Abstract
Objective:
The objective was to conduct a systematic review and network meta-analysis (NMA) of existing treatments for ABSSSI focusing on the novel lipoglycopeptide oritavancin.
Methods:
EMBASE, MEDLINE, MEDLINE in Process, CENTRAL (Cochrane), and select conferences were searched for randomized controlled trials investigating antimicrobial agents for the treatment of ABSSSI. NMA was used to estimate the odds ratios of the Test-Of-Cure (TOC) and Early Clinical Response (ECR) outcomes for treatments relative to vancomycin in the ITT populations. Sub-group analyses in MRSA and MSSA populations were conducted for TOC; sensitivity analyses investigated the use of the clinically evaluable (CE) populations and the restriction to trials following the recent FDA guidelines for clinical trials.
Results:
The systematic review identified 52 trials. The most commonly investigated treatments were vancomycin and linezolid; most trials reported TOC, but not ECR. The posterior mean and 95% credible intervals for odds ratios of TOC for antimicrobial agents relative to vancomycin were: linezolid (1.55; 0.91–2.57), daptomycin (2.18; 0.90–5.42), and oritavancin 1200 mg (1.06; 0.80–1.43). The odds ratio of ECR for oritavancin 1200 mg was 1.02 (0.23–4.33). In the MRSA sub-group the odds ratios relative to vancomycin for TOC were: linezolid (1.55; 0.96–2.46), daptomycin (0.74; 0.13–3.66), and oritavancin 1200 mg (0.94; 0.44–2.02). In the MSSA sub-group they were linezolid (1.36; 0.15–13.34) and oritavancin 1200 mg (0.82; 0.08–7.83). These results were robust to the sensitivity analyses.
Conclusions:
This NMA provides a unified framework for the comparison of all available antimicrobial agents used in the treatment of ABSSSI and is the first to assess the ECR end-point. The results suggest equivalence of clinical efficacy between vancomycin, daptomycin, linezolid, and novel antimicrobial agents including oritavancin for the treatment of ABSSSI at TOC. The wide uncertainty margins indicate the heterogeneity of the available evidence and the need for further research.
Introduction
Acute bacterial skin and skin structure infections (ABSSSI) incidence is rising globallyCitation1, with recent annual incidence estimates requiring healthcare intervention in the US of 4.96%Citation2, which represents an increased healthcare and economic burdenCitation3,Citation4. The 2010 FDA guidelines define ABSSSI as bacterial infections of the skin with a lesion size of at least 75 cm2 (measured by the area of redness, edema, or induration) and include cellulitis, wound infection, and abscesses with surrounding cellulitisCitation5. ABSSSI are caused by many different bacteria, most commonly gram-positive bacteria including methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S. aureus (MSSA)Citation6,Citation7 and with increasing antibiotic resistance treatment is becoming more difficultCitation8–10. A surveillance study found 44.6% of ABSSSI in North America were caused by S. aureus and 80% of these exhibited methicillin resistanceCitation11. Current treatment can include administration of broad-spectrum antibiotics; however, in the case of known or suspected MRSA targeted antibiotics are administered over 7–14 daysCitation12–14.
Vancomycin, linezolid, and daptomycin are among the most common antibiotics used in the treatment of ABSSSI, but there is limited evidence on their comparative effectivenessCitation15,Citation16. Recently approved antibiotics for the treatment of ABSSSI, such as oritavancinCitation17, dalbavancinCitation18, and tedizolidCitation19, further highlight the need for comparative effectiveness research. The majority of published randomized controlled trials (RCTs) have been compared to vancomycin and have not achieved statistically significant differencesCitation15, although, arguably, the strongest results have been found in favor of linezolid over vancomycinCitation20–23. Thirteen meta-analyses have been conducted comparing the efficacy of vancomycin, linezolid, and other anti-microbial agents, the majority comparing linezolid with vancomycin and finding clinical superiority of linezolidCitation7,Citation12,Citation24–34. However, most of these considered only direct comparisons of two treatments and neglected the impact of indirect evidence.
Bayesian network meta-analysis (NMA) is a statistical method used to combine the results from multiple studies and indirectly compare multiple treatments, thus incorporating all available direct and indirect evidenceCitation35–37. Two Bayesian NMAs have been conducted in ABSSSI caused by MRSA. The first compared telavancin, daptomycin, vancomycin, linezolid, tigecycline, and dalbavancin and found higher success rates for linezolid, dalbavancin, and telavancin, but the analysis only included studies published by 2010, and omitted recently approved antimicrobial agents such as oritavancinCitation7. The second was conducted in 2012 and compared vancomycin, linezolid, and ceftarolineCitation26. Since the publication of these analyses, the FDA has issued guidance on the conduct of clinical trials in ABSSSI, and studies following these guidelines are expected to be more homogeneous and appropriate for NMACitation5.
These limitations of the current evidence on the comparative efficacy of antimicrobial agents, particularly with regards oritavancin and other novel therapies for the treatment of ABSSSI, motivates the conduct of a systematic literature review and Bayesian NMA.
Methods
A systematic literature review and Bayesian NMA was conducted to compare the efficacy and safety of common antimicrobial agents used in the treatment of ABSSSI.
Systematic literature review
RCTs meeting inclusion criteria were identified by searching the EMBASE, MEDLINE, and MEDLINE in Process and the Cochrane Central Register of Controlled Trials databases. Searches were conducted from database inception to January 2, 2014 (available on request). In addition, conference proceedings from the 2012 and 2013 Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Infectious Diseases Week (ID Week), and European Society for Clinical Microbiology and Infectious Diseases (ECCMID) conferences were searched for relevant abstracts describing RCTs meeting inclusion criteria.
Publications were included if they recruited adult patients with ABSSSI and if the investigated interventions included oritavancin, vancomycin, linezolid, tigecycline, dalbavancin, daptomycin, ceftaroline, teicoplanin, clindamycin, or telavancin; additional treatments were included if they were investigated in a study that included one of the listed interventions. The following outcomes were considered: clinical response, clinical cure, Early Clinical Response (ECR), lesion size reduction, relapse/recurrence, sustained clinical response, overall incidence of adverse events (AEs), treatment emergent AEs, serious AEs, and the most commonly reported AEs across studies (nausea, vomiting, diarrhea, pain, fever, headache, and pruritus). Studies were restricted to English language RCTs.
The risk of bias of the included full publications was assessed using the Cochrane risk of bias toolCitation38. Important aspects of risk of bias are not normally reported in conference abstracts, so a critical appraisal of trials reported in abstracts only was not conducted.
Definitions and outcomes
The FDA guidance for ABSSSI recommends four study populations for analyses. The primary population that should be considered is the intention to treat (ITT) population, which includes all randomized patients; other populations are the clinically evaluable (CE) population, which includes patients from the ITT population who follow important components of the trial such as attendance at follow-up examinations and demonstrate compliance to treatment (definitions vary by study), the microbiologically evaluable (ME) population which includes ITT patients with a confirmed baseline bacterial pathogen, and the safety population which includes all patients who received at least one dose of study drug.
Base case analyses were conducted using trials reporting efficacy of antimicrobial agents in the treatment of ABSSSI due to a mixture of microbial causes. Two key outcomes were chosen for these analyses. The first is treatment success at the ‘Test-Of-Cure’ (TOC) visit, commonly 7–14 days after the end of treatment, in the intention to treat (ITT) population (as recommended by the FDA guidance). Treatment success in ABSSSI can be determined either clinically or microbiologically. Clinical success is generally characterized as resolution of the symptoms associated with the infection present at admission or improvement to the extent that no further therapy was necessary; in the absence of a culture it is presumed that microbiological eradication has occurred. When a culture is taken, microbiological success is defined as eradication or presumed eradication of the baseline pathogen in a culture taken at a follow-up visit. These measures of success were judged equivalent for the purposes of this analysis. The second outcome was ECR. As of October 2013, this is the primary end-point for the clinical assessment of treatment success in ABSSSI of the FDA in the US and is defined as cessation of spread of skin lesion erythema and absence of fever at 48–72 h after treatment initiationCitation5.
Sub-group analyses were performed for the microbiologically confirmed MRSA and MSSA populations for the TOC and ECR outcomes. By definition, these were conducted in the microbiologically evaluable (ME) populations.
Sensitivity analyses assessing robustness of patient population definitions were conducted. To clinicians, the response of patients who are compliant to treatment (i.e. CE population) can be seen as more informative than response in the ITT population. Therefore, analyses of the TOC and ECR end-points were conducted using the CE population in preference to the ITT population. In addition, the TOC end-point in the ITT and CE populations using the sub-set of trials that followed recent FDA guidance on patient inclusion in clinical trials were analyzedCitation5. This guidance recommends including patients with the previously noted FDA definition of ABSSSI and a mixture of both male and female patients and ABSSSI types, such as wound infection or major cutaneous abscess. A number of patient types to exclude are also recommended, such as those who have received more than 24 h of successful treatment for the current episode of ABSSSI or those with other medical conditions, for example neutropenia, that may interfere with the interpretation of the primary end-point. This reduces the heterogeneity of ABSSSI patients across trials.
Statistical analysis
The Bayesian NMA was conducted using recommendations from the NICE Decision Support Unit for count data modeling, and adopted a generalized linear model with a binomial outcome and logistic link functionCitation39. Vague priors were used for baseline and treatment effects. Treatment effects were estimated as mean odds ratios compared to vancomycin, alongside Bayesian credible intervals (CrI). The model code is provided in the Supplementary Appendix.
Random treatment effects (RE) models were used to account for heterogeneity across trialsCitation40. The Deviance Information Criterion (DIC)Citation41 was used to compare the model fit. A random effects model was used for each analysis unless its DIC was five units higher than that of the fixed effects modelCitation42. The exception is if less than three studies were included in the analysis or if the posterior estimates were unstable, in which case the fixed effects model was preferred.
Inconsistency between direct and indirect treatment effect estimates may also be present in an evidence networkCitation43,Citation44. The consistency between direct and indirect evidence was assessed for the best fitting model for each end-point using the node-splitting approachCitation45.
Analyses were conducted using OpenBUGS (Version 3.2.2 rev 1063 2012-07-15, Medical Research Council and Imperial College, London, UK)Citation42,Citation46. Samples from 50,000 iterations of the posterior distribution of two chains, with a 50,000 iteration burn-in to ensure convergence, were used for parameter estimates. Caterpillar and Brooks-Gelman-Rubin (BGR) plots of the estimated parameters were examined to ensure that the models converged satisfactorilyCitation42.
Results
Results of systematic literature review
The systematic review identified 52 trials in 50 published articles (46 trials) and eight conference abstracts (six trials) (). Two of the six trials identified in conference abstracts were found to have been published by June 5, 2014 and the clinical study reports for SOLO ICitation3 and SOLO IICitation17 were provided by The Medicines Company. Vancomycin and linezolid were the most frequently reported treatments, being included in 26 and 18 out of the 52 trials, respectively.
Figure 1. PRISMA flowchart illustrating systematic literature review strategy and article selection process.
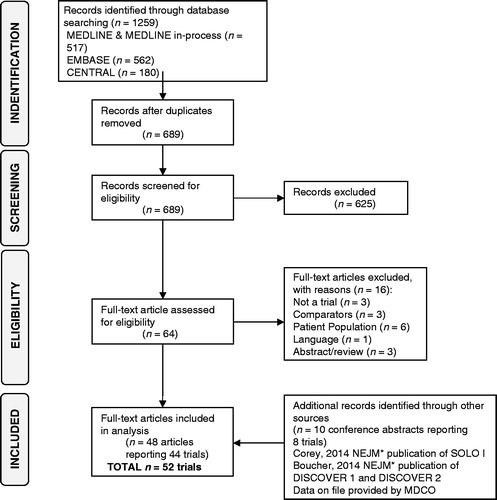
The trials were generally judged to be at low risk of bias (results presented in the Supplementary Appendix). An exception was in studies where TOC was assessed clinically but the assessors were inadequately blinded. Treatment doses and administration across trials were considered clinically homogenous. Two studies were excluded from the analysis for administering doses not used in clinical practice; the daptomycin arm of Katz et al.Citation47 and the dalbavancin arm of Seltzer et al.Citation48. Oritavancin doses were considered separately. Six trials with comparator arms where patients continued current therapy or received an option of treatments after randomization were considered too heterogeneous and were excluded from the analysisCitation48–53. Five trials were excluded as TOC or ECR were not reported in the ITT populationCitation21,Citation54–57. A further five trials, all published in 1990 or earlier, investigated treatments that could not be connected to the evidence network and, thus, were not included in the analysisCitation58–62. The Teras et al.Citation63 study was excluded as it was a sub-group analysis of the Breedt et al.Citation64 trial. Summaries of the 33 trials included in the NMA are provided in .
Table 1. Summary of studies include in network meta-analysis with results for TOC ITT outcomes.
The definition of ECR varied widely across studies; definitions included characterization solely by absence of fever or absence of fever as well as absence of need for rescue antibiotics. The evidence network for ECR included only 10 studies, while TOC included 26 studies.
Results of base case mixed treatment comparisons
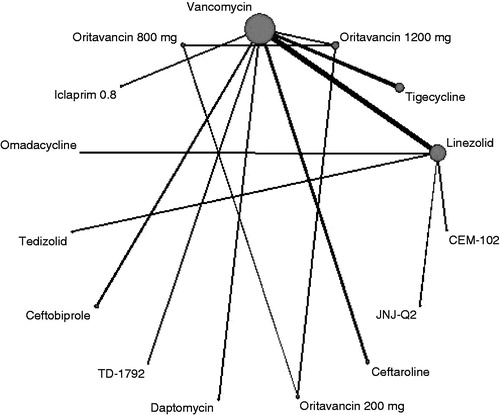
In total, there were 19 treatments in 26 studies that reported TOC and were connected to the evidence network (). Clindamycin (and combinations) and telavancin did not connect to the evidence network, so relative treatment effects could not be calculated. Treatment effects are presented as odds ratios vs vancomycin, with ratios greater than one indicating a superior TOC outcome for the comparator (). The posterior mean and 95% credible intervals (CrI) for odds ratios of TOC for antimicrobial agents relative to vancomycin were linezolid (OR = 1.55; 95% CrI = 0.91–2.57), dalbavancin (OR = 1.76; 95% CrI = 0.84–3.61), daptomycin (OR = 2.18; 95% CrI = 0.90–5.42), and oritavancin 1200 mg (OR = 1.06; 95% CrI = 0.80–1.43), showing no evidence of a difference in efficacy. Omadacycline showed a statistically significant improvement in TOC over vancomycin (OR = 2.48; 95% CrI = 1.10–5.43). All other 95% credible intervals crossed one and, therefore, none of the other treatments showed a statistically significant difference in TOC compared to vancomycin.
Figure 2. Evidence network for TOC end-point ITT analysis. The thickness of the lines represents the number of studies available for each comparison and the size of the nodes represents the number of studies available for each treatment.
Table 2. Mean odds ratios (95% credible intervals) of treatments vs vancomycin for base case and sub-group analyses.
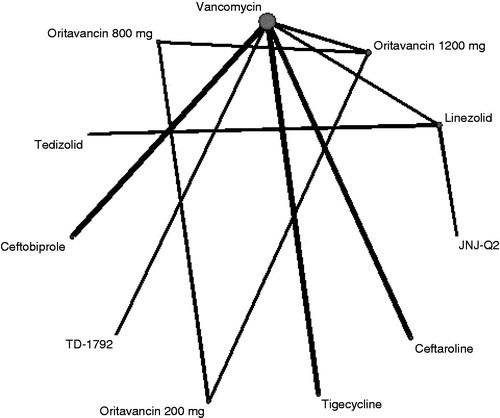
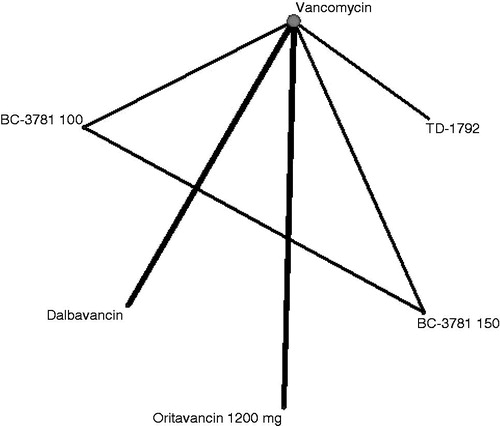
The network for ECR included six treatments in 10 studies so was much more limited than the TOC end-point, allowing fewer indirect comparisons, in particular omitting linezolid (). The 95% credible intervals around the mean odds ratio relative to vancomycin of ECR for dalbavancin (OR = 1.01; 95% CrI = 0.24–4.41), oritavancin 1200 mg (OR = 1.02; 95% CrI = 0.23–4.33), and all other treatments crossed unity. There was, therefore, no statistically significant evidence of improvement in ECR compared to vancomycin ().
Figure 3. Evidence network for ECR end-point ITT analysis. The thickness of the lines represents the number of studies available for each comparison and the size of the nodes represents the number of studies available for each treatment.
Fixed effects results for both these analyses were similar and are presented in the Supplementary Appendix.
Results of subgroup analyses—MRSA and MSSA populations
The evidence networks of the sub-groups for the TOC end-point in the ME population analysis are shown in the Supplementary Appendix. The MRSA network included 15 treatments in 25 studies, while the MSSA network was more limited, with 11 treatments in 13 studies. The ECR outcome was reported in only five studies with MRSA sub-groups and four with MSSA sub-groups, and this evidence was too limited to conduct a meaningful network meta-analysis ().
For the MRSA sub-group (), the odds ratios relative to vancomycin for TOC were linezolid (OR = 1.55; 95% CrI = 0.96–2.46), daptomycin (OR = 0.74; 95% CrI = 0.13–3.66), and oritavancin 1200 mg (OR = 0.94; 95% CrI = 0.44–2.02). In the MSSA sub-group () they were linezolid (OR = 1.36; 95% CrI = 0.15–13.34) and oritavancin 1200 mg (OR = 0.82; 95% CrI = 0.08–7.83). The large uncertainties around some of the results were due to the sparse data available for these treatments. All of the 95% credible intervals for MRSA and MSSA populations crossed unity so none of the treatments led to a statistically significant difference in TOC response compared to vancomycin. The MRSA results for iclaprim 0.8 mg are not presented in the table as they were based on only four MRSA patients in a single study. Fixed effects results are presented in the Supplementary Appendix.
Results of the four sensitivity analyses
The TOC mixed CE population analysis included 14 treatments in 20 studies. All 95% credible intervals included unity, indicating no strong evidence of a difference in efficacy between vancomycin and any of the comparators ().
Only the SOLO I and SOLO II trials were included in the ECR mixed CE populations analysisCitation3,Citation17, so only oritavancin 1200 mg and vancomycin were comparable on this end-point and population. Fixed effects results are presented in , as there were fewer than three studies in the analysis. The posterior mean and 95% credible intervals odds ratio of oritavancin 1200 mg relative to vancomycin was OR = 0.89; 95% CrI = 0.65–1.16, and gave no evidence of difference in efficacy.
The TOC mixed ITT FDA analysis was limited to five treatments in four studies. The limited evidence caused the random effects analysis to be unstable. Fixed effects results are, therefore, presented in . There was again no evidence of a difference in efficacy for TOC between each of the comparators and vancomycin.
The TOC mixed CE FDA populations analysis was limited to eight treatments in seven studies, so the random effects results were again unstable. , therefore, presents the fixed effects analysis. There was no evidence of superiority of any treatment over vancomycin.
For all sensitivity analyses, results from the alternative, random, or fixed effects, models are presented in the Supplementary Appendix.
Consistency checking
The evidence networks were inspected to identify loops, not due solely to three arm trials; only one case where inconsistency could be present was identified in the TOC mixed (CE) network. This loop consisted of vancomycin, linezolid, and dalbavancin. The node-splitting method found no substantial differences in the direct and indirect treatment effect estimates and, therefore, no evidence of inconsistency in this network.
Discussion
The objective of this study was to conduct a systematic review and formal comparison to demonstrate similar efficacy of oritavancin to other available antimicrobial agents for the treatment of ABSSSI, due to a mixture of microbial causes, on the basis of clinical and microbiological success at TOC and on the basis of ECR. The systematic review identified 52 trials. The Bayesian NMA provided a framework for the formal comparison of treatments included in the identified trials. This NMA found evidence that oritavancin 1200 mg was equivalent to vancomycin (OR = 1.06; 95% CrI = 0.80–1.43) at TOC. The indirect evidence also suggests that oritavancin 1200 mg demonstrates equivalence to linezolid (OR = 1.55; 95% CrI = 0.91–2.57) and daptomycin (OR = 2.18; 95% CrI = 0.90–5.42) at TOC. The only significant finding was that omadacycline was superior to vancomycin for TOC in the mixed population (OR = 2.48; 95% CrI = 1.10–5.43). However, only two trials (a total of 359 patients) compared omadacycline to linezolid and these studies had contradictory findingsCitation65,Citation66. There were no other statistically significant findings of differences in efficacy between the anti-microbial agents in the mixed populations for TOC or ECR or for TOC in the MRSA or MSSA sub-groups. Sensitivity analyses found the results of the base case to be robust to choice of ITT over CE population and the choice to include studies that did not conform to recent FDA guidance on clinical trials.
This is the first systematic review and NMA to include oritavancin and an assessment of ECR in ABSSSI patients. The results for all outcomes and populations were largely consistent with the head-to-head findings of the included clinical trials. Three previous systematic reviews and meta-analyses found linezolid to be more efficacious for the treatment of ABSSSI than vancomycin, with mean and 95% confidence intervals (CI) for the odds ratios of OR = 1.57; 95% CI = 1.18–2.10Citation28, OR = 1.40; 95% CI = 1.01–1.95Citation31, and OR = 1.67; 95% CI = 1.31–2.12Citation33. However, the present study is based on a larger and more recent set of trials and includes indirect evidence via the NMA methodology. A recent large meta-analysisCitation24 of 53 studies in the treatment of ABSSSI comparing vancomycin with linezolid and other anti-microbial agents found only significant differences in efficacy for linezolid (OR = 1.61; 95% CI = 1.07–2.43). This study also neglected indirect evidence and was restricted to comparisons with vancomycin. These results from earlier analyses are consistent with the mean and 95% credible interval for the odds ratio relative to vancomycin for linezolid (OR = 1.55; 95% CrI = 0.96–2.46) found in the present NMA.
One previous NMA assessed the comparative efficacy of vancomycin, dalbavancin, linezolid, telavancin, daptomycin, and tigecycline for MRSA complicated skin and soft tissue infectionsCitation7. The search was conducted in 2008 and was, thus, limited to only 14 studies in MRSA, while the present study identified 25 studies in MRSA. Their odds ratios for linezolid (1.83), daptomycin (1.21) and tigecycline (0.81) were comparable to the estimates in the MRSA sub-group of the present study.
Two further meta-analyses have compared daptomycin with non-daptomycin treatments in skin and soft-tissue infections, one due only to MRSACitation29 and another due to both MRSA and MSSACitation12. The study in only MRSA caused infections found a mean and 95% confidence interval for the odds ratio of daptomycin relative to other treatments of OR = 0.89; 95% CI = 0.63–1.25Citation29, consistent with the odds ratio for daptomycin relative to vancomycin in the MRSA sub-group of the present study (OR = 0.74; 95% CrI = 0.13–3.66). The study in ABSSSI due to a mixture of MRSA and MSSA found a mean and 95% confidence interval for the odds ratio of daptomycin relative to vancomycin of OR = 1.05; 95% CI = 0.84–1.31) for clinical successCitation12, consistent with the odds ratio for TOC of daptomycin relative vancomycin in ABSSSI in the present study (OR = 2.18; 95% CrI = 0.90–5.42).
Limitations
Across all outcomes and populations, wide credible intervals were seen, pointing to the heterogeneity of available evidence. For the ECR end-point, it was only possible to indirectly compare five treatments to the common comparator, vancomycin. The systematic review was limited to English language publications; it is possible non-English language trials have been missed.
As recommended by FDA guidance, the base case analyses for the TOC and ECR end-points were conducted in the ITT populations. Using the ITT population, when a high numbers of dropouts across the studies are observed, could be considered a potential limitation. The impact of this was assessed in sensitivity analyses including patients in the CE populations, i.e. excluding patients who dropped out of the studies or were otherwise not evaluable; results were similar to the base case. Differences in the definition of ABSSSI and other patient characteristics across trials were a further limitation. Sensitivity analyses restricted to studies conducted in accordance with recent FDA guidelinesCitation5, thus ensuring greater similarity in populations, gave results similar to the base case.
The NMA methodology suffers from several limitations due to the assumptions necessary to conduct the indirect comparisonsCitation39,Citation67. Fixed effects models must assume that the relative treatment effect across studies is the same and this may not be the case due to heterogeneityCitation38,Citation40. Random effects models weaken this to an assumption of exchangeability of treatment, but these models can be difficult to fit or lead to highly uncertain estimates. NMA makes a further assumption of consistency of treatment effects across studiesCitation43, although no evidence of inconsistency in the networks was identifiedCitation45.
Further research
As discussed, wide credible intervals were seen across the results of these analyses and several important comparisons in TOC, ECR, and in the sub-groups of MRSA and MSSA infections were not possible due to limitations in the evidence base. A large and high quality trial directly comparing several antimicrobial agents could be of high value to clinical practice and healthcare decision-making.
Conclusion
This systematic review and NMA has provided an up-to-date and unified framework for the comparison of available antimicrobial agents used in the treatment of ABSSSI; this is the first such analyses to include novel agents such as oritavancin and to look at the ECR end-point. The results suggest equivalence between oritavancin 1200 mg, and other novel antimicrobial agents including daptomycin and linezolid, compared with vancomycin for both TOC and ECR.
Comparative_Effectiveness_antibiotics_for_ABSSSI_13Apr2015_supplementary_documents_BUGS_code.docx
Download MS Word (40.7 KB)Comparative_Effectiveness_antibiotics_for_ABSSSI_13Apr2015_supplementary_documents_table_1.docx
Download MS Word (44.7 KB)Comparative_Effectiveness_antibiotics_for_ABSSSI_13Apr2015_supplementary_documents_table_2.docx
Download MS Word (41.7 KB)Transparency
Declaration of funding
The Medicines Company funded ICON plc to complete this research.
Declaration of financial/other relationships
HT was a paid consultant of ICON Plc for the duration of this project. DAS, JCT and NH are employees of ICON Plc which was funded by The Medicines Company to complete this research. KS is an employee of The Medicines Company. GRC is an advisor/consultant to The Medicines Company, Pfizer, Merck, GSK, Cubist and others. CMRO peer reviewers on this manuscript have received an honorarium from CMRO for their review work, but have no other relevant financial relationships to disclose.
References
- Hersch AL, Chambers HF, Maselli JH, et al. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 2008;168:1585-91
- Ray GT, Suaya JA, Baxter R. Incidence, microbiology, and patient characteristics of skin and soft-tissue infections in a U.S. population: a retrospective population-based study. BMC Infect Dis 2013;13:252
- Corey GR, Kabler H, Mehra P, et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. New Engl J Med 2014;370:2180-90
- Landrum ML, Neumann C, Cook C, et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005-2010. JAMA 2012;308:50-9
- FDA. Guidance for industry acute bacterial skin and skin structure infections: developing drugs for treatment. Food and Drug Administration. Silver Spring, Maryland, USA, 2013
- Shor AF. Epidemiology and economic impact of methicillin-resistant Staphylococcus aureus: review and analysis of the literature. Pharmacoeconomics 2007;25:751-68
- Logman JF, Heeg B, Haider S, et al. Comparative effectiveness of antibiotics for the treatment of MRSA complicated skin and soft tissue infections. Curr Med Res Opin 2010;26:1565-78
- Dryden M. Complicated skin and soft tissue infection. J Antimicrob Chemother 2010;65(Suppl 3):iii35-44
- Wilcox MH. The tide of antimicrobial resistance and selection. J Antimicrob Agents 2009;34(Suppl 3):S6-10
- Nathwani D. New antibiotics for the management of complicated skin and soft tissue infections: are they any better? Int J Antimicrob Agents 2009;34:S24-9
- Moet GJ, Jones RN, Biedenbach DJ, et al. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004). Diagnost Microb Infect Dis 2007;57:7-13
- Wang SH, Hu JT, Zhang C, et al. The safety and efficacy of daptomycin versus other antibiotics for skin and soft-tissue infections: a meta-analysis of randomised controlled trials. BMJ Open 2014;4:e004744
- Liu C, Bayer A, Cosgrove SE. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clinl Infect Dis 2011;52:e18-55
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005;41:1373-406
- Eckmann C, Dryden M. Treatment of complicated skin and soft-tissue infections caused by resistant bacteria: value of Linezolid, Tigecycline, Daptomycin and Vancomycin. Eur J Medl Res 2010;15:554-63
- Itani KM, Dryden MS, Bhattacharyya H, et al. Efficacy and safety of linezolid versus vancomycin for the treatment of complicated skin and soft-tissue infections proven to be caused by methicillin-resistant Staphylococcus aureus. Am J Surg 2010;199:804-16
- Corey R, Wikler M, Moeck G, et al. Single-dose oritavancin compared to 7–10 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections; the SOLO II study. 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). Denver, Colorado, USA, 2013
- Boucher HW, Wilcox M, Talbot GH, et al. DISCOVER 1: A randomized, double-blind study of Dalbavancin (DAL) compared to Vacomycin (V) (with an option to switch to Linezolid (L)) in treatment of acute bacterial skin and skin structure infections (abSSSI) (L-201). 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). Denver, Colorado, USA, 2013
- Prokocimer P, De AC, Fang E, et al. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections. The ESTABLISH-1 randomized trial. JAMA 2013;309:559-69
- Weigelt J, Itani K, Stevens D, et al. Linezolid versus vancomycin in the treatment of complicated skin and soft tissue infections. Antimicrob Agents Chemother 2005;49:2260-6
- Stevens DL, Herr D, Lampiris H, et al. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis 2002;34:1481-90
- Sharpe JN, Shively EH, Polk HCJ. Clinical and economic outcomes of oral linezolid versus intravenous vancomycin in the treatment of MRSA-complicated, lower-extremity skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. Am J Surg 2005;189:425-28
- Kohno S, Yamaguchi K, Aikawa N, et al. Linezolid versus vancomycin for the treatment of infections caused by methicillin-resistant Staphylococcus aureus in Japan. J Antimicrob Chemother 2007;60:1361-9
- Vardakas KZ, Mavros MN, Roussos N, et al. Meta-analysis of randomized controlled trials of vancomycin for the treatment of patients with gram-positive infections: focus on the study design. Mayo Clinic Proceed 2012;87:349-63
- Fu J, Ye X, Chen C, et al. The efficacy and safety of linezolid and glycopeptides in the treatment of staphylococcus aureus infections. PLoS ONE 2013;8:e58240
- Bally M, Dendukuri N, Sinclair A, et al. A network meta-analysis of antibiotics for treatment of hospitalised patients with suspected or proven meticillin-resistant Staphylococcus aureus infection. Int J Antimicrobl Agents 2012;40:479-95
- Polyzos KA, Mavros MN, Vardakas KZ, et al. Efficacy and safety of telavancin in clinical trials: a systematic review and meta-analysis. PLoS One 2012;7:e41870
- Guo Z, Lin Z, Huang P, et al. Linezolid versus glycopeptides in the treatment of complicated skin and soft tissue infections: a meta-analysis of randomized controlled trials. Chin J Infect Chemother 2011;11:3-12
- Bliziotis IA, Plessa E, Peppas G, et al. Daptomycin versus other antimicrobial agents for the treatment of skin and soft tissue infections: a meta-analaysis. Ann Pharmacother 2010;44:97-106
- Bounthavong M, Hsu DI. Efficacy and safety of linezolid in methicillin-resistant Staphylococcus aureus (MRSA) complicated skin and soft tissue infection (cSSTI): a meta-analysis. Curr Med Res Opin 2010;26:407-21
- Beibei L, Yun C, Mengli C, et al. Linezolid versus vancomycin for the treatment of Gram-positive bacterial infections: meta-analysis of randomised controlled trials. Int J Antimicrob Agents 2010;35:3-12
- Dodds TJ, Hawke CI. Linezolid versus vancomycin for MRSA skin and soft tissue infections (systematic review and meta-analysis). ANZ J Surg 2009;79:629-35
- Falagas ME, Siempos II, Vardakas KZ. Linezolid versus glycopeptide or beta-lactam for treatment of Gram-positive bacterial infections: meta-analysis of randomised controlled trials. Lancet Infect Dis 2008;8:53-66
- An MM, Shen H, Zhang JD, et al. Linezolid versus vancomycin for meticillin-resistant Staphylococcus aureus infection: a meta-analysis of randomised controlled trials. Int J Antimicrob Agents 2013;41:426-33
- Dias S, Welton N, Sutton A, et al. NICE DSU Technical Support Document 1: introduction to evidence synthesis for decision making; 2011. Sheffield, UK: National Institute for Health and Care Excellence, 2012. Available at: http://www.nicedsu.org.uk. Accessed April 2012
- Dias S, Welton N, Sutton A, et al. NICE DSU Technical Support Document 2: a generalised linear modelling framework for Pairwise and network meta-analysis of randomised controlled trials. 2011. Sheffield, UK: National Institute for Health and Care Excellence, 2014. Available at: http://www.nicedsu.org.uk. Accessed April 2014
- Ades A, Sculpher M, Sutton A, et al. Bayesian methods for evidence synthesis in cost-effectiveness analsis. Pharmacoeconomics 2006;24:1-19
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London, UK: The Cochrane Collaboration, 2011. Available at: www.cochrane-handbook.org. Accessed March 2011
- Dias S, Sutton A, Ades A, et al. Evidence Synthesis for Decision Making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607-17
- Dias S, Sutton A, Welton N, et al. Evidence Synthesis for Decision Making 3: heterogeneity-subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making 2013;33:618-40
- Spiegelhalter DJ, Best NG, Carlin BP, et al. Bayesian measures of model complexity and fit. J R Statist Soc B 2002;64:583-639
- Lunn DJ, Jackson CH, Best N, et al. The BUGS Book. New York: CRC Press, 2013
- Dias S, Welton N, Sutton A, et al. Evidence Synthesis for Decision Making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making 2013;33:641-56
- Lu G, Ades A. Assessing evidence inconsistency in mixed treatment comparisons. J Am Statist Assoc 2006;101:447-59
- Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Statist Med 2010;29:932-44
- Lunn DJ, Thomas A, Best N, et al. WinBUGS - a Bayesian modelling framework: concepts, structure, and extensibility. Statist Comput 2000;10:325-37
- Katz DE, Lindfield KC, Steenbergen JN, et al. A pilot study of high-dose short duration daptomycin for the treatment of patietns with complicated skin and skin structure infections caused by gram-positive bacteria. Int J Clin Pract 2008;62:1455-64
- Seltzer E, Dorr MB, Goldstein BP, et al. Once-weekly dalbavancin versus standard-of-care antimicrobial regimens for treatment of skin and soft-tissue infections. Clin Infect Dis 2003;37:1298-303
- Stryjewski ME, O'Riordan WD, Lau WK, et al. Telavancin versus standard therapy for treatment of complicated skin and soft-tissue infections due to gram-positive bacteria. Clin Infect Dis 2005;40:1601-7
- Stryjewski ME, Chu VH, O'Riordan WD, et al. Telavancin versus standard therapy for treatment of complicated skin and skin structure infections caused by gram-positive bacteria: FAST 2 study. Antimicrob Agents Chemother 2006;50:862-7
- Stein GE, Schooley SL, Havlichek DH, et al. Outpatient intravenous antibiotic therapy compared with oral linezolid in patients with skin and soft tissue infections: a pharmacoeconomic analysis. Infect Dis Clin Pract 2008;16:235-9
- Mathews P, Alpert M, Rahav G, et al. A randomized trial of tigecycline versus ampicillin-sulbactam or amoxicillin-clavulanate for the treatment of complicated skin and skin structure infections. BMC Infect Dis 2012;12:297
- Konychev A, Heep M, Mortiz RKC, et al. Safety and efficacy of daptomycin as first-line treatment for complicated skin and soft tissue infections in elderly patients: an open-label, multicentre, randomized phase IIIb trial. Drugs Aging 2013;30:829-36
- Evers R, Antony NI, Alozie O, et al. Pilot study comparing daptomycin and telavancin in the treatment of skin and soft tissue infections. Intern J Infect Dis 2013;12
- Stevens DL. Teicoplanin for skin and soft-tissue infections: An open study and a randomized comparative trial versus cefazolin. J Infect Chemother 1999;5:40-5
- Lin D, Zhang Y, Wu J, et al. Linezolid for the treatment of infections caused by Gram-positive pathogens in China. Int J Antimicrob Agents 2008;32:241-9
- Blaszczyk-Kostanecka M, Dobozy A, Dominguez-Soto L, et al. Comparison of two regimens of oral clindamycin versus dicloxacillin in the treatment of mild-to-moderate skin and soft-tissue infections. Curr Therapeut Res Clin Exp 1998;59:341-53
- Stromberg BV, Reines HD, Hunt P. Comparative clinical study of Sulbactam and ampicillin and clindamycin and tobramycin in infections of soft tissues. Surg Gynecol Obstet 1986;162:575-8
- Pusponegoro EHD, Wirvadi BE. Clindamycin and cloxacillin compared in the treatment of skin and soft-tissue infections. Clin Therapeut 1990;12:236-41
- Chessick KC, Begley LA, Curtis LE, et al. Efficacy of intravenous clindamycin and methicillin in gram-positive soft tissue infections. Ann Surg 1975;181:203-6
- Stone HH, Geheber CE, Kolb LD, et al. Clinical comparison of cefotaxime versus the combination of gentamicin plus clindamycin in the treatment of peritonitis and similar polymicrobial soft-tissue surgical sepsis. Clin Therapeut 1981;4(Suppl):67-80
- Gabel-Hughes KS, Geelhoed GW. A prospective randomized clinical trial of antimicrobial therapy for soft tissue infections in surgical patients. Infect Surg 1990;9:21-4
- Teras J, Gardovskis J, Vaasna T, et al. Overview of tigecycline efficacy and safety in the treatment of complicated skin and structure infections - A European perspective. J Chemother 2008;20(Suppl 1):20-7
- Breedt J, Teras J, Gardovskis J, et al. Safety and efficacy of tigecycline in treatment of skin and skin structure infections: results of a Double-Blind Phase 3 Comparison Study with Vancomycin-Aztreonam. Antimicrob Agents Chemother 2005;49:4658-66
- Noel GJ, Draper MP, Hait H, et al. A randomized, evaluator-blind, phase 2 study comparing the safety and efficacy of omadacycline to those of linezolid for treatment of complicated skin and skin structure infections. Antimicrob Agents Chemother 2012;56:5650-4
- Noel GJ, Draper MP, Hait H, et al. Safety and efficacy of PTK 0796 (omadacycline) as treatment of complicated skin and soft tissue infection (P694). 22nd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID). London, UK: European Society for Clinical Microbiology and Infectious Diseases, 2012
- Dias S, Welton N, Sutton A, et al. Evidence Synthesis for Decision Making 1: introduction. Med Dec Making 2013;33:597-606
- Aikawa N, Kusachi S, Mikamo H, et al. Efficacy and safety of intravenous daptomycin in Japanese patients with skin and soft tissue infections. J Infect Chemother 2013;19:447-55
- Corey GR, Wilcox M, Talbot GH, et al. CANVAS 1: the first Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother 2010;65(Suppl 4):41-51
- Covington P, Davenport JM, Andrae D, et al. Randomized, double-blind, phase II, multicenter study evaluating the safety/tolerability and efficacy of JNJ-Q2, a novel fluoroquinolone, compared with linezolid for treatment of acute bacterial skin and skin structure infection. Antimicrob Agents Chemother 2011;55:5790-7
- Craft JC, Moriarty SR, Clark K, et al. A randomized, double-blind phase 2 study comparing the efficacy and safety of an oral fusidic acid loading-dose regimen to oral linezolid for the treatment of acute bacterial skin and skin structure infections. Clin Infect Dis 2011;52(Suppl 7):S520-6
- Fang E, De Anda C, Das A, et al. Safety profile of tedizolid phosphate compared to linezolid in a Phase 3 ABSSSI Study (L1-1664). San Francisco, California, USA: Interscience Conference on Antimicrobial Agents and Chemotherapy, 2012
- Dunbar LM, Milata J, McClure T, et al. Comparison of the efficacy and safety of oritavancin front-loaded dosing regimens to daily dosing: An analysis of the SIMPLIFI trial. Antimicrob Agents Chemother 2011;55:3476-84
- Fang E, De Anda C, Das A, Prokocimer P. Efficacy and safety results from the ESTABLISH 2 ABSSSI study comparing IV and oral tedizolid phosphate and linezolid. Berlin: ECCMID, 2013
- Florescu I, Beuran M, Dimov R, et al. Efficacy and safety of tigecycline compared with vancomycin or linezolid for treatment of serious infections with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci: a Phase 3, multicentre, double-blind, randomized study. J Antimicrob Chemother 2008;62(Suppl 1):17-28
- Jauregui LE, Babazadeh S, Seltzer E, et al. Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin Infect Dis 2005;41:1407-15
- Krievins D, Brandt R, Hawser S, et al. Multicenter, randomized study of the efficacy and safety of intravenous iclaprim in complicated skin and skin structure infections. Antimicrob Agents Chemother 2009;53:2834-40
- Noel GJ, Strauss RS, Amsler K, et al. Results of a double-blind randomized trial of ceftobiprole treatment of complicated skin and skin structure infections caused by gram-positive bacteria. Antimicrob Agents Chemother 2008;52:37-44
- Noel GJ, Bush K, Bagchi P, et al. A randomized, double-blind trial comparing ceftobiprole medocaril with vancomycin plus ceftazidime for the treatment of patients with complicated skin and skin-structure infections. Clin Infect Dis 2008;46:647-55
- Pertel PE, Eisenstein BI, Link AS, et al. The efficacy and safety of daptomycin vs vancomycin for the treatment of cellulitis and erysipelas. Int J Clin Pract 2009;63:368-75
- Postier RG, Green SL, Klein SR, et al. Results of a multicenter, randomized, open-label efficacy and safety study of two doses of tigecycline for complicated skin and skin-structure infections in hospitalized patients. Clin Therapeut 2004;26:704-14
- Prince WT, Ivezic-Schoenfeld Z, Lell C, et al. Phase II clinical study of BC-3781, a pleuromutilin antibiotic, in treatment of patients with acute bacterial skin and skin strucutre infections. Antimicrob Agents Chemother 2013;57:2087-94
- Sacchidanand S, Penn RL, Embil JM, et al. Efficacy and safety of tigecycline monotherapy compared with vancomycin plus aztreonam in patients with complicated skin and skin structure infections: results from a phase 3, randomized, double-blind trial. Int J Infect Dis 2005;9:251-61
- Stevens D, Smith CT, Bruss JB, et al. Randomized comparison of linezolid (PNU-100766) versus oxacillin-dicloxacillin for treatment of complicated skin and soft tissue infections. Antimicrob Agents Chemother 2000;44:3408-13
- Stryjewski ME, Potgieter PD, Yu-Ping L, et al. TD-1792 versus Vancomycin for Treatment of Complicated Skin and Skin Structure Infections. Antimicrob Agents Chemother 2012;56:5476-83
- Talbot GH, Thye D, Das A, et al. Phase 2 study of ceftaroline versus standard therapy in treatment of complicated skin and skin structure infections. Antimicrob Agents Chemother 2007;51:3612-16
- Wilcox M, Nathwani D, Dryden M. Linezolid compared with teicoplanin for the treatment of suspected or proven Gram-positive infections. J Antimicrob Chemother 2004;53:335-44
- Wilcox M, Corey GR, Talbot GH, et al. CANVAS 2: the second Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother 2010;65(Suppl 4):iv53-iv65
- Wilcox M, Boucher HW, Talbot GH, et al. DISCOVER 2: a randomized, double-blind study of dalbavancin (DAL) compared to Vancomycin (V) (with an option to switch to Linezolid (L)) in treatment of acute bacterial skin and skin structure infections (abSSSI) (L-202). 53rd Annual ICAAC Meeting; 2013; Chicago, IL