Abstract
Background and objective:
Neuropathic pain (NP) is a common type of chronic pain in which 60% of patients present with localized symptoms. Early diagnosis of NP is often a challenge in primary care. Moreover, so far no standard diagnostic procedure for localized NP (LNP) is available. To help general practitioners, a screening tool was developed and evaluated.
Research design and methods:
The development of the screening tool was based on the grading system principles for NP proposed by the IASP, focusing on medical history and distribution of painful symptoms and sensory signs. It was tested by 31 general practitioners and evaluated against the NP diagnosis of three pain specialists as reference in a single center prospective study in Spain using a cohort study design including an adult population of chronic pain patients. This design avoids spectrum bias where the spectrum of disease is not correctly reflected in the study population.
Main outcome measures:
General practitioners rated usefulness, simplicity, and time requirements of the tool. Diagnostic accuracy was expressed by sensitivity, specificity, and positive and negative predictive values.
Results:
General practitioners consecutively screened 2079 chronic pain patients (mean age 60.7 ± 11.1 years, 69.9% female). Using the tool, 394 patients were diagnosed with LNP. Screening including sensory examination took 7 min (median). General practitioners rated the tool as useful (24/31; 77.4%) or very useful (7/31; 22.6%) for diagnosing LNP and facilitating clinical practice (30/31; 96.8%). Under daily practice conditions, sensitivity and specificity of the tool for detecting LNP was 46.7% and 86.6%, respectively.
Conclusions:
The proposed screening tool was shown to be easy and useful for detecting NP and LNP in chronic pain patients as a fast first assessment tool in primary care, thus facilitating the choice of a topical treatment.
Limitations and strengths:
The drop-out rate was high but was accounted for by using correction factors in the diagnostic accuracy calculations. A strength is the unselected chronic patient population: spectrum of disease correctly reflects day-to-day clinical practice and is not biased. Diagnostic accuracy of the tool therefore appears to be realistic.
Introduction
Neuropathic pain (NP), defined as ‘pain arising as a direct consequence of a lesion or disease affecting the somatosensory system’Citation1,Citation2 is a debilitating condition originating in the peripheral or central nervous system. It has a serious negative impact on patient functioning and quality of lifeCitation3–5, with an overall disease burden frequently higher than for non-neuropathic painCitation4,Citation6. NP is characterized by abnormal sensory perceptions generally distinguished as positive signs such as burning or shooting pain, tingling, prickling, tightness, or electrical sensations, and negative signs such as loss of noxious, mechanical, or thermal perception with the very frequent concomitant occurrence of paresthesias, allodynia, or hyperalgesiaCitation7. These clinical manifestations are relatively similar across the different neuropathic pain conditionsCitation7 which may exhibit different combinations of signs and symptoms in several patients. The true prevalence of NP is difficult to estimateCitation5, possibly affecting approximately 26 million patients worldwideCitation8 but particularly high among patients with diabetes (20.3%)Citation9, cancer (10.8%)Citation10, after surgical procedures (up to 50%)Citation11, or following herpes zoster (8%)Citation12. The annual NP incidence was evaluated at almost 1% of the general Dutch populationCitation13.
The topographical distribution of NP varies among patients according to NP etiology; in some pain conditions, pain is clearly localized in a circumscribed area of the body for the majority of patients resulting in the use of the term ‘localized neuropathic pain’ (LNP) (note that ‘focal’ is sometimes used instead of ‘localized’ for the same situation). The lack of a definition for LNP in the literature and a poor understanding of the term by physicians of several Western European countriesCitation14 led to the recent proposal of a core definition: “Localized neuropathic pain is a type of neuropathic pain that is characterized by consistent and circumscribed area(s) of maximum pain”Citation14. Patients with LNP should be able to point to the location(s) of their pain or abnormal sensations on the skin in order to distinguish LNP from widely distributed or even widespread pain, the area of maximal pain remaining relatively constant over timeCitation14. A survey in Western Europe showed that LNP may be common: averaging across all NP conditions, physicians reported that pain was localized in approximately 60% of NP patientsCitation14.
General practitioners are the ‘gatekeepers’ of the health system. Pain is the main reason for about 40% of patient consultations in primary care each year in Western countries, and 20% of these patients suffered from pain for more than 6 monthsCitation15. Only 2% of chronic pain patients are treated by a pain specialistCitation16; the challenge to diagnose neuropathic pain early thus generally rests with primary care physicians. The supposed complexity of the clinical evaluation of signs and symptoms by general practitioners may result in failures in the diagnosis of NPCitation17. NP can hence remain untreated for months or years. Moreover, when treated, 40–60% of patients obtain only partial pain reliefCitation18. Several screening or diagnostic tools for NP have thus been developedCitation19,Citation20. However, mainly due to time constraints, the use of specific tools is not usual practice in primary care. In addition, there is no comprehensive classification for disorders associated with NP to provide specific and accurate diagnostic groupsCitation21. Finally, so far no standard diagnostic procedure for LNP, the most common type of NP, is available.
Pharmacotherapy remains the most important treatment option for NPCitation22; however, the patient may benefit from accompanying physical (e.g. transcutaneous electrical nerve stimulation) or psychological interventions (e.g. cognitive and behavioral techniques). With different treatment algorithms for different types of chronic pain, taking into account different pathophysiological mechanisms and modes of action of medications, a correct diagnosis of pain type facilitates an evidence-based treatment approach and avoids iterative treatment failures. As most NP treatment guidelines consider pain localization in the choice of first-line therapeutic approachCitation18,Citation23, the diagnosis of LNP appears essential. In order to support easy diagnosis of LNP in patients presenting with chronic pain in primary care, a screening tool was developed and subsequently evaluated in a single center trial in general daily practice.
Patients and methods
Development of LNP screening tool
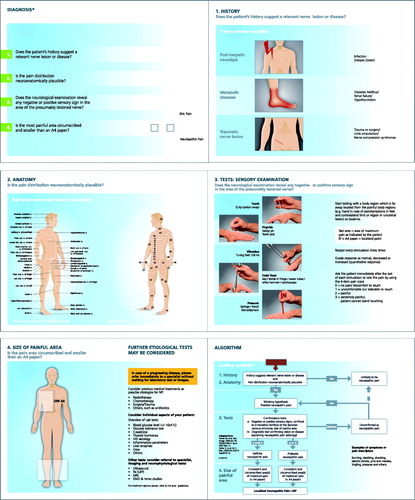
A screening tool for the identification of probable LNP in patients presenting with chronic pain in general clinical practice was developed by five of the authors (G.M., R.B., G.C.-I., G.H., V.M.), based on the grading system principles for NP proposed by the Neuropathic Pain Special Interest Group of the International Association for the Study of Pain expert committeeCitation1,Citation24, with the main focus turned towards medical history and distribution of painful symptoms and sensory signs. It was stipulated that the tool should be easy to understand and easy to use, and that it should not be time-consuming. The tool, in the format of a pocket card (9 cm × 14 cm), consists of four screening questions, as presented in (English version). The first two questions refer to the patient’s pain history (e.g. herpes zoster infection as a cause of chronic pain) and the neuroanatomical plausibility of pain distribution (documentation of painful area, painful symptoms, and sensory alterations). Answering ‘yes’ to these two questions leads to the working hypothesis of ‘possible NP’ which is then subsequently tested by sensory examination which should reveal any deficits if present. This third question was therefore associated with explanations of how to carry out simple sensory tests. If neurological examination reveals any positive or negative sensory signs in an innervation territory of a nervous structure, NP is at least considered probable. Measuring the size of the painful area relates to the fourth question: is pain circumscribed and smaller than the size of an A4 paper (as a given reference)? The pocket card additionally provides examples for diagnostic tests confirming the existence of a nervous lesion or disease underlying NP.
Figure 1. Screening tool for probable neuropathic pain and localized neuropathic pain; A6 pocket card (translation from Spanish to English including design adaptations from the study version). The tool consists of four screening questions plus information/examples regarding these questions, and a diagnosis algorithm.
Study design for the evaluation of the screening tool
In a prospective single center study, a Spanish version of the screening tool was tested by 31 general practitioners in Badalona (Spain) and evaluated against the NP diagnosis of three pain specialists at Badalona Municipal Hospital as reference. The study was approved by the ethics committee of the Hospital Universitari Germans Trias I Pujol and conducted from November 2012 to March 2013 with written informed patient consent required prior to enrolment.
A realistic cohort study design which included a general population of chronic pain patients was chosen over a case control design in which unclear pain conditions are excluded. This approach avoided spectrum bias (i.e. the spectrum of disease is not correctly reflected in the study population), the most relevant bias in diagnostic studies which usually leads to overestimation of diagnostic accuracyCitation25.
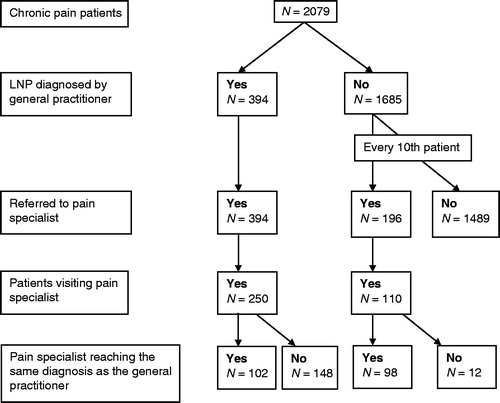
In order to assess the diagnoses made by the general practitioners using the screening tool, all patients diagnosed with LNP and every tenth consecutive patient diagnosed as not having LNP (i.e. having NP but widespread/diffuse, but not localized NP [nLNP] or a type of pain other than NP [nNP]) were referred to pain specialists without stating the patients’ type of chronic pain and thus blinding the pain specialists to the general practitioners’ diagnoses. Using their experience and the IASP criteria, the pain specialists provided their own pain diagnoses of the patients. They also noted putative etiology of pain and possible localized character of the pain. Pain specialists additionally used the DN4 questionnaire for comparison in terms of screening for NPCitation26. The visit to the pain specialist had to take place within 14 days after general practitioner’s diagnosis.
Patients
Adult patients suffering from chronic pain, defined as at least 50% of days with significant pain affecting quality of life within the previous 3 months, were included in this study if they provided written informed consent and were able to sufficiently communicate and differentiate location, intensity, and quality of their pain. Patients participating in an interventional study involving investigational drugs were excluded.
Documentation
Data documentation was conducted using a web application providing case report forms. General practitioners documented demographics, medical history, comorbidities, and current analgesic medication, and then performed pain diagnosis according to the screening tool looking for location and size of the most painful area, and finally evaluated the tool itself. Pain specialists did not evaluate the screening tool and only served as reference for the evaluation of diagnostic accuracy of the tool.
Study outcomes
The primary study objective was the evaluation of the usefulness of the screening tool for the diagnosis of NP and of LNP in an unselected chronic pain population, as rated by the participating general practitioners (possible answers: very useful, useful, minimally useful, not useful). Secondary objectives included diagnostic accuracy of the tool for LNP expressed as sensitivity and specificity as well as positive and negative predictive values, and the assessment by participating general practitioners of several items: usefulness of the tool for sensory examination, simplicity of sensory examination proposed by the tool, simplicity of diagnosing LNP with the tool, time needed for performing sensory examination, time needed to diagnose LNP, facilitation of clinical work. In addition, the accuracy of the tool for diagnosing NP was compared to the accuracy of the DN4 questionnaire.
Statistical analysis
Sample size calculations were based on the assumption that 6–8% of adults in the general population suffer from chronic pain with neuropathic characteristicsCitation12, among those about 60% present with LNPCitation14. As a result, one in 25 patients experiencing chronic pain might suffer from LNP. Consequently, a study population of 2500 evaluable patients was necessary to identify about 100 patients probably suffering from LNP which is suitable to estimate proportions with an appropriate uncertainty expressed by 95% CIs. In order to obtain 2500 patient accepting to participate, screening of 7500 patients was requested for a realistic approach (two out of three were expected to refuse to take part in the study).
The primary study endpoint was the proportion of general practitioners rating the tool as ‘very useful’ or ‘useful’ for diagnosing the presence or absence of NP and of LNP in each individual patient with chronic pain.
The secondary endpoint ‘diagnostic accuracy of the tool’ was assessed within the context of binary test results (diagnoses of general practitioners according to screening tool) and binary disease status (diagnoses of pain specialists as gold standard) using 2 × 2 contingency tables. The primary analysis was the comparison of LNP vs. (nLNP + nNP). Measures included sensitivity and specificity of the tool as well as predictive values calculated as follows (TP = true positive, FP = false positive, TN = true negative, FN = false negative): sensitivity = TP/(TP + FN), specificity = TN/(TN + FP), positive predictive value = TP/(TP + FP), and negative predictive value = TN/(TN + FN). The analysis took into account incomplete verification according to the design of the study (one out of ten patients with a diagnosis ‘nLNP’ or ‘nNP’ were allocated to the pain specialist) as well as due to observed drop-outs (a proportion of referred patients did not visit the pain specialist). The raw counts were corrected by inverse probability weighting assuming that the ‘missing at random’ (MAR) assumption heldCitation27: counts were multiplied with the correction factors Nall/Nverified per subgroup according to the diagnosis of the general practitioner, and confidence intervals of sensitivity and specificity were corrected accordinglyCitation28.
A comparison between screening tool and DN4 questionnaire regarding the diagnostic accuracies of diagnosis of NP was carried out using the analysis of NP vs. nNP and the pain specialist diagnosis as a reference.
Results
Patients
A total of 2079 patients with chronic pain were consecutively screened by the 31 general practitioners. Mean age was 60.7 ± 11.1 years, with 69.9% female. The majority (76.3%) had suffered from chronic pain for more than 1 year. Using the tool, LNP was diagnosed in 394 patients (19%); of the other 1685 patients with chronic pain, 234 (11.3%) were diagnosed with non-localized neuropathic pain (nLNP) and 1451 (69.8% of 2079 patients) with pain other than NP (nNP). Among the 394 LNP patients and 196 nLNP + nNP patients referred to pain specialist, 250 LNP and 110 nLNP + nNP patients complied with this visit ().
The majority of chronic pain patients (74.5%) suffered from comorbidities; these were observed in 73.8% of LNP patients (data available for 381 patients) and consisted mainly of cardiovascular (59.1%), central nervous system and psychiatric (45.2%), or endocrine (44.8%) disorders. The most frequent etiologies reported for LNP by general practitioners were back pain (27.4%) and carpal tunnel syndrome (22.1%) (). Analgesic medication of LNP patients consisted mainly of WHO I medication (74.9%) but 15.0% received weak opioids and 4.3% strong opioids (). General practitioners documented evidence-based medication for NP in 21.8% of the patients.
Table 1. Pain diagnoses of the patients suffering from localized neuropathic pain according to general practitioners (n = 394).
Table 2. Analgesic medication of the patients suffering from localized neuropathic pain according to general practitioner diagnosis (n = 394). Multiple answers possible.
Screening tool assessment by general practitioners
Assessment of the usefulness of the tool for diagnosing NP and LNP was available for 564 of the 590 patients referred to the pain specialist. General practitioners considered the tool useful or very useful for the majority of individual patient diagnoses for both NP (94.5%) and LNP (94.3%) (). Evaluation of usefulness by the number of participating general practitioners showed that the majority (96.8%) considered the screening tool as useful (24/31; 77.4%) or very useful (6/31; 19.4%) for diagnosing NP. All 31 general practitioners rated the tool useful or very useful for the diagnosis of LNP (). Further assessment results are provided in . General practitioners spent a median of 7 min to carry out the diagnosis with the tool including sensory examination (inter-quartile range: 5–10 min). Overall, 30 general practitioners (96.8%) stated that the tool facilitated their clinical work ().
Figure 3. Usefulness of the screening tool for diagnosing neuropathic pain (NP) and localized neuropathic pain (LNP) rated for all individual patient diagnoses by the general practitioners (all patients referred to pain specialists excluding missing ratings, n = 564; primary study endpoint). Data are shown with 95% confidence intervals.
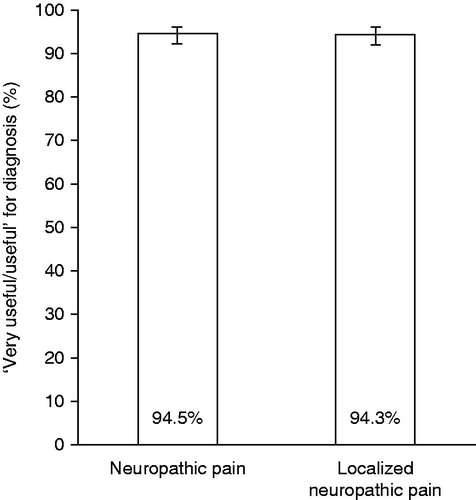
Figure 4. Usefulness of the screening tool for diagnosing localized neuropathic pain (LNP) and facilitation of clinical work as rated by the 31 participating general practitioners.
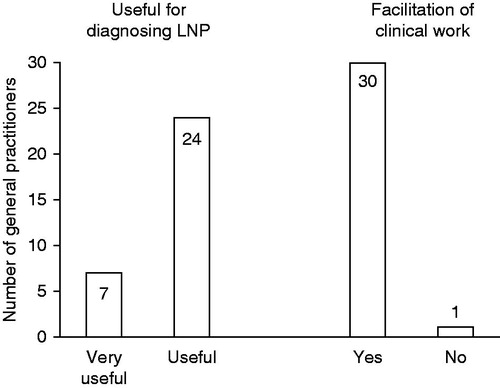
Table 3. Further assessments of the screening tool by the participating general practitioners (n = 31).
Diagnostic accuracy of the tool
Among the 250 patients diagnosed with LNP by the general practitioners who visited the pain specialists, this diagnosis was confirmed in 102 patients (40.8%). In the nLNP + nNP group (110 patients), the diagnosis of nLNP/nNP was confirmed by pain specialists in 98 patients (89.1%). Cross-tabulation of general practitioner diagnosis with pain specialist diagnosis for the comparison of LNP vs. (nLNP + nNP) showed raw counts (, upper part) and numbers corrected for incomplete verification (, lower part). Using these data, the following estimates for diagnostic accuracy of the tool were obtained: sensitivity = 46.7% (95% CI: 33.2–60.6), specificity = 86.6% (95% CI: 84.5–88.3), positive predicted value = 40.8%, and negative predictive value = 89.1%. According to the tool and confirmed by pain specialists, LNP prevalence among NP patients was 55.3%, close to the approximation of 60% previously reportedCitation14. The comparison of diagnostic accuracy in the detection of NP in chronic pain patients between the evaluated tool and the DN4 questionnaire showed comparable predictive values for both instruments, but DN4 exhibited greater sensitivity whereas specificity was higher with the screening tool ().
Table 4. Agreement in diagnosis between general practitioners and pain specialists (raw counts, and counts and percentages corrected for incomplete verification). Estimates for diagnostic accuracy are shown in bold.
Table 5. Estimates for accuracy in diagnosing neuropathic pain using the screening tool (general practitioners) and with the DN4 questionnaire. Analyses were NP vs. nNP with pain specialist diagnosis as reference standard.
Discussion
Neuropathic pain is a common condition but often under- or misdiagnosed in daily practice, and neuropathic pain with localized symptoms may require a specific treatment regimen, often known only to pain specialists. Several screening or diagnostic questionnaires or tools for NP (e.g., Leeds Assessment of Neuropathic Symptoms and Signs [LANSS]Citation29, DN4Citation26, painDETECTCitation30) are available which are all based on procedures chosen by the developers and involve time dedicated to clinical examination and score calculation. The screening tool evaluated in the present study allows simple and fast screening of NP according to IASP diagnostic criteria for NP, and helps physicians to perform a clinical examination, a basic part of NP diagnosis often considered difficult by non-neurologists. A large majority of general practitioners found this tool useful for detecting both NP and LNP and found that it facilitated their daily clinical practice: the tool may thus help at the primary care level for early initial diagnosis of probable NP or LNP in chronic pain patients, and for the choice of adequate treatment.
Analgesic treatment of LNP patients in the present study consisted mainly of nonsteroidal anti-inflammatory drugs (NSAIDs) and paracetamol. Neuropathic pain guidelines recommend first-line treatment with tricyclic antidepressants or calcium channel α2-δ ligands, and topical compounds (in case of LNP)Citation18,Citation23,Citation31. However, evidence-based medications were only documented for 22% of patients with LNP in the present study. General pain treatment usually begins with NSAIDs or paracetamol which can provide relief for the nociceptive component in mixed pain such as low back pain but were found to be largely ineffective in neuropathic painCitation32, and are neither suitable nor safe for long-term chronic pain treatment. An early diagnosis of NP and LNP allows a revision of this initial treatment decision.
The tool evaluated in the present study exhibited a sensitivity of 47% and a specificity of 87% for diagnosing LNP with positive and negative predictive values at 41% and 89%, respectively, indicating a good accuracy in excluding LNP as a diagnosis. To discuss diagnostic accuracy of the evaluated tool in comparison with available NP screening tools, designs used in the validation studies must be considered, since they influenced study outcomes. Diagnostic accuracy of the DN4 questionnaire was assessed within a selected pain population and only with pain specialists, excluding patients with painful syndromes of unknown origin or associated with diffuse pains, or pains of presumably mixed origin or of visceral originsCitation26, thus leading to high sensitivity (83%), specificity (90%), and positive predictive value (86%) under such specific conditions. However, DN4 diagnostic performance was reduced in chronic pain patients with a broad range of pain situationsCitation33. This pre-selection effect was also observed for the original LANSS questionnaireCitation33 which exhibited high sensitivity (85%), specificity (80%), and positive predictive value (82%) in a study design excluding patients with mixed pain components or uncertain diagnosisCitation29. For the painDETECT questionnaire, only patients diagnosed independently by two experienced pain specialists with ‘typical’ neuropathic or nociceptive entities could participate in the validation studyCitation30. Here, predominantly neuropathic pain conditions included postherpetic neuralgia, painful polyneuropathy, nerve traumas, and low back pain (solely of the lumbar vertebrae, sacrum and coccyx), and predominantly nociceptive pain conditions included visceral pain, osteoarthritis, inflammatory arthropathies, and mechanical low back pain. Patients with pain of assumed mixed origin (e.g., malignancy, compression fractures, ankylosing spondylitis) or fibromyalgia, or unclear symptomatology were excluded. This led to high sensitivity (85%), specificity (80%), and positive predictive accuracy (83%) of the questionnaire (paper version). To mimic daily general practice conditions in which a broad range of pain situations should be evaluated by non-specialists, we decided to use a study design that included an unselected population of chronic pain patients. This design minimized spectrum bias and avoided overestimation of diagnostic accuracy associated with this situation. Sensitivity and specificity of the tool evaluated for diagnosing NP and LNP in the present study may thus correctly reflect accuracy of a tool under daily clinical practice conditions in primary care setting.
Furthermore, for a comparison of diagnostic accuracy of the tool in the detection of NP with a well established diagnostic tool but in an unselected chronic pain patient population, we considered the evaluation by the pain specialist as a reference and included the use of the DN4 questionnaire. A greater sensitivity of DN4 and a higher specificity with the evaluated tool were calculated, the proportion of patients correctly identified with NP by practitioners and pain specialists being in the same range (51% with the tool, 48% with DN4). This indicates that the tool evaluated in the present study, using a diagnostic procedure based on an algorithm proposed by the IASP for NPCitation1,Citation24, achieves comparable diagnostic accuracies to a well established diagnostic tool and can thus also be used as a screening instrument for the detection of NP. It is a strength of this study that for the first time, the evaluated tool narrowly adhered to IASP diagnostic criteria.
The study was performed in a given area of Spain, the Badalona area which is a highly populated municipality and was considered representative for Spain by the local investigators. The drop-out rate in the study was high but not surprising for the study objective in a population with three quarters of patients suffering from chronic pain for more than one year. Chronic pain patients frequently report anxiety, depression, sleep disturbances, and a reduction of quality of life. They are substantially affected in their social and working lifeCitation16; and hence not always compliant with study requirements such as attending a pain specialist within two weeks of consultation of a general practitioner. We used correction factors in the calculation for diagnostic accuracy to account for this. It should also be noted that there is so far no definition of the upper limit for the size of LNPCitation14. Discussions with pain experts and general practitioners revealed that in clinical practice an area not larger than a DIN A4 piece of paper fits most appropriately as a first approximation. However, there will always be patients with larger but circumscribed areas of pain.
Conclusion
The screening tool evaluated in the present study is easy and useful for detecting neuropathic pain and localized neuropathic pain in chronic pain patients in primary care. This tool, the first screening instrument based on the IASP algorithm, may therefore be commonly used for a fast assessment of chronic pain by general practitioners in daily practice, and as a help to orientate their treatment strategy.
Transparency
Declaration of funding
The study was supported by Grünenthal GmbH, Aachen, Germany.
Declaration of financial/other relationships
G.M. has disclosed that he is a periodical consultant for Grünenthal. R.B. has disclosed that he is a consultant for Pfizer, Genzyme, Grünenthal, Mundipharma, Allergan, Sanofi Pasteur, Medtronic, Eisai, Lilly, Boehringer Ingelheim, Astellas, Novartis, Bristol-Myers Squibb, Biogenidec, AstraZeneca, Merck, and Abbvie. He is on the speakers’ bureau for Pfizer, Genzyme, Grünenthal, Mundipharma, Sanofi Pasteur, Medtronic, Eisai, Lilly, Boehringer Ingelheim, Astellas, Desitin, Teva Pharma, Bayer-Schering, and MSD, and received grants/research funding from Pfizer, Genzyme, Grünenthal, the German Federal Ministry of Education and Research, and the German Research Foundation. He is also a member of the IMI ‘European’ collaboration. G.C.-I. has disclosed that he was previously a consultant for Grünenthal. G.H. has disclosed that he received travel/accommodation expenses from Grünenthal for this project. V.M. has disclosed that he is a consultant for Grünenthal and has received sponsorship from Grünenthal. X.F. and D.S. have both disclosed that they received grants/research funding from Grünenthal. T.K. has disclosed that he is a consultant for Grünenthal and received travel/accommodation expenses from Grünenthal.
CMRO peer reviewers on this manuscript have received an honorarium from CMRO for their review work. Peer reviewer 1 has no other relevant financial or other relationships to disclose; peer reviewer 2 has disclosed that he is on the speakers’ bureau for Merck.
Acknowledgments
The authors would like to thank all physicians participating in the study. Writing and editorial assistance was provided by Elke Grosselindemann and Birgit Brett. The study and the preparation of the manuscript were supported by Grünenthal GmbH, Aachen, Germany.
Previous presentation: Part of the manuscript data were presented as a poster at the XXI World Congress of Neurology (WCN), Vienna, Austria, 21–26 September 2013 (abstract 3145; Mick et al. J Neurol Sci 2013;333:e519-36).
List of investigators: General practitioners: A. Artuñedo, J.M. Badia, C. Burgos, J. Caballero, G. Casas, J. Claramunt, J.M. Cruz, M. Ferrer, X. Ferrer, X. Frías, A. Garcia, F. Gavilan, M. Gilaberte, M. Gomez, I. Gonzalez, A. Gregorio, A. Herrero, A. León, J. Mallafre, G. Martínez, I. Matey, M. Morales, N. Moran, X. Ordoñez, A. Oriol, B. Raspall, F. Salomon, T. Sayrol, N. Vazquez, A. Ventura, M. Vila. Pain specialists: E. Barceló, M. Pijoan, D. Sintes.
References
- Treede R-D, Jensen TS, Campbell JN, et al. Neuropathic pain. Redefinition and a grading system for clinical and research purposes. Neurology 2008;70:1630-5
- IASP Taskforce on Taxonomy. Available at: http://www.iasp-pain.org/Content/NavigationMenu/GeneralResourceLinks/PainDefinitions/#Neuropathicpain [Last accessed 19 November 2013]
- O’Connor AB. Neuropathic pain. Quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics 2009;27:95-112
- Attal N, Lanteri-Minet M, Laurent B, et al. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain 2011;152:2836-43
- Smith BH, Torrance N. Epidemiology of neuropathic pain and its impact on quality of life. Curr Pain Headache Rep 2012;16:191-8
- Ohayon MM, Stingl JC. Prevalence and comorbidity of chronic pain in the German general population. J Psychiatr Res 2012;46:444-50
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010;9:807-19
- Pal M, Angaru S, Kodimuthali A, et al. Vanilloid receptor antagonists: emerging class of novel anti-inflammatory agents for pain management. Curr Pharm Des 2009;15:1008-26
- Bouhassira D, Letanoux M, Hatemann A. Chronic pain with neuropathic characteristics in diabetic patients: a French cross-sectional study. PLoS One 2013;8:e74195
- Rayment C, Hjermstad MJ, Aass N, et al. Neuropathic cancer pain: prevalence, severity, analgesics and impact from the European Palliative Care Research Collaborative – Computerised Symptom Assessment study. Palliat Med 2013;27:714-21
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618-25
- Smith BH, Torrance N. Epidemiology of neuropathic pain. Pain Manage 2011;1:87-96
- Dieleman JP, Kerklaan J, Huygen FJ, et al. Incidence rates and treatment of neuropathic pain conditions in the general population. Pain 2008;137:681-8
- Mick G, Baron R, Finnerup NB, et al. What is localized neuropathic pain? A first proposal to characterize and define a widely used term. Pain Manage 2012;2:71-7
- Mäntyselkä P, Kumpusalo E, Ahonen R, et al. Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain 2001;89:175-80
- Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287-333
- Vadalouca A, Siafaka I, Argyra E, et al. Therapeutic management of chronic neuropathic pain. An examination of pharmacologic treatment. Ann NY Acad Sci 2006;1088:164-86
- Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 2007;132:237-51
- Haanpää M, Attal N, Backonja M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain 2011;152:14-27
- Bennett MI, Attal N, Backonja M, et al. Using screening tools to identify neuropathic pain. Pain 2007;127:199-203
- Finnerup NB, Scholz J, Attal N, et al. Neuropathic pain needs systematic classification. Eur J Pain 2013;17:953-6
- Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain 2010;150:573-81
- Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc 2010;85(Suppl):S3-14
- Geber C, Baumgärtner U, Schwab R, et al. Revised definition of neuropathic pain and its grading system: an open case series illustrating its use in clinical practice. Am J Med 2009;122:S3-12
- Lijmer JG, Mol BW, Heisterkamp S, et al. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 1999;282:1061-6
- Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005;114:29-36
- Little RJA, Rubin DB. Statistical Analysis with Missing Data, 1st edn. New York: John Wiley, 1987
- Begg CB, Greenes RA. Assessment of diagnostic tests when disease verification is subject to selection bias. Biometrics 1983;39:207-15
- Bennett MI. The LANSS pain scale: the Leeds assessment of neuropathic symptoms and signs. Pain 2001;92:147-57
- Freynhagen R, Baron R, Gockel U, et al. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911-20
- Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol 2010;17:1113-e88
- Haanpää ML, Backonja MM, Bennett MI, et al. Assessment of neuropathic pain in primary care. Am J Med 2009;122(10 Suppl):S13-21
- Sadler A, Wilson J, Colvin L. Acute and chronic neuropathic pain in the hospital setting. Use of screening tools. Clin J Pain 2013;29:507-11