inferiority limit of −1.5. The robustness of the primary analysis on the FAS was supported by similar results for the PP population.
The primary endpoint focused on weekly mean changes of the patient’s average daily pain measured using the BS-11 pain score. Farrar et al.Citation37 analyzed the pooled results of 10 placebo-controlled studies involving patients with chronic pain syndromes (OA, diabetic neuropathy, postherpetic neuralgia, chronic low back pain and fibromyalgia) to substantiate the association between change in pain intensity on an 11-point numeric rating scale and an improvement in quantifiable measures of clinical status. The analysis showed that, on average, a reduction of approximately 2 points from baseline on the 11 point pain rating scale (equivalent to a 30% reduction on pain severity from baseline) corresponds to a clinically meaningful improvement. A subsequent studyCitation38 has also demonstrated that on a 0 to 10 numeric rating scale of pain intensity for patient-reported ‘average’ pain, a percentage change of 34% most accurately represented a clinically important difference, namely the Patient Global Impression of Improvement (PGI-I) category of ‘much better’ or higher. The BS-11 uses a similar 11 point scale to that used by Farrar et al.Citation37,Citation38, but on a box scale. The non-adjusted mean reduction in BS-11 pain scores in the FAS in the current study was 1.99 (30.1% reduction from baseline) in the younger group and 1.87 (31.7% reduction from baseline) in the elderly group, with larger mean reductions observed in the PP population (2.37/35.9% in the younger group and 2.09/35.6% in the elderly group). Although the numerical reduction from baseline was slightly larger in the younger group, baseline scores were higher for the younger group than the elderly group (6.49 vs. 5.91, respectively) resulting in very similar percentage reductions from baseline for both age groups. Overall, both age groups showed a >30% mean reduction in pain intensity compared to baseline, which can be considered to be clinically significant.
The statistically significant and clinically relevant changes from baseline to last visit for the primary endpoint were further supported by significant improvements from baseline to the last visit in WOMAC total score, patients’ overall health state (EQ-5D Index and EQ-5D VAS) and sleep quality, and significant reductions in the mean number of nights with sleep disturbances due to pain and rescue medication use for both age groups. This provides strong evidence that patients experienced greater pain relief following the switch to buprenorphine patch compared with the maximal tolerated dose of paracetamol taken during the screening period. No significant differences were found between the age groups for any of the secondary efficacy endpoints, with the exception of WOMAC pain subscale score in the PP population, which showed a significantly larger decrease in the younger group. However, since several statistical significance tests were performed and no adjustment for multiplicity was implemented, the results from the secondary efficacy analyses should be interpreted with some caution.
A limitation of the study is the lack of any active control, but no other opioid analgesics were considered appropriate due to their pharmacokinetic profile in the elderly or due to the indication, strong opioids being indicated for severe pain. Although the duration of the study was limited to 12 weeks, this was in accordance with EMEA guidelinesCitation31, and the study was performed at the patients’ usual clinic and closely reflected normal clinical practice. It is also noted that the elderly group of patients included in this study had additional co-morbidity and concomitant medications, as they would in normal practice, and patients were not excluded from the study for these reasons. Overall the results of this study are expected to translate well into a real-life clinical setting.
The results from the current study are supported by the results from several other studies of transdermal buprenorphine in the elderlyCitation39. Likar et al.Citation40 studied the efficacy and safety of buprenorphine transdermal patches in patients with moderate to severe chronic pain in three age groups: ≥65 years, 51 to 64 years and ≤50 years. Buprenorphine patches were shown to be at least as effective at relieving pain in the elderly group (≥65 years) as in patients in the two younger age groups, with no age-related differences in safety or tolerability. Buprenorphine transdermal patches were also found to be equally effective for patients with chronic pain aged ≤65 years, those aged between 65 and 75 years, and those aged ≥75 years in a prospective, observational studyCitation41. A further open, observational study of over 13,000 patients with moderate to severe cancer or non-cancer pain in Germany found buprenorphine transdermal patches provided effective, sustained and dose-dependent analgesia irrespective of the patient’s ageCitation42.
The incidence of adverse events was relatively high in the present study, but this is not unexpected given the slightly older population of generally opioid-naive patients, and the events reported were consistent with the expected adverse event profile of buprenorphine transdermal patchesCitation21 and opioid analgesics in general. The number of adverse events reported was higher in the younger group than the elderly group, indicating that elderly individuals tolerated the buprenorphine patches at least as well as the younger individuals. Although five elderly patients reported serious adverse events during the study, only one had events (laboratory test abnormalities) with a likely relationship to study treatment. Adverse events were generally mild or moderate and patients demonstrated good treatment persistence compared with other opioidsCitation43, suggesting that the benefits of treatment in terms of pain relief and improved function outweighed the effect of adverse events. Other studies have also shown no increase in adverse events with increasing ageCitation41,Citation42. In common with the current study, more adverse events were reported by subjects in the younger group (50–60 years) than the elderly group (≥75 years) in the previous buprenorphine pharmacokinetic studyCitation28.
Swedish guidelines for treatment of chronic pain in the elderly state that if paracetamol is not giving enough pain relief, a high-potency opioid in low doses should be considered. The guidelines state that weak opioids, such as tramadol and codeine, should be avoided for long-term treatment among the elderly due to the risk of side effectsCitation19. It has already been established that, unlike other opioids, buprenorphine pharmacokinetics are not altered by increasing ageCitation28. This is further strengthened by the present study. The risk of respiratory depression is lower than with other opioidsCitation44, and buprenorphine is probably not associated with immunosuppression and does not activate the hypothalamic–pituitary axisCitation45. In support of this, Pergolizzi et al.Citation46 describes buprenorphine’s effect on the immune system as ‘neutral’ and that opioid agonists vary in their influence on the HPA axis. Polypharmacy is common in the elderly and drug–drug interactions through cytochrome P450 enzymes are common in patients who are using multiple medicationsCitation47; however, buprenorphine is rapidly conjugated, and glucuronidation is associated with few drug interactionsCitation48. Transdermal buprenorphine formulations have improved the clinical use of buprenorphine, offering a convenient form of administration with a continuous release rate that is not affected by the absorption rate in the gut, which can be physiologically impaired in elderly patients. All these factors combined indicate that buprenorphine patches can be considered a particularly suitable method of pain relief in the elderly.
Data from the current and previous studies show no difference in analgesic efficacy and safety of buprenorphine transdermal patches in elderly patients compared with younger patients, confirming buprenorphine to be well suited for chronic pain management in the elderly patient population, with no requirement for dose adjustment due to age.
Conclusions
The study demonstrated good efficacy and tolerability of buprenorphine patches in patients with chronic OA pain regardless of age, and supports the conclusions from the previous pharmacokinetic study of buprenorphine transdermal patches in patients aged 50–60 years and ≥75 years, that no dose adjustment of transdermal buprenorphine is needed due to age in a clinical setting.
Transparency
Declaration of funding
This study was sponsored by Mundipharma AB, Gothenburg, Sweden.
Declaration of financial/other relationships
J.K., A.S., and B.G.A. have disclosed that they have no significant relationships with or financial interests in any commercial companies related to this study or article. A.C.B. has disclosed that she is an employee of Mundipharma.
CMRO peer reviewers may have received honoraria for their review work. The peer reviewers on this manuscript have disclosed that they have no relevant financial relationships.
Acknowledgments
The authors thank the participating study sites in Sweden for their contribution: Dr Bengt-Olov Tengmark, Stockholm; Dr Katarina Berndtsson Blom, Skene; and Dr Anders Kempe, Härnösand; and Norma CRO, Lund, for statistical analyses.
This manuscript was written with the assistance of Karen Paine, Scientific Editorial, in accordance with CONSORT and European Medical Writers Association (EMWA) guidelines.
Previous presentations: Karlsson J, Söderström A, Berggren A-C. Evaluation of Buprenorphine TransDermal System (BTDS) among elderly subjects with chronic, moderate to severe osteoarthritis (OA) pain of the hip and/or knee. Poster presented at: British Geriatrics Society (BGS) meeting, 17–19 April 2013. Poster 52.
References
- Heel RC, Brogden RN, Speight TM, et al. Buprenorphine: a review of its pharmacological properties and therapeutic efficacy. Drugs 1979;17:81-110
- Lewis JW. Buprenorphine. Drug Alcohol Depend 1985;14:363-72
- Hoskin PJ, Hanks GW. Opioid agonist–antagonist drugs in acute and chronic pain states. Drugs 1991;41:326-44
- Budd K. Buprenorphine and the transdermal system: the ideal match in pain management. Int J Clin Pract Suppl 2003;133:9-14, discussion 23-4
- Conaghan PG, O’Brien CM, Wilson M, et al. Transdermal buprenorphine plus oral paracetamol vs an oral codeine–paracetamol combination for osteoarthritis of hip and/or knee: a randomised trial. Osteoarthritis Cartilage 2011;19:930-8
- Karlsson M, Berggren AC. Efficacy and safety of low-dose transdermal buprenorphine patches (5, 10 and 20 microg/h) versus prolonged release tramadol tablets (75, 100, 150 and 200 mg) in subjects with chronic osteoarthritis pain: a 12-week, randomized, open-label, controlled, parallel-group noninferiority study. Clin Ther 2009;31:503-13
- James IG, O’Brien CM, McDonald CJ. A randomised, double-blind, double-dummy comparison of the efficacy and tolerability of low dose transdermal buprenorphine (BuTran s sevenday patches) with buprenorphine sublingual tablets (Temgesic) in patients with osteoarthritis pain. J Pain Symptom Manage 2010;40:266-78
- Breivik H, Ljosaa TM, Stengaard-Pedersen K, et al. A 6-months, randomised, placebo-controlled evaluation of efficacy and tolerability of a low-dose 7-day buprenorphine transdermal patch in osteoarthritis subjects naïve to potent opioids. Scand J Pain 2010;1:122-41
- Murray CJL, Lopez AD, eds. The Global Burden of Disease. A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. Cambridge (MA): Harvard School of Public Health on behalf of the World Health Organization and The World Bank, 1996
- Symmons D, Mathers C, Pfleger B. Global burden of osteoarthritis in the year 2000. Geneva: World Health Organization, 2003. Available at: www.who.int/healthinfo/statistics/bod_osteoarthritis.pdf [Last accessed 29 April 2013]
- Arthritis Care. OA Nation: the most comprehensive UK report of people with osteoarthritis. Available at: http://www.arthritiscare.org.uk/PublicationsandResources/Forhealthprofessionals/OANation/Downloads/main_content/@114177 [Last accessed 29 April 2013]
- Information from the Medical Products Agency. Treatment of osteoarthritis: treatment recommendations [in Swedish]. 2004;3(15):19-25. Available at: www.mpa.se [Last accessed 16 December 2013]
- NICE Clinical Guidelines for Care and Management in Adults – Osteoarthritis. Available at: http://www.nice.org.uk/nicemedia/pdf/CG59NICEguideline.pdf [Last accessed 29 April 2013]
- The Swedish Council on Technology Assessment in Health Care. Pharmaceutical treatments among the elderly – how can it be improved? [in Swedish]. Stockholm, Sweden: SBU, 2009
- American Geriatric Society (AGS) Panel on pharmacological management of persistent pain in older persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009;57:1331-46
- Page J, Henry D. Consumption of NSAIDs and the development of congestive heart failure in elderly patients: an underrecognized public health problem. Arch Intern Med 2000;160:777-84
- Information from the Medical Products Agency. Use of opioids for chronic non-malignant pain -- recommendations [in Swedish]. 2002;1:17-30 . Available at: www.mpa.se [Last accessed 16 December 2013]
- The Swedish Council on Technology Assessment in Health Care. Methods for treatment of chronic pain [in Swedish]. Stockholm, Sweden: SBU, 2006
- The National Board of Health and Welfare. Swedish guidelines 2010 [in Swedish]: Indikatorer för god läkemedelsterapi hos äldre. 2010, pp 61-65. Available at: www.socialstyrelsen.se [Last accessed 16 December 2013]
- European Guidelines for primary care management of chronic osteoarthritis pain. Available at: http://eGuidelines.co.uk [Last accessed 16 December 2013]
- Norspan Transdermal Patches. Summary of Product Characteristics; November 2009. Available at www.mpa.se [Last accessed 16 December 2013]
- Vadivelu N, Hines RL. Management of chronic pain in the elderly: focus on transdermal buprenorphine. Clin Interv Aging 2008;3:421-30
- Brewster D, Humphrey MJ, McLeavy MA. Biliary excretion, metabolism and enterohepatic circulation of buprenorphine. Xenobiotica 1981;11:189-96
- Cone EJ, Gorodetzky CW, Yousefnejad D, et al. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos 1984;12:577-81
- Filitz J, Griessinger N, Sittl R, et al. Effects of intermittent hemodialysis on buprenorphine and norbuprenorphine plasma concentrations in chronic pain participants treated with transdermal buprenorphine. Eur J Pain 2006;10:743-8
- Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Cline Pharmacol Ther 1994;55:569-80
- Johnson RE, Fudala PJ, Payne R. Buprenorphine: considerations for pain management. J Pain Symptom Manage 2005;29:297-326
- Al-Tawil N, Odar-Cederlöf I, Berggren AC, et al. Pharmacokinetics of transdermal buprenorphine patch in the elderly. Eur J Clin Pharmacol 2013;69:143-9
- European Medicines Agency, International Conference on Harmonization–World Health Organization. Guideline for Good Clinical Practice [EMA website]. ICH Topic E6. Geneva, Switzerland: WHO, 2002. Available at: www.ema.europa.eu/pdfs/human/ich/013595en.pdf [Last accessed 29 April 2013]
- World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects [WMA website]. Ferney-Voltaire, France: WMA, 1989. Available at: www.wma.net/en/30publications/10policies/b3/17c.pdf [Last accessed 29 April 2013]
- EMEA: CPMP/EWP/612/00. Note for guidance on clinical investigation of medicinal products for treatment of nociceptive pain. London, 21 November 2002. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003525.pdf [Last accessed 29 April 2013]
- Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain 1986;27:117-26
- Jensen MP, Karoly P, O’Riordan EF, et al. The subjective experience of acute pain. An assessment of the utility of 10 indices. Clin J Pain 1989;5:153-9
- Kremer E, Atkinson JH, Ignelzi RJ. Measurement of pain: patient preference does not confound pain measurement. Pain 1981;10:241-8
- Szende A, Oppe M, Devlin N (eds). EQ-5D Value Sets: Inventory, Comparatory Review and User Guide. EuroQol Group Monographs Volume 2. The Netherlands: Springer, 2007
- Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105-21
- Farrar JT, Young JP Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical rating scale. Pain 2001;94:149-58
- Farrar JT, Pritchett YL, Robinson M, et al. The clinical importance of changes in the 0 to 10 Numeric Rating scale for Worst, Least, and Average Pain Intensity: analyses of data from clinical trials of duloxetine in pain disorders. J Pain 2010;11:109-18
- Davis MP. Twelve reasons for considering buprenorphine as a frontline analgesic in the management of pain. J Support Oncol 2012;10:209-19
- Likar R, Vadlau EM, Breschan C, et al. Comparable analgesic efficacy of transdermal buprenorphine in patients over and under 65 years of age. Clin J Pain 2008;24:536-43
- Muriel Villoria C, Pérez-Castejón Garrote JM, et al. Effectiveness and safety of transdermal buprenorphine for chronic pain treatment in the elderly: a prospective observational study [in Spanish]. Med Clin (Barc) 2007;128:204-10
- Griessinger N, Sittl R, Likar R. Transdermal buprenorphine in clinical practice – a post-marketing surveillance study in 13,179 patients. Curr Med Res Opin 2005;21:1147-56
- Gallagher AM, Leighton-Scott J, van Staa TP. Utilization characteristics and treatment persistence in participants prescribed low-dose buprenorphine patches in primary care in the United Kingdom: a retrospective cohort study. Clin Ther 2009;31:1707-15
- Chevillard L, Mégarbane B, Risède P, et al. Characteristics and comparative severity of respiratory response to toxic doses of fentanyl, methadone, morphine, and buprenorphine in rats. Toxicol Lett 2009;191:327-40
- Gomez-Flores R, Weber R. Differential effects of buprenorphine and morphine on immune and neuroendocrine functions following acute administration in the rat mesencephalon periaqueductal gray. Immunopharmacology 2000;48:145-56
- Pergolizzi J, Aloisi AM, Dahan A, et al. Current knowledge of buprenorphone and its unique pharmacological profile. Pain Practice 2010;10:428-50
- Seripa D, Pilotto A, Panza F, et al. Pharmacogenetics of cytochrome P450 (CYP) in the elderly. Ageing Res Rev 2010;9:457-74
- Mistry M, Houston JB. Glucuronidation in vitro and in vivo. Comparison of intestinal and hepatic conjugation of morphine, naloxone and buprenorphine. Drug Metab Dispos 1987;15:710-17