Abstract
Objectives:
Luseogliflozin is a selective sodium glucose cotransporter 2 inhibitor under development for the treatment of type 2 diabetes mellitus (T2DM). This phase II study was conducted to confirm the efficacy and safety of luseogliflozin monotherapy at doses of up to 10 mg in Japanese patients with T2DM.
Patients and methods:
Patients with hemoglobin A1c (HbA1c) of 6.9–10.5% on diet therapy were randomized in a double-blind manner to treatment with 1, 2.5, 5, or 10 mg luseogliflozin or placebo for 12 weeks (n = 56, 56, 54, 58, and 58, respectively).
Trial registration:
Japan Pharmaceutical Information Center (identifier: Japic CTI-101191).
Main outcome measures:
The primary endpoint was the change in HbA1c from baseline to the end of treatment. Other endpoints included fasting plasma glucose (FPG), postprandial plasma glucose (PPG) and body weight. Adverse events were recorded throughout the study.
Results:
HbA1c decreased significantly at the end of treatment in the 1, 2.5, 5, and 10 mg luseogliflozin groups compared with placebo (−0.29, −0.39, −0.46, and −0.43%, respectively, versus +0.22%; all P < 0.001), as did FPG and PPG (all P < 0.001). Body weight also decreased significantly in all luseogliflozin groups compared with placebo (all P < 0.001). The incidence rates of adverse events (40.0–50.0%) were not significantly different among the five groups. The overall incidence of hypoglycemia was low. Limitations of this study include the short study duration and the relatively small sample size.
Conclusions:
In Japanese patients with T2DM, luseogliflozin was well tolerated, improved glycemic control, and reduced body weight over 12 weeks of treatment at all tested doses. Doses of ≥2.5 mg achieved similar improvements in glycemic control.
Introduction
Recent statistics indicate that as many as 371 million people have diabetesCitation1 and that its prevalence is increasing rapidly, especially in AsiaCitation2. Existing therapies, while effective in many patients, are often associated with limited durability or adverse effects such as hypoglycemia and weight gainCitation3–5. Therefore, there is a need for new treatments to overcome the perceived limitations of existing drugs.
The kidney has a major role in glucose homeostasis. Although about 180 g of glucose is filtered by the renal glomerulus, most of the filtered glucose is reabsorbed through sodium glucose cotransporter 2 (SGLT2) in the proximal tubuleCitation6–8. SGLT2-deficient animal models showed increased urinary glucose excretionCitation9,Citation10. Similarly, in humans, patients with familial renal glucosuria caused by mutations in the SGLT2 gene show glucosuriaCitation11 although serious problems associated with SGLT2 mutation have not been reported. From these observations, inhibition of SGLT2 was expected to increase urinary glucose excretion.
Because of these expectations, several SGLT2 inhibitors have been developed as novel treatments for type 2 diabetes mellitus (T2DM)Citation12–14, including luseogliflozin [TS-071; (1S)-1,5-anhydro-1-[5-(4-ethoxybenzyl)-2-methoxy-4-methylphenyl]-1-thio-D-glucitol hydrate]. Preclinical studies have shown that luseogliflozin is a specific and selective SGLT2 inhibitorCitation15 and that luseogliflozin improved glucose tolerance and reduced hyperglycemia in animal models of diabetesCitation16.
In a phase I study of luseogliflozin in healthy individualsCitation17 and a clinical pharmacology study that assessed the pharmacokinetic and pharmacodynamic profiles of luseogliflozin in patients with T2DMCitation18, luseogliflozin dose dependently increased urinary glucose excretion without causing hypoglycemia.
In an exploratory phase II studyCitation19, luseogliflozin monotherapy at doses of 0.5, 2.5, and 5 mg together with ongoing diet therapy for 12 weeks significantly reduced hemoglobin A1c (HbA1c), fasting plasma glucose (FPG), and 2 h postprandial plasma glucose (PPG) compared with placebo. In addition, 2.5 and 5 mg of luseogliflozin significantly reduced body weight compared with placebo. No safety concerns were identified and luseogliflozin was well tolerated in the exploratory study. Thus, the exploratory study provided evidence supporting the efficacy and safety of luseogliflozin at doses of up to 5 mg. However, the differences in efficacy and tolerability between the 2.5 and 5 mg doses are unclear, and no studies have determined the efficacy and safety of doses exceeding 5 mg in patients with T2DM. Therefore, it is necessary to evaluate the clinical efficacy and safety of 10 mg luseogliflozin. In addition, considering that the prior exploratory phase II study demonstrated that luseogliflozin reduced body weight, and because SGLT2 inhibitors promote caloric loss by increasing urinary glucose excretion, it is important to determine the effects of luseogliflozin on plasma lipids to obtain a deeper understanding of the pharmacological effects of luseogliflozin.
Based on these findings, the present phase II study was designed to verify the dose-dependent efficacy and safety of treatment with luseogliflozin at 1, 2.5, 5 and 10 mg for 12 weeks in Japanese patients with T2DM. In addition, physiological effects and the change in metabolism with treatment of luseogliflozin were explored using more detailed clinical laboratory tests than in prior studies.
Patients and methods
Eligibility criteria
Japanese outpatients with type 2 diabetes diagnosed according to the Japan Diabetes Society (JDS) guidelinesCitation20,Citation21 were eligible if they met the following criteria: age 20–74 years; HbA1c 6.9–10.5% at Weeks -6 and -2 with a maximum change of 1.0% between these visits; FPG ≥126 mg/dL at Weeks -6 or -2; maximum change in body weight of 3.0% between Weeks -6 and -2; and on stable diet therapy for ≥6 weeks before Week -6. Major exclusion criteria included an insulin-dependent state; another form of diabetes; use of an oral antidiabetic drug or insulin ≤6 weeks before Week -6; complication of a renal disorder or history of chronic renal disease, nephrectomy, or renal transplantation; complication of or history of repeated urinary tract/genital infections; obvious urination disorder; clinically evident hepatic disorder; complication of severe gastrointestinal, cardiac disorders, or diabetic microangiopathy. Additional exclusion criteria are listed in the Online Supplementary Materials. To protect patients, patients were to discontinue the study if their HbA1c was >11.5% and/or FPG was >270 mg/dL on two consecutive visits. All patients provided written informed consent before enrollment.
Study design and treatments
This was a phase II, randomized, placebo-controlled, double-blind, five-way parallel-group study performed at 41 institutions distributed throughout Japan between June 2010 and January 2011. This study was registered with the Japan Pharmaceutical Information Center (identifier: Japic CTI-101191). The study was conducted according to the Declaration of Helsinki, Good Clinical Practice, and Japanese law, and was approved by the institutional review board at each institution. The study consisted of a 6-week observation period, in which patients did not receive any diabetes treatment, other than continuing their diet therapy, followed by a 12-week treatment period. The rationale for the doses used is presented in the Online Supplementary Materials.
The study drugs were taken orally, once daily before breakfast. Patients who satisfied the eligibility criteria were randomized to 1, 2.5, 5, or 10 mg luseogliflozin or placebo at a 1:1:1:1:1 ratio. The study drug controller randomly allocated study drugs to the study groups and then prepared the randomization schedule. The Subject Registration Center allocated the study drugs to each medical institution in a serial manner according to the study drug numbers for eligible patients. Investigators prescribed the study drug to each patient according to the study drug numbers. The study drugs were provided in packaging and tablets that were indistinguishable from each other.
The patients were to continue their prescribed diet therapy throughout the entire study period (observation and treatment periods), and the prescribed number of calories was not to be changed after Week -6. Oral antidiabetic drugs, insulin, corticosteroids (except for topical use), intravenous fluids containing sugars, diuretics, and other investigational drugs were prohibited during the entire study period. Drugs used to treat concomitant disorders (e.g., antidyslipidemic/antihypertensive drugs) could be continued if these drugs were being used from before Week -6, and their doses and types were to be continued unchanged during the study. If hypoglycemic symptoms appeared, glucose was to be administered orally or intravenously depending on the symptoms.
Endpoints and measurements
The primary efficacy endpoint was the change in HbA1c from baseline to the end of treatment. Secondary efficacy endpoints included plasma glucose, insulin, glucagon, C-peptide immunoreactivity, intact proinsulin, glycosylated albumin, body weight, and urinary glucose.
HbA1c was measured in JDS units, which were converted to National Glycohemoglobin Standardization Program (NGSP) units using the certified equationCitation22: HbA1c (NGSP) (%) = 1.02 × HbA1c (JDS) (%) + 0.25%.
Meal tolerance tests were performed at Weeks 0 (before study drug administration) and 12 (Online Supplementary Materials). All laboratory tests were conducted at an independent laboratory (Mitsubishi Chemical Medience Corp., Tokyo, Japan). To maintain blinding, the laboratory did not disclose the quantitative urinary glucose levels measured during the meal tolerance test until after the database had been locked. Furthermore, qualitative urinary glucose tests were not to be performed during the study at each institution.
Adverse events (AEs) were assessed throughout the treatment period. Clinical laboratory parameters and vital signs were assessed at each visit. Twelve-lead electrocardiography was performed at Weeks -6, 0, and 12. AEs were coded using the Japanese version of the Medical Dictionary for Regulatory Activities, version 13.1, in terms of system organ class and preferred term. AEs were evaluated in terms of their causal relationship to the study drug (definitely related, probably related, possibly related, unrelated, or unknown) and severity (mild, moderate, or severe) by the participating investigators, who recorded this information on their patients’ case-report forms.
Statistical analyses
Baseline characteristics were compared among the five groups using the χ2 test and analysis of variance. A significance level of 15% (two-sided) was used to examine the heterogeneity of patient characteristics.
For the primary and secondary efficacy endpoints, the changes from baseline to each point of evaluation and to the end of treatment were calculated. Missing data at the end of treatment were imputed using the last observation carried forward method. The least squares mean and 95% confidence interval for the changes in efficacy variables in each group, as well as the differences between each luseogliflozin group and the placebo group, were calculated. For HbA1c, plasma glucose, area under the concentration–time curve (AUC) for plasma glucose, and glycosylated albumin, analysis of covariance was applied using the baseline value as a covariate. For other secondary efficacy endpoints, the unrestricted least significant difference method was used. For these analyses, a significance level of 5% (two-sided) was used.
For the change in HbA1c from baseline to the end of treatment, the closed test procedure was used to determine the significance of differences between the placebo group and each luseogliflozin group, in the following order: (1) placebo vs. 10 mg; (2) placebo vs. 5 mg; (3) placebo vs. 2.5 mg; and (4) placebo vs. 1 mg.
The frequency of AEs is presented as the number (%) of patients with each event. The incidences of AEs and adverse drug reactions (ADRs) were compared among the five groups using the χ2 test. Changes in safety variables were examined as described for the efficacy variables.
All statistical analyses were performed using SAS version 9.1.3 (SAS Inc., Cary, NC, USA). The sample size calculation and efficacy/safety analysis populations are described in the Online Supplementary Materials.
Results
Baseline characteristics
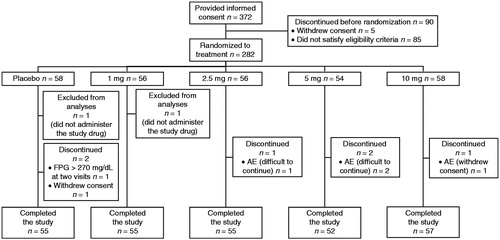
summarizes the disposition of patients. Of 372 patients who provided informed consent, 282 were allocated to the five study groups. One patient in each of the placebo and 1 mg groups did not receive the study drug. One patient randomized to placebo actually received 2.5 mg luseogliflozin, and one patient in the 2.5 mg group actually received placebo; these patients were analyzed according to the allocated treatment rather than treatment received in efficacy analyses, and according to actual treatment received in safety analyses. The baseline characteristics of patients were generally comparable across the five groups, although body weight, body mass index, fasting serum insulin, 2 h serum insulin, glycosylated albumin, HOMA-R, and HOMA-β showed heterogeneity at P < 0.15 among the five groups ( and ). The mean age and HbA1c ranged from 57.1 to 59.6 years and from 7.77 to 8.05%, respectively, among the study groups.
Table 1. Patient characteristics (full analysis set).
Table 2. Changes in efficacy variables from baseline to the end of treatment (LOCF, full analysis set).
HbA1c
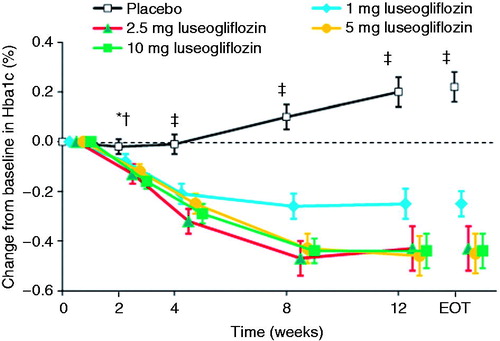
The mean decrease in HbA1c from baseline to the end of treatment was significantly greater in the 1, 2.5, 5, and 10 mg luseogliflozin groups than in the placebo group (−0.29, −0.39, −0.46, and −0.43%, respectively, vs. +0.22%; all P < 0.001; ). The magnitude of the reduction tended to be greater in the 2.5, 5, and 10 mg groups than in the 1 mg group. Significant reductions in HbA1c in the luseogliflozin groups relative to the placebo group were observed as early as Week 2, and were maintained until Week 12 (except for 1 mg at Week 2, ). Among patients with HbA1c ≥7.0% at baseline, 3.9% (2/51), 11.1% (6/54), 15.7% (8/51), and 17.5% (10/57) of patients in the 1, 2.5, 5, and 10 mg luseogliflozin groups, respectively, achieved HbA1c <7.0% at the end of treatment versus 1.8% (1/55) in the placebo group.
Figure 2. Changes in HbA1c from baseline to each visit or end of treatment. Values are means with standard error. The last observation carried forward method was applied to data at EOT. Differences between each luseogliflozin group and placebo were analyzed by the unrestricted least significant difference method. *P < 0.05 for 2.5 and 5 mg luseogliflozin vs. placebo. †P < 0.001 for 10 mg luseogliflozin vs. placebo. ‡P < 0.001 for all luseogliflozin groups vs. placebo. All data are shown for the full analysis set. HbA1c, hemoglobin A1c; EOT, end of treatment.
Fasting plasma glucose
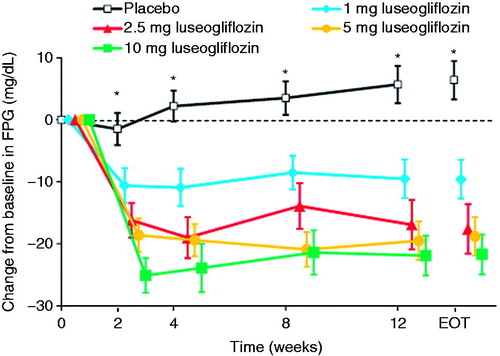
Consistent with the change in HbA1c, FPG levels decreased significantly from baseline at each visit, with significant differences compared with placebo noted as early as Week 2 (). The change in FPG from baseline to the end of treatment was also significantly greater in each luseogliflozin group than in the placebo group (all P < 0.001; ). The reductions in FPG tended to be greater in the 2.5 mg and higher dose groups compared with the 1 mg group. However, there were no marked differences in the reduction among the 2.5 mg and higher dose groups.
Figure 3. Changes in FPG from baseline to each visit or end of treatment. Values are means with standard error. The last observation carried forward method was applied to data at EOT. Differences between each luseogliflozin group and placebo were analyzed by the unrestricted least significant difference method. *P < 0.001 for all luseogliflozin groups vs. placebo. All data are shown for the full analysis set. FPG, fasting plasma glucose; EOT, end of treatment.
Meal tolerance test
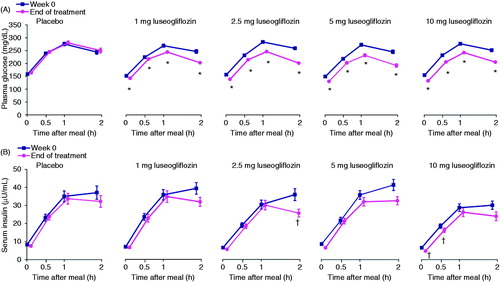
presents the results of the meal tolerance tests in terms of plasma glucose () and serum insulin () measured during each test at Week 0 and at the end of treatment. At the end of treatment, plasma glucose levels at each measurement time (0, 0.5, 1, and 2 h post-meal) and the plasma glucose AUC0–2h during the meal tolerance test were significantly lower in all four luseogliflozin groups than in the placebo group (all P < 0.001, , ). Serum insulin concentrations and insulin AUC0–2h at the end of treatment tended to be lower in all four luseogliflozin groups than in the placebo group, although the differences were only significant (at P < 0.05) at 2 h in the 2.5 mg group, and at 0 h and 0.5 h in the 10 mg group (, ). All doses of luseogliflozin significantly increased urinary glucose excretion during the meal tolerance test compared with placebo (all P < 0.001; ).
Figure 4. Plasma glucose (A) and insulin (B) levels during the meal tolerance tests performed at Week 0 and at the end of treatment. Values are means with standard error. The last observation carried forward method was applied to data at EOT. Differences in the change from baseline to EOT between each luseogliflozin group and placebo were analyzed by the unrestricted least significant difference method. *P < 0.001 vs. placebo. †P < 0.05 vs. placebo. All data are shown for the full analysis set. EOT, end of treatment.
Body weight
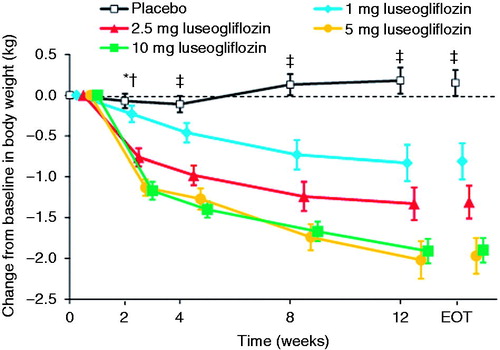
The reductions in body weight from baseline to the end of treatment were significantly greater in all four luseogliflozin groups than in the placebo group (all P < 0.001; , ). The reduction in body weight tended to increase as the luseogliflozin dose increased up to 5 mg. As shown in , the reductions in body weight in the luseogliflozin groups were significantly greater than those in the placebo group as early as Week 2 (Week 4 in the 1 mg group), and the progressive reductions were maintained until Week 12.
Figure 5. Changes in body weight from baseline to each visit or end of treatment. Values are means with standard error. The last observation carried forward method was applied to data at EOT. Differences between each luseogliflozin group and placebo were analyzed by the unrestricted least significant difference method. *P < 0.001 for 2.5, 5, and 10 mg luseogliflozin vs. placebo. †P < 0.05 for 1 mg luseogliflozin vs. placebo. ‡P < 0.001 for all luseogliflozin groups vs. placebo. All data are shown for the full analysis set. EOT, end of treatment.
Adverse events
AEs occurred in 40.0–50.0% of patients and ADRs occurred in 16.1–24.1% of patients in the luseogliflozin groups. AEs and ADRs occurred in 42.1% and 10.5% of patients in the placebo group, respectively (). The incidence rates of AEs and ADRs were not significantly different among the five groups. Most of the events were classified as mild, with only five in the luseogliflozin and two in the placebo groups classified as moderate; none of the events were classified as severe. Of three serious AEs, two (Prinzmetal angina, 2.5 mg group; laryngeal cancer, 5 mg group) occurred in the luseogliflozin groups. None of the serious events or events classified as moderate were causally related to the study drug. Five events led to discontinuation in four patients (serious AEs in two patients in the luseogliflozin groups; generalized rash in the 5 mg group; thirst and malaise in the 10 mg group). The most common AEs occurring in ≥3% of patients in any luseogliflozin group are shown in (e.g., preferred terms of nasopharyngitis, pollakiuria, increased urinary β2-microglobulin).
Table 3. List of adverse events (safety analysis set).
Considering the results of other studies of SGLT2 inhibitors and pharmacological action of SGLT2 inhibitors, we also assessed the incidence of certain types of AEs of special interest, including hypoglycemia, urinary tract infections, genital infections, AEs related to pollakiuria, AEs related to renal function, and AEs related to volume depletion (). Hypoglycemia occurred in one patient treated with 5 mg luseogliflozin; however, it was classified as mild, recovered after the patient ate some sweets, and did not lead to study discontinuation. Urinary tract infection occurred in one patient treated with 1 mg luseogliflozin (cystitis). This event was mild in severity and recovered after appropriate treatment. Genital infection did not occur in any of the luseogliflozin groups but occurred in one patient treated with placebo (genital herpes). All AEs related to renal function were classified as mild and most of these resolved during the study.
Clinical and laboratory variables
The changes from baseline to the end of treatment in clinical and laboratory variables are presented in . Systolic blood pressure decreased significantly in all four luseogliflozin groups compared with the placebo group (all P < 0.05). Diastolic blood pressure decreased significantly in the 10 mg group compared with the placebo group (P < 0.05). Hypotension and postural hypotension did not occur.
Table 4. Changes in clinical/laboratory variables from baseline to the end of treatment (safety analysis set).
Urine volume increased significantly in all four luseogliflozin groups compared with placebo (all P < 0.05). In addition, there were significant increases in the red blood cell count, hemoglobin, and hematocrit in all four luseogliflozin groups and blood urea nitrogen in the 1 and 10 mg groups compared with the placebo group. However, the changes were small and were not judged as AEs by investigators. Serum uric acid decreased significantly and hepatic enzyme levels tended to decrease in the luseogliflozin groups compared with the placebo group.
The mean triglyceride levels tended to decrease in the 1–5 mg luseogliflozin groups compared with the placebo group and tended to increase in the 10 mg group. The changes in median triglyceride levels from baseline to the end of treatment were −13, −31.0, −13.0, and −14.5 mg/dL in the 1, 2.5, 5 and 10 mg luseogliflozin groups, respectively, versus +1 mg/dL in the placebo group. High-density lipoprotein cholesterol (HDL-C) levels showed a trend to increase in all of the luseogliflozin groups compared with the placebo group, with significant increases in the 2.5 and 10 mg groups.
Fasting and 2 h postprandial acetoacetic acid and β-hydroxybutanoic acid levels increased significantly in the 2.5–10 mg groups compared with placebo (all P < 0.05) except for 2 h postprandial β-hydroxybutanoic acid in the 2.5 mg group. However, the magnitude of the increases in 2 h postprandial levels was smaller than the increases in fasting levels. In addition, the serum fasting acetoacetic acid and β-hydroxybutanoic acid levels did not continue to increase, and the changes were not clinically significant (data not shown). There were no episodes of ketoacidosis.
There were no clinically meaningful changes in serum creatinine, cystatin C, or electrolytes, nor were there any notable changes in 12-lead electrocardiography (data not shown).
Discussion
The present study revealed that treatment with 1–10 mg luseogliflozin monotherapy for 12 weeks significantly improved glycemic control in terms of HbA1c, FPG, and PPG in Japanese patients with T2DM. The improvements in HbA1c and FPG were apparent within 2–4 weeks of starting treatment, and were maintained throughout the study. In addition, the improvements in glycemic control tended to be greater at luseogliflozin doses of ≥2.5 mg compared with the lower dose. These effects were consistent with the results of our prior exploratory phase II studyCitation19 in which the changes in HbA1c from baseline to the end of treatment were −0.36, −0.62, and −0.75% in the 0.5, 2.5, and 5 mg luseogliflozin groups, respectively, versus +0.06% in the placebo group. Therefore, the results of both studies indicate that once-daily treatment with 2.5 mg luseogliflozin shows sufficient glucose-lowering effects in Japanese patients with T2DM.
Treatments for diabetes that do not place an excessive burden on pancreatic β-cells could be useful for long-term administration. This is because the chronic hyperglycemic state in T2DM places a burden on pancreatic β-cells, which may lead to β-cell dysfunction, worsening of glycemic control expressed as glucotoxicity. Accordingly, the results of the meal tolerance tests performed in this study revealed that luseogliflozin improved PPG without excess insulin secretion, which suggests that luseogliflozin might have favorable properties in the treatment of T2DM. Similar results were observed in the prior exploratory phase II studyCitation19 and in several preclinical studies in Zucker fatty ratsCitation16. Furthermore, luseogliflozin prevented decreases in pancreatic β-cells in streptozotocin-treated rats, an animal model characterized by dysfunctional insulin secretion. Thus, luseogliflozin is expected to be useful in clinical practice because of this insulin-independent mechanism of action.
Luseogliflozin was associated with significantly greater reductions in body weight compared with placebo, with some evidence for a dose-dependent effect at doses of 1–5 mg. The reduction was apparent as early as Week 2, and the progressive reduction was sustained until Week 12. One mechanism for the reduction in body weight may involve an increase in urine output because the increase in urinary glucose may have an osmodiuretic effect. The ongoing reductions in body weight throughout the study might reflect a constant caloric loss attributable to maintained urinary glucose excretion.
The reductions in body weight associated with the constant caloric loss are particularly interesting, and luseogliflozin might promote fat oxidation because reductions in triglyceride levels and increases in fasting ketone bodies levels were observed in the luseogliflozin groups. Therefore, luseogliflozin seemed to promote lipolysis in fat cells and the synthesis of ketone bodies in the liver. Although many of the patients in this study only showed slight impairments in insulin secretion (the median fasting CPR level ranged from 1.1–1.4 ng/mL among the study groups), the serum levels of fasting ketone bodies did not continue to increase during the study, but rather decreased rapidly after the meal. In addition, none of the patients experienced ketoacidosis. This indicates that the induction of lipolysis was moderate and the reduction in body weight was within a physiological range. Considering that luseogliflozin will be administered to various types of patients in clinical practice, it is important to obtain further understanding of the metabolic changes that occur during treatment with luseogliflozin.
In this study, plasma lipids tended to improve in the luseogliflozin groups. Patients with T2DM often show dyslipidemia, including hypertriglyceridemia and low HDL-C, because insulin resistance causes a decrease in peripheral lipoprotein lipase activity. Notably, luseogliflozin improved both of these parameters. Although the mean triglyceride level increased in the 10 mg group, unlike in the other groups, this was mainly due to an outlier in this group because one patient had concomitant dyslipidemia, and the investigator considered that the elevated triglyceride level in this patient was related to food intake. The median triglyceride level decreased in all of the luseogliflozin groups. Because low HDL-C and hypertriglyceridemia are associated with increased risk of coronary heart diseaseCitation23, the improvements in plasma lipids during treatment with luseogliflozin are expected to be beneficial for patients with T2DM, although these effects need to be confirmed in a longer study.
At all tested doses up to 10 mg, treatment with luseogliflozin for 12 weeks was well tolerated, and the safety profile was similar to that observed in the prior phase II studyCitation19. There were no significant differences in the incidences of AEs and ADRs among the five groups, and most AEs were classified as mild in severity. Although this was the first study to determine the safety of a high dose of luseogliflozin (i.e., 10 mg), there was no increase in the incidence of AEs at this dose compared with lower doses, supporting the good safety profile of luseogliflozin.
In terms of AEs of special interest, there was only one episode of hypoglycemia, which was classified as mild. The incidence of hypoglycemia was also low in other Japanese studies of SGLT2 inhibitorsCitation24,Citation25. It is thought that hypoglycemia is relatively rare in monotherapy using SGLT2 inhibitors, especially compared with other drugs, such as sulfonylureas, which increase insulin secretionCitation26.
A by-product of enhanced urinary glucose excretion seems to be an increased risk of genital and urinary tract infectionsCitation27,Citation28. In a study of canagliflozin, another SGLT2 inhibitor, the incidence of genital mycotic infections was higher in the canagliflozin group than in the placebo groupCitation29. Nevertheless, the incidence of genital and urinary tract infections was low, and they were generally mild and easily treated in the present study and in the prior phase II studyCitation19. Low incidences of these events were also reported for other SGLT2 inhibitors in Japanese patients with T2DMCitation24,Citation25.
The small increases in red blood cell count, hemoglobin and hematocrit, and the occurrence of thirst in a few patients in this study suggest that the osmodiuretic effect of increased urinary glucose excretion might lead to slight volume depletion. Nevertheless, there were no serious events, such as dehydration, which indicates that there were no major concerns in relation to volume depletion in this study.
The low incidence of AEs of special interest may have been due to the short study duration and the relatively small sample size. Longer and larger phase III studies were recently completed and the results will help provide further insight into the safety profile of luseogliflozin.
Conclusions
This study revealed that monotherapy with 1–10 mg luseogliflozin for 12 weeks significantly improved glycemic control compared with placebo, and the effects were similar at doses of ≥2.5 mg. Luseogliflozin also significantly reduced body weight. Luseogliflozin was well tolerated with no difference in the incidence of AEs among the five study groups. These results suggest that luseogliflozin at doses of ≥2.5 mg will be useful in clinical practice for treating Japanese patients with T2DM.
Transparency
Declaration of funding
This study was supported by Taisho Pharmaceutical Co. Ltd.
Declaration of financial/other relationships
Y.Se. has disclosed that he has received consultancy fees or lecture fees from Sanofi, Novo Nordisk, Eli Lilly and Company, GlaxoSmithKline, Astellas Pharma, Takeda Pharmaceuticals, Boehringer Ingelheim, Johnson & Johnson, Becton Dickinson and Company, AstraZeneca, and Taisho Pharmaceutical Co. Ltd. T.S. has disclosed that he has received joint research funds from Canon Inc. and consultancy fees from Taisho Pharmaceutical Co. Ltd. A.F. has disclosed that he has received consultancy fees from Taisho Pharmaceutical Co. Ltd. M.U., S.S. and Y.Sa. have disclosed that they are employees of Taisho Pharmaceutical Co. Ltd, which is developing luseogliflozin.
CMRO peer reviewers on this manuscript have received an honorarium from CMRO for their review work, but have no relevant financial or other relationships to disclose.
Supplementary Material
Download PDF (70.2 KB)Acknowledgments
We wish to thank Nicholas D. Smith PhD for editorial support.
Previous presentation: Parts of this study were reported as an abstract and poster (abstract 1039-P) at the 72nd Annual Scientific Sessions of the American Diabetes Association, 8–12 June 2012, Philadelphia, PA, USA, and as an abstract and oral presentation (abstract 740) at the 48th Annual Meeting of the European Association for the Study of Diabetes, 1–5 October 2012, Berlin, Germany.
References
- International Diabetes Federation. IDF Diabetes Atlas 5th Edition 2012 Update: New Estimates for 2012 of Diabetes Prevalence, Mortality, and Healthcare Expenditure. 2012
- Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann NY Acad Sci 2013;1281:64-91
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364-79
- Ahren B. DPP-4 inhibition and islet function. J Diabetes Investig 2012;3:3-10
- Seino Y, Yabe D. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1: incretin actions beyond the pancreas. J Diabetes Investig 2013;4:108-30
- Bakris GL, Fonseca VA, Sharma K, et al. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int 2009;75:1272-7
- Sabolic I, Vrhovac I, Eror DB, et al. Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am J Physiol Cell Physiol 2012;302:C1174-88
- Harada N, Inagaki N. Role of sodium-glucose transporters in glucose uptake of the intestine and kidney. J Diabetes Investig 2012;3:352-3
- Jurczak MJ, Lee HY, Birkenfeld AL, et al. SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta-cell function. Diabetes 2011;60:890-8
- Vallon V, Rose M, Gerasimova M, et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 2013;304:F156-67
- Santer R, Calado J. Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clin J Am Soc Nephrol 2010;5:133-41
- Abdul-Ghani MA, DeFronzo RA. Inhibition of renal glucose reabsorption: a novel strategy for achieving glucose control in type 2 diabetes mellitus. Endocr Pract 2008;14:782-90
- DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 2012;14:5-14
- Nair S, Wilding JP. Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab 2010;95:34-42
- Kakinuma H, Oi T, Hashimoto-Tsuchiya Y, et al. (1S)-1,5-anhydro-1-[5-(4-ethoxybenzyl)-2-methoxy-4-methylphenyl]-1-thio-D-glucitol (TS-071) is a potent, selective sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for type 2 diabetes treatment. J Med Chem 2010;53:3247-61
- Yamamoto K, Uchida S, Kitano K, et al. TS-071 is a novel, potent and selective renal sodium-glucose cotransporter 2 (SGLT2) inhibitor with anti-hyperglycaemic activity. Br J Pharmacol 2011;164:181-91
- Sasaki T, Seino Y, Fukatsu A, et al. Safety, pharmacokinetics, and pharmacodynamics of single and multiple luseogliflozin dosing in healthy Japanese males: a randomized, single-blind, placebo-controlled trial. Adv Ther 2014: published online 18 February 2014, doi:10.1007/s12325-014-0102-3
- Sasaki T, Seino Y, Fukatsu A, et al. TS-071, a novel potent and highly selective renal sodium-glucose co-transporter 2 (SGLT2) inhibitor, increases urinary glucose excretion and reduces plasma glucose levels in Japanese patients with type 2 diabetes mellitus. 47th European Association for the Study of Diabetes (EASD) Annual Meeting, 12–16 September 2011. Lisbon, Portugal, 2011
- Seino Y, Sasaki T, Fukatsu A, et al. Efficacy and safety of luseogliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a 12-week, randomized, placebo-controlled, phase II study. Curr Med Res Opin: 2014;30:1219-30
- Kuzuya T, Nakagawa S, Satoh J, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract 2002;55:65-85
- Seino Y, Nanjo K, Tajima N, et al. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. J Diabetes Investig 2010;1:212-28
- Kashiwagi A, Kasuga M, Araki E, et al. International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. Diabetol Int 2012;3:8-10
- Koba S, Sasaki J. Treatment of hyperlipidemia from Japanese evidence. J Atheroscler Thromb 2006;13:267-80
- Inagaki N, Kondo K, Yoshinari T, et al. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab 2013;15:1136-45
- Kaku K, Inoue S, Matsuoka O, et al. Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control: a phase II multicentre, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab 2013;15:432-40
- Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care 2011;34:2015-22
- Nicolle LE, Capuano G, Ways K, et al. Effect of canagliflozin, a sodium glucose co-transporter 2 (SGLT2) inhibitor, on bacteriuria and urinary tract infection in subjects with type 2 diabetes enrolled in a 12-week, phase 2 study. Curr Med Res Opin 2012;28:1167-71
- Nyirjesy P, Zhao Y, Ways K, et al. Evaluation of vulvovaginal symptoms and Candida colonization in women with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co-transporter 2 inhibitor. Curr Med Res Opin 2012;28:1173-8
- Stenlof K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 2013;15:372-82