Abstract
Objective:
To provide real-world data on caregiver and physician perceptions of the advantages and disadvantages of rotigotine transdermal patch (Neupro) versus oral Parkinson’s Disease (PD) medication.
Methods:
Cross-sectional, non-interventional study in routine clinical practice in Germany (NCT01330290). Patients had PD with documented need for care, and had received rotigotine transdermal patch as add-on to oral PD treatment for ≥1 month. Caregivers/nurses and physicians assessed rotigotine transdermal patch versus oral PD medications using questionnaires. Specific questions regarding the possible benefits of transdermal application were asked and comprised questions on: swallowing dysfunction, nausea/vomiting, monitoring therapy, once daily application, application independently from meals, application to sleeping patients, caregiving efforts (caregivers only) and clinical aspects (physicians only). Each question was assessed on a 5 point scale ranging from -2 (major disadvantage) to 2 (major advantage) compared with oral treatment. Primary outcomes were mean total scores of all questions for caregivers/nurses and physicians who provided responses for ≥4 questions. As there are no validated tools to assess physician/caregiver preference in the PD setting, there is no reference against which the current findings can be compared; this study serves to pilot the questionnaires.
Results:
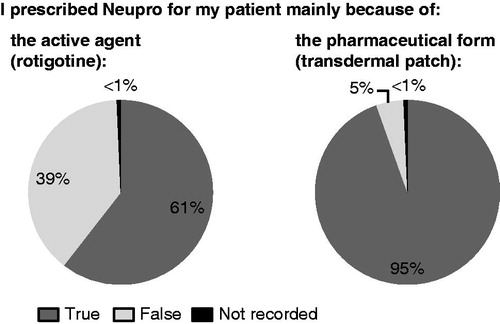
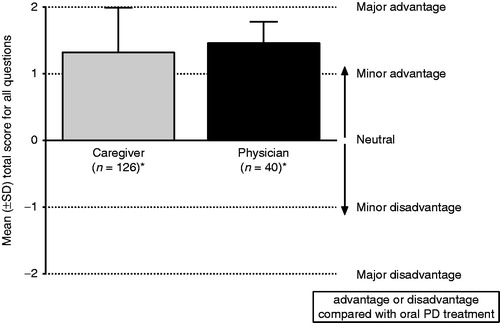
Questionnaire responses from 128 caregivers/nurses and 41 physicians were documented for 147 patients. One hundred (68%) patients had a caregiving family member; 40 (27%) were cared for by a nurse. Mean PD duration was 8.2 (SD 6.3) years; 136 (93%) patients were taking levodopa. Mean total score of caregivers’/nurses’ questionnaires was 1.32 (SD 0.67) and of physicians’ questionnaires was 1.46 (0.32) indicating a perceived advantage of rotigotine transdermal patch over oral PD therapy. Mean scores for individual questions were in the range 1.03–1.54 for caregivers/nurses and 1.15–1.87 for physicians. When given a choice about rationale to prescribe, physicians cited pharmaceutical form (patch) in 139 (95%) cases and active agent (rotigotine) in 89 (61%) cases.
Conclusion:
Caregivers/nurses and physicians perceived advantages with rotigotine transdermal patch compared to an oral PD medication as add-on therapy in patients with PD; advantages were observed in aspects of medical treatment as well as in everyday situations of caregiving of PD patients.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by motor impairments. In addition to the cardinal motor symptoms of PD, non-motor symptoms such as mood and neuropsychiatric disorders, cognitive impairment, sleep disturbances, pain and autonomic dysfunction have a significant impact on patients’ quality of lifeCitation1–3. The symptoms of PD are heterogeneous with varying comorbidities, and may result in the need for a caregiver as the disease progresses. Family members are usually the primary caregivers of patients with moderate PDCitation4. However, support may also be needed from nurses working in outpatient care or in nursing homesCitation5–7. Medication schemes for patients with PD are often very complex; inadequate compliance with medication is an issue and can have clinical consequencesCitation8,Citation9. Moreover, swallowing dysfunction and cognitive impairment can also lead to inaccurate medication dosing and/or a lack of complianceCitation10,Citation11. Managing medication can be a serious burden for caregivers. An improved understanding of physicians’ and caregivers’ treatment preferences in patients with PD may help maximize intervention effectiveness.
Rotigotine, a dopamine receptor agonist, is the only available medication for PD that is delivered via a transdermal patch. Continuous transdermal delivery of rotigotine maintains stable plasma levels over 24 hours with a single daily applicationCitation12. Significant treatment effects of rotigotine transdermal patch (Neupro) have been observed in five major double-blind, placebo-controlled studies as monotherapy in patients with early-stage PDCitation13–15 and as add-on therapy to levodopa in advanced-stage PDCitation16,Citation17, and in patients with PD and unsatisfactory control of early-morning motor functionCitation18.
This cross-sectional, non-interventional study was conducted to assess caregiver and physician perceptions of the real-world advantages and disadvantages of rotigotine transdermal patch compared with oral PD medications in patients with PD in documented need of care. Preferences for rotigotine transdermal patch vs oral medications were evaluated using questionnaires. It was anticipated that transdermal application may offer benefits in comparison with an oral therapy to the physician and/or caregiver in certain aspects of administering/managing medications, and thus questions relating to potential benefits of transdermal delivery were asked.
Patients and methods
Design
The CARE-ACT study (SP0939; ClinicalTrials.gov NCT01330290) was a multicenter, non-interventional, cross-sectional evaluation of caregivers’ and physicians’ preferred route of PD drug administration (rotigotine transdermal patch vs oral PD medication) in patients requiring caregiver support, and the physicians’ rationale in daily practice for the choice of rotigotine transdermal patch as add-on therapy in these patients.
The evaluation was carried out in routine clinical settings in Germany. Physicians treating patients with PD in outpatient care or in nursing homes were invited to participate. Patients and their corresponding caregivers were selected by the physician; caregivers could be family members at home, or professional nurses working in nursing homes or outpatient care. Eligible patients had a diagnosis of idiopathic PD and were in need of care as documented in their medical record (e.g. this could be based on the German level of care intensity of at least 1 [Pflegestufe ≥1]). Other inclusion criteria required that the patients had been treated for at least 1 month with a combination therapy oral PD medication and rotigotine transdermal patch. The decision to prescribe rotigotine was made by the treating physician according to regular clinical practice, and was reached prior to and independently of the decision to include the patient in the study. Treatment according to the European Summary of Product Characteristics was recommendedCitation19.
The observational plan, amendments, and patient data consent form were reviewed and approved by the Institutional Ethics Committee of the Ernst-Moritz-Arndt University, Greifswald, Germany, and the study was conducted in compliance with local legal requirements for non-interventional studies. All patients provided written informed consent regarding use of data, and were free to withdraw consent for use of their data at any time during this study.
Questionnaire assessments
Caregivers and physicians were asked to complete questionnaires to assess rotigotine transdermal patch compared with oral PD medication for each of their patients. The questionnaires were developed by physicians participating in the study (including the authors) in association with UCB Pharma, and based on clinical experience with PD patients in need of care. In the absence of instruments that specifically address caregiver preference/satisfaction for different PD medications, the validated Alzheimer’s Disease Caregivers Preference Questionnaire, developed to assess Alzheimer’s Disease caregivers’ satisfaction with and preference for transdermal patch or capsule treatments in patients with Alzheimer’s DiseaseCitation20,Citation21, was used as a sample questionnaire and adapted to the needs of patients with PD. This study is the first to pilot these questionnaires assessing rotigotine transdermal patch compared with oral PD medication. The questionnaires were devised and completed in German. Separate questionnaires were developed for physicians (10 questions) and for caregivers/nurses (7 questions), covering clinical aspects and questions of caregiving (); a patient could have a questionnaire completed by a caregiver only, a physician only or by both caregiver and physician. Each physician and caregiver/nurse could assess multiple patients. To avoid a bias resulting from multiple identical answers given by the same person, the analysis was based primarily on the number of physicians and caregivers, rather than the number of patients; multiple assessments of a single question were first averaged per caregiver/nurse or physician to obtain a single response per caregiver/nurse or physician. These averages were used for calculation of the mean scores. The mean total score of a questionnaire was calculated if at least four questions had been answered; this cut-off was used because a minimum of four questions were applicable to every patient. Each question was assessed on a 5 point scale: ‘major disadvantages’ (-2), ‘minor disadvantages’ (1), ‘neutral’ (0), ‘minor advantages’ (1), and ‘major advantages’ (2), compared with oral PD treatment.
Table 1. Caregiver and Physician Questionnaire (translated from the original German language version).
Two primary variables were defined: (1) mean score (all items) of caregivers’/nurses’ assessment of rotigotine transdermal patch compared to an oral PD therapy, and (2) mean score (all items) of physicians’ assessment of rotigotine transdermal patch compared to an oral PD therapy. The secondary variables were: (1) single item scores of caregivers’/nurses’ and physicians’ assessment of rotigotine patch compared to an oral therapy, and (2) medical rationale for physicians’ prescription of rotigotine transdermal patch. For this, physicians answered true or false for the following: (i) ‘I have prescribed Neupro for my patient mainly because of the active agent contained (rotigotine)’; (ii) ‘I have prescribed Neupro for my patient mainly because of the pharmaceutical form (transdermal patch)’.
Other assessments
Demographic characteristics (age, gender, duration of PD, Hoehn and Yahr stage, level of German care intensity) were recorded. Tests assessing patient mobility (timed up-and-go test), activities of daily living (Barthel Index), and cognitive function (Mini Mental State Examination [MMSE], Montreal Cognitive Assessment [MoCA] test and Parkinson Neuropsychometric Dementia Assessment [PANDA]) were reported only if the data were already documented in the patient’s medical files. Safety was assessed by incidence of adverse events (AEs) and serious AEs. Physicians were asked to report AEs documented at the time of data collection.
Statistical analyses
Data were recorded at a single point of time for each patient. Patient demographic characteristics and assessments of primary and secondary variables were reported using the full analysis set, which comprised all patients who provided informed consent and who had at least one assessment in the physicians’ or caregivers’/nurses’ questionnaires. Safety was assessed using the safety set, which comprised all patients who had been treated with rotigotine. All variables are presented using descriptive summary statistics, and no formal statistical testing was performed.
Results
Patients
Overall, 41 sites in Germany enrolled patients between 16 March 2011 and 14 August 2012. Of 148 patients enrolled, 147 were included in the full analysis set (one patient was excluded due to missing informed consent). In total, 128 caregivers/nurses were enrolled in this study, and completed questionnaires for 143 of 147 patients in the full analysis set. A total of 41 physicians completed questionnaires for the 147 patients in the full analysis set. Patients included in this observational study reported a mean duration of PD of 8.2 years, and were recorded as Hoehn and Yahr stages 1–5, representing all stages of motor impairment, with the majority being in stages 3–4. Most (88%) patients were older than 65 years, and all required caregiver support. Patient demographics and disease characteristics are reported in , and available data for tests assessing mobility, activities of daily living and cognitive function are reported in . MoCA and PANDA test data were only available for two and 16 patients respectively, and are therefore not reported due to the small sample size. The mean ± SD daily dose of rotigotine was 6.7 ± 3.8 mg/24 h, and patients had been receiving rotigotine on average for approximately 1 year 9 months ( ±1 year 10 months; range 0 months – 8 years 5 months). A total of 136 (93%) patients were additionally taking levodopa, and 26 (18%) were taking other dopamine receptor agonists.
Table 2. Patient demographics and disease characteristics.
Table 3. Patient mobility, activities of daily living and cognitive function§.
The majority of patients (68%) had a caregiving family member, and 27% were cared for by a nurse working in either outpatient care or in a nursing home ().
Table 4. Caregiver relation.
Primary variables
Mean total score (all items) of (1) caregivers’/nurses’ and (2) physicians’ assessment of rotigotine patch compared to an oral therapy
The mean (±SD) total score recorded from the caregivers’/nurses’ questionnaire was 1.32 ± 0.67, indicating a perceived advantage of rotigotine transdermal patch over oral PD therapy. The results were similar for the physicians’ assessment; the mean (±SD) total score recorded from the physicians’ questionnaire was 1.46 ± 0.32, also indicating an advantage of the patch over oral PD therapy ().
Figure 1. Mean score of all questions for assessment of rotigotine transdermal patch (Neupro) compared with oral PD treatment. The mean was calculated if at least four questions were answered. *Multiple patient assessments provided by one person were averaged to obtain a single response per caregiver/physician. PD, Parkinson’s disease; SD, standard deviation.
Secondary outcomes
Single item scores of the assessment of PD medication as a patch (rotigotine) versus oral medication by caregivers/nurses and physicians
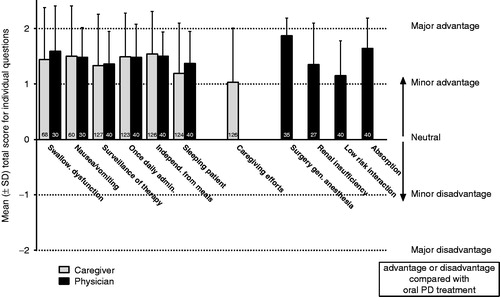
The mean scores for individual questions were in the range 1.03–1.54 for caregivers/nurses and 1.15–1.87 for physicians (), representing at least a minor advantage of rotigotine over oral PD therapy for all questions.
Figure 2. Mean score of individual questions for assessment of rotigotine transdermal patch (Neupro) compared with oral PD treatment. Number of patients for each question displayed in the bars; multiple patient assessments provided by one person were averaged to obtain a single response per caregiver/physician. PD, Parkinson’s disease; SD, standard deviation.
Medical rationale for physicians’ prescription of rotigotine transdermal patch
When given a choice about their rationale to prescribe rotigotine transdermal patch for each patient, physicians selected the pharmaceutical form (patch) in 139/147 (95%) cases and the active agent (rotigotine) in 89/147 (61%) cases ().
Safety
Due to the cross-sectional design of this observational study, assessments were made at only a single time point. One patient experienced non-serious palpitations and hallucinations. These AEs were considered to be related to treatment; rotigotine was temporarily interrupted and the patient recovered.
Discussion
This is the first study to report caregiver and physician assessments of the advantages and disadvantages of a transdermal patch compared with oral PD medications in patients with PD. Managing medications can contribute to the workload associated with caring for patients with PD, and therefore a simple, convenient medication for PD may reduce caregiver burdenCitation8,Citation9. Overall, caregivers/nurses and physicians provided positive feedback on the treatment with rotigotine transdermal patch in this observational cross-sectional study.
The mean score of all questions of both the caregivers’ and physicians’ questionnaires indicated perceived advantages of rotigotine transdermal patch in both the treatment of patients with PD and in situations of daily caregiving in comparison to an oral medication. Notably, the opinions of caregivers/nurses were very similar to those of physicians with comparable ratings for all questions asked. The visibility of the patch, its once daily application, and the ability to administer it independently from meals and to sleeping patients were all considered to be advantageous. Caregivers and physicians also perceived advantages of rotigotine over oral therapy when treating patients with specific PD symptoms, e.g. swallowing dysfunction, or problems with nausea/vomiting. There are various challenges and difficulties faced by caregivers of patients with PDCitation22. It is therefore notable that, when asked, caregivers/nurses felt that the rotigotine transdermal patch offered advantages over oral therapy in terms of caregiving efforts, suggesting that the caregivers felt satisfied with this medication.
The results from this evaluation also suggest that physicians may consider the rotigotine transdermal patch to have advantages over oral PD medications in certain clinical situations, for example in patients undergoing surgery under general anesthesia, and in special populations, e.g. in those with impaired renal function, as no adaptation of dose is necessary. This is in support of previous findingsCitation23–25. The physicians participating in this observational study reported prescribing rotigotine transdermal patch as treatment for their patients (>60%) because it contained rotigotine as the active substance. However, notably, the physicians reported prescribing rotigotine in more than 95% of patients because of the pharmaceutical form, i.e. the transdermal patch, suggesting that the transdermal route of administration strongly influenced treatment preference. Overall, these results suggest that the active agent, rotigotine, as well as the transdermal patch were well accepted by the physicians who participated in this study. No new safety concerns regarding therapy with the rotigotine transdermal patch were observed during the course of this observational study. However, due to the cross-sectional design, no safety conclusions can be drawn.
In addition to caregiver and physician assessments of PD therapy, knowledge of patients’ treatment needs is also an important requirement for comprehensive long-term patient care. The findings from the current study are in line with a questionnaire-based patient survey, which indicated that patients’ preferred PD therapy formulations were a once daily patch or a once daily tabletCitation26. Moreover, most patients rated continuous drug delivery over 24 hours, 24 hour symptom control and non-dependency on meals as important properties of a PD medicationCitation26. It is notable that similar results have been observed in terms of treatment preferences in studies of patients with Alzheimer’s disease, a common neurodegenerative disorder which also requires a high level of caregiver support. Observational studies of patients with Alzheimer’s disease and their caregivers demonstrated that caregivers preferred rivastigmine transdermal patch to a rivastigmine capsuleCitation21, and that the patch formulation may also improve caregiver burden and treatment adherence of patientsCitation27. Once daily transdermal patches may thus offer a convenient medication for the treatment of patients with neurodegenerative disorders such as Alzheimer’s disease and PD, and may also reduce caregiver burden. It is notable that a multicenter European study found significantly better adherence with once daily PD medication compared to medication prescribed more frequentlyCitation9. Furthermore, the rotigotine transdermal patch was associated with high treatment compliance in a non-interventional, multicenter study of patients with PD under clinical practice conditionsCitation28.
This study has a number of inherent limitations. It is the first to pilot the current questionnaires developed to assess the advantages and disadvantages of a transdermal patch compared with oral PD medications in patients with PD. As there are no available validated tools to assess physician/caregiver preference in the PD setting, there is therefore no reference against which the current findings can be compared. Moreover, the questionnaires have not been validated. However, the variability of the results appeared low, suggesting that there was a reasonable level of consistency. Secondly, caregivers/nurses and physicians were asked specific questions regarding the possible advantages of rotigotine; this may have created a potential bias. A less directed questionnaire may have identified disadvantages or additional perceived advantages of rotigotine compared with oral PD therapies. Finally, a number of patients were included in the analyses without reaching a German level of care intensity of at least 1 (Pflegestufe ≥ 1). However, all patients included in the study were documented as in need of care in their medical record, and received caregiver support.
Conclusion
In summary, the results from this observational study suggest that caregivers/nurses and physicians may prefer the rotigotine transdermal patch to an oral PD medication as add-on therapy in patients with PD. The findings of the questionnaire indicated perceived advantages with rotigotine transdermal patches in medical treatment as well as in everyday situations of caregiving of PD patients.
Transparency
Declaration of funding
This study was supported by UCB Pharma, Monheim am Rhein, Germany. The questionnaires were devised by the authors in association with UCB Pharma.
Declaration of financial/other relationships
J.P.S. has disclosed that he has received honoraria for lectures and for advisory efforts from Boehringer Ingelheim, Teva and UCB Pharma prior to 2012; however, since 2012 he receives no honoraria from any pharmaceutical companies. P.T. has disclosed that he has received lecture fees from Abbvie, TEVA, UCB Pharma, Orion Pharma, Novartis and Desitin. T.W. has disclosed that he has received lecture fees from Abbvie, Teva, Bayer and UCB Pharma; worked as a consultant for Archimedes and UCB Pharma; and receives a research grant from Deutsche Parkinson Vereinigung eV (dPV). S.L. has disclosed that he has received honoraria from UCB Pharma, Boehringer, Teva, Gruenenthal and Orion Pharma. T.L., R.B. and F.G. have disclosed that they are employees of UCB Pharma, Monheim am Rhein, Germany.
CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors acknowledge Emily Thompson PhD (Evidence Scientific Solutions, London, UK) for writing support which was funded by UCB Pharma, Brussels, Belgium, and Cédric Laloyoux PhD (Global Publications Manager, UCB Pharma, Brussels, Belgium) for publication coordination.
Previous presentation: Abstract presented at the World Parkinson Congress, 1–4 October 2013, Montreal, Canada: Sieb JP, Themann P, Warnecke T, et al. Non-interventional study of caregivers’ and physicians’ attitudes to drug administration via a transdermal patch in patients with Parkinson’s disease who require caregiver support.
Notes
*Neupro is a registered trade name of UCB Manufacturing Ireland Ltd, Shannon, Ireland
*Neupro is a registered trade name of UCB Manufacturing Ireland Ltd, Shannon, Ireland
References
- Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson's disease. Mov Disord 2011;26:399-406
- Chaudhuri KR, Odin P, Antonini A, et al. Parkinson's disease: the non-motor issues. Parkinsonism Relat Disord 2011;17:717-23
- Antonini A, Barone P, Marconi R, et al. The progression of non-motor symptoms in Parkinson's disease and their contribution to motor disability and quality of life. J Neurol 2012;259:2621-31
- Martinez-Martin P, Rodriguez-Blazquez C, Forjaz MJ. Quality of life and burden in caregivers for patients with Parkinson's disease: concepts, assessment and related factors. Expert Rev Pharmacoecon Outcomes Res 2012;12:221-30
- Tse W, Frisina PG, Halbig TD, et al. The effects of withdrawal of dopaminergic medication in nursing home patients with advanced parkinsonism. J Am Med Dir Assoc 2008;9:670-5
- van Rumund A, Weerkamp N, Tissingh G, et al. Perspectives on Parkinson Disease Care in Dutch Nursing Homes. J Am Med Dir Assoc 2014;15:732-7
- Aarsland D, Larsen JP, Tandberg E, et al. Predictors of nursing home placement in Parkinson's disease: a population-based, prospective study. J Am Geriatrics Soc 2000;48:938-42
- Grosset D. Therapy adherence issues in Parkinson's disease. J Neurol Sci 2010;289:115-18
- Grosset D, Antonini A, Canesi M, et al. Adherence to antiparkinson medication in a multicenter European study. Mov Disord 2009;24:826-32
- Fargel M, Grobe B, Oesterle E, et al. Treatment of Parkinson's disease: a survey of patients and neurologists. Clin Drug Investig 2007;27:207-18
- Leopold NA, Polansky M, Hurka MR. Drug adherence in Parkinson's disease. Mov Disord 2004;19:513-17
- Elshoff JP, Braun M, Andreas JO, et al. Steady-state plasma concentration profile of transdermal rotigotine: an integrated analysis of three, open-label, randomized, phase I multiple dose studies. Clin Therapeut 2012;34:966-78
- Giladi N, Boroojerdi B, Korczyn AD, et al. Rotigotine transdermal patch in early Parkinson's disease: a randomized, double-blind, controlled study versus placebo and ropinirole. Mov Disord 2007;22:2398-404
- Jankovic J, Watts RL, Martin W, et al. Transdermal rotigotine: double-blind, placebo-controlled trial in Parkinson disease. Arch Neurol 2007;64:676-82
- Watts RL, Jankovic J, Waters C, et al. Randomized, blind, controlled trial of transdermal rotigotine in early Parkinson disease. Neurology 2007;68:272-6
- Poewe WH, Rascol O, Quinn N, et al. Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson's disease: a double-blind, double-dummy, randomised controlled trial. Lancet Neurol 2007;6:513-20
- LeWitt PA, Lyons KE, Pahwa R. Advanced Parkinson disease treated with rotigotine transdermal system: PREFER Study. Neurology 2007;68:1262-7
- Trenkwalder C, Kies B, Rudzinska M, et al. Rotigotine effects on early morning motor function and sleep in Parkinson's disease: A double-blind, randomized, placebo-controlled study (RECOVER). Mov Disord 2011;26:90-9
- European Medicines Agency. Neupro: EPAR Product Information. Summary of Product Characteristics. UCB Manufacturing Ireland Ltd, Ireland; first published 21 December 2009
- Abetz L, Rofail D, Mertzanis P, et al. Alzheimer's disease treatment: assessing caregiver preferences for mode of treatment delivery. Adv Ther 2009;26:627-44
- Winblad B, Kawata AK, Beusterien KM, et al. Caregiver preference for rivastigmine patch relative to capsules for treatment of probable Alzheimer's disease. Int J Geriatr Psychiatr 2007;22:485-91
- Martinez-Martin P, Forjaz MJ, Frades-Payo B, et al. Caregiver burden in Parkinson's disease. Mov Disord 2007;22:924-31; quiz 1060
- Wüllner U, Kassubek J, Odin P, et al. Transdermal rotigotine for the perioperative management of Parkinson's disease. J Neural Transmission 2010;117:855-9
- Cawello W, Ahrweiler S, Sulowicz W, et al. Single dose pharmacokinetics of the transdermal rotigotine patch in patients with impaired renal function. Br J Clin Pharmacol 2012;73:46-54
- Korczyn AD, Reichmann H, Boroojerdi B, et al. Rotigotine transdermal system for perioperative administration. J Neural Transmission 2007;114:219-21
- Wullner U, Fuchs G, Reketat N, et al. Requirements for Parkinson's disease pharmacotherapy from the patients' perspective: a questionnaire-based survey. Curr Med Res Opin 2012;28:1239-46
- Adler G, Mueller B, Articus K. The transdermal formulation of rivastigmine improves caregiver burden and treatment adherence of patients with Alzheimer's disease under daily practice conditions. Int J Clin Pract 2014;68:465-70
- Schnitzler A, Leffers KW, Hack HJ. High compliance with rotigotine transdermal patch in the treatment of idiopathic Parkinson's disease. Parkinsonism Relat Disord 2010;16:513-16