Abstract
Background:
Chronic kidney disease is commonly associated with type 2 diabetes mellitus (T2DM) and may impact the efficacy and safety of glucose-lowering therapies. Canagliflozin, a sodium glucose co-transporter 2 inhibitor, reduces blood glucose levels in patients with T2DM by lowering the renal threshold for glucose, thereby promoting urinary glucose excretion. This review describes the pharmacology, efficacy and safety of canagliflozin according to kidney function in participants with T2DM.
Methods:
Published articles that reported efficacy, safety and pharmacokinetics/pharmacodynamics data for canagliflozin in patients with T2DM and impaired renal function, and renal safety data with canagliflozin in various populations of patients with T2DM through May 2015 were included.
Results:
Early transient reductions in estimated glomerular filtration rate were observed with canagliflozin; these changes generally stabilized or attenuated over time and reversed after discontinuation, suggesting no renal (glomerular or tubular) damage with canagliflozin treatment. Urinary albumin-to-creatinine ratios were reduced with canagliflozin. Canagliflozin was generally well tolerated in patients with normal or mild to moderately impaired renal function, with a modestly higher incidence of renal-related adverse events and volume depletion–related adverse events in patients with moderate renal impairment. Adverse events related to potassium elevations were infrequent with canagliflozin 100 mg regardless of kidney function status; however, patients with moderately impaired kidney function experienced hyperkalemia more frequently with canagliflozin 300 mg compared with patients treated with either canagliflozin 100 mg or placebo. Canagliflozin was not associated with increased cardiovascular risk across studies; however, relatively few events among patients with impaired renal function meant that the analysis was not adequately powered to examine this outcome, and results from separate trials are awaited.
Conclusions:
Overall, canagliflozin is associated with small, transient changes in kidney function, and is well tolerated in patients with T2DM with varying kidney function status.
Introduction
Chronic kidney disease (CKD) is one of the most important challenges in patients with type 2 diabetes mellitus (T2DM), as it affects more than 40% of all patients and more than 60% of patients over the age of 65Citation1. People with T2DM and CKD have much higher mortality and morbidity rates than either condition aloneCitation2, which highlights the need for better treatments for affected individuals. T2DM is the most common cause of kidney failure that requires dialysis in the United States and in most developed countriesCitation3, and recent studies suggest that glucose control may have a key role in preventing kidney failureCitation4,Citation5. Therefore, it is important to understand the efficacy and safety of individual glucose-lowering therapies on the kidney in patients with varying baseline kidney function status.
Sodium glucose co-transporter 2 (SGLT2) inhibitors are a new class of drugs for the treatment of hyperglycemia in adults with T2DMCitation6. Drugs in this class act on the kidney to lower blood glucose levels by lowering the renal threshold for glucose, which in turn increases urinary glucose excretion (UGE) and leads to a mild osmotic diuresisCitation6. SGLT2 inhibitors have been associated with improvements in glycemic control, weight loss and blood pressure (BP) reductions in patients with T2DMCitation6; however, because the rate of UGE is proportional to glomerular filtration rate (GFR) as well as plasma glucose concentrationCitation7,Citation8, the glucose-lowering efficacy of SGLT2 inhibitors may be reduced in patients with comorbid CKD.
Canagliflozin is approved in many countries as an adjunct to diet and exercise for improving glycemic control in adults with T2DMCitation9–21. Although the renal excretion of canagliflozin is lowCitation22, the hemodynamic changes related to osmotic diuresis, reduction in BP and altered intrarenal hemodynamics (e.g., improved tubuloglomerular feedback) seen with canagliflozin may also contribute to changes in estimated GFR (eGFR). This review article summarizes the pharmacokinetics and pharmacodynamics of canagliflozin in patients with renal impairment, the effects of canagliflozin on the kidney in patients with T2DM across a range of levels of kidney function, including those with Stage 3 CKD, and the efficacy and important safety findings from randomized, Phase 3 studies of canagliflozin in a broad range of patients with T2DM and varying baseline kidney function status.
Effects of T2DM and SGLT2 inhibition on the kidney
In patients with T2DM, the filtered glucose load is increased, leading to increased reabsorption of glucose and sodium, which is further compounded by the upregulation of SGLT2 transportersCitation23. These physiologic adaptations paradoxically sustain hyperglycemia and exacerbate the volume expansion that is characteristically seen in people with T2DM. Intravascular volume expansion contributes to elevated BP and renal hyperfiltration, as well as atrial stretch, which in turn activates the release of atrial natriuretic peptide (ANP), a contributor to altered intrarenal hemodynamics, leading to further hyperfiltration and compounding the observed renal abnormalitiesCitation24.
Hyperfiltration in the early stages of diabetic nephropathy is associated with enhanced sodium reabsorption in the proximal renal tubule, which reduces sodium delivery to the juxtaglomerular apparatus (i.e., macula densa)Citation25; this impairs tubuloglomerular feedback and results in afferent arteriolar vasodilation, which leads to elevated intraglomerular pressure and increased GFRCitation23,Citation26. The net effect of these changes is volume expansion, increased BP and renal hyperfiltration, all of which increase the risk of kidney damage and progressive loss of kidney function.
Inhibition of SGLT2 in the proximal tubule increases delivery of sodium to the macula densa, thereby normalizing GFR and restoring tubuloglomerular feedback, which leads to decreased hyperfiltration ()Citation25,Citation27–30. Evidence from preclinical and clinical studies has shown that treatment with SGLT2 inhibitors may lead to improvements in markers of diabetic nephropathy, thereby suggesting a potential renoprotective role for this class in patients with diabetes ()Citation27–40. Specifically, SGLT2 inhibitors may confer renoprotection through direct action on glucose lowering, activation of tubuloglomerular feedback and/or potentially weight loss, through volume normalization, reduced intraglomerular pressure and improved BP controlCitation27–40. Although lowering of ANP is another mechanism that could mitigate the progression of kidney damage in diabetes, the relationship between ANP and SGLT2 inhibition has not been described to date. While the effects of SGLT2 inhibition are similar to those seen with angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), these classes of agents may be complementary since the primary effect of SGLT2 inhibition is to counter dilation of the afferent arteriole, whereas ACE inhibitors and ARBs primarily counter the vasoconstrictive effects of angiotensin 2 on the efferent arterioleCitation23,Citation41.
Figure 1. Renal effects of SGLT2 inhibition. SGLT2i, sodium glucose co-transporter 2 inhibitor; eGFR, estimated glomerular filtration rate; ACEi; angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker. Adapted from http://en.wikipedia.org/wiki/Renal_physiology, user Madhero88. This file is licensed under the Creative Commons Attribution 3.0 Unported license.
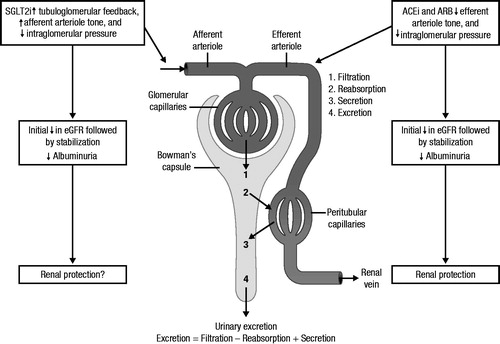
Table 1. Summary of evidence suggesting renoprotection with SGLT2 inhibition.
The BP and blood glucose lowering achieved with SGLT2 inhibition are also likely to be beneficial in offsetting or reversing the progression of CKDCitation4,Citation42–44. Improving glycemic control has been shown to delay the progression of albuminuriaCitation4 and may prevent end-stage renal disease (ESRD)Citation4,Citation5, while intensive BP lowering has been shown to confer renoprotection in patients with albuminuria; however, the effects in those without albuminuria remain uncertainCitation44. Overall, SGLT2 inhibition induces a suite of changes in people with T2DM that suggests a strong likelihood of renoprotection.
Methods
This non-systematic review article reports efficacy, safety and pharmacokinetics/pharmacodynamics data for canagliflozin in patients with T2DM and impaired renal function, and renal safety data with canagliflozin in various populations of patients with T2DM published through May 2015. Data were summarized from published primary articles and pooled analyses of Phase 3 data from the canagliflozin clinical trial program, as well as from publicly available sources of canagliflozin data; select data were provided by the sponsor of canagliflozin (Janssen Research & Development, LLC).
Specific endpoints of interest included changes in eGFR, serum creatinine, blood urea nitrogen (BUN) and albumin/creatinine ratio, as well as renal safety (e.g., renal-related adverse events [AEs]) and other safety outcomes (e.g., hyperkalemia, AEs related to volume depletion, bone fractures, cardiovascular safety). This manuscript summarizes renal data from the pooled, placebo-controlled population, including patients with normal renal function from four placebo-controlled studies of canagliflozinCitation9,Citation11–13; active-controlled studies of canagliflozin as add-on to metformin versus glimepiride and sitagliptinCitation11,Citation14,Citation18 and as add-on to metformin plus sulfonylurea versus sitagliptinCitation15; and a placebo-controlled study in older adultsCitation17,Citation20 (). This manuscript also reports data from patients with impaired renal function, including those enrolled in a dedicated placebo-controlled study of canagliflozin in patients with moderate renal impairmentCitation16,Citation19 and a pooled population of patients with Stage 3 CKD who were enrolled in four placebo-controlled studiesCitation9,Citation16,Citation17,Citation47 (); assessments of kidney function are also reported for patients in the Stage 3A and Stage 3B CKD subgroups of the Stage 3 CKD populationCitation45.
Table 2. Study design and patient populationsCitation11,Citation14,Citation15,Citation17,Citation18,Citation45,Citation46.
Effect of renal function on the pharmacokinetics and pharmacodynamics of canagliflozin
Reductions in renal function lead to moderate increases in canagliflozin exposure. As part of the canagliflozin clinical development program, an open-label, Phase 1 pharmacokinetic/pharmacodynamic study was conducted in non-diabetic subjects (N = 40) with normal renal function (eGFR ≥90 mL/min/1.73 m2), mild (eGFR ≥60 and <90 mL/min/1.73 m2), moderate (eGFR ≥30 and <60 mL/min/1.73 m2) and severe (eGFR <30 mL/min/1.73 m2) renal impairment, and ESRDCitation48. All subjects received a single dose of canagliflozin 200 mg, except those with ESRD, who received one dose post-dialysis and another dose pre-dialysis, 10 days later. In this study, the mean elimination half-life of canagliflozin was somewhat longer in subjects with impaired renal function (range, 17.2–23.9 hours) compared with subjects with normal renal function (14.2 hours)Citation48. Compared with the normal renal function group, geometric mean values for systemic exposure to canagliflozin (AUC∞) were ∼7%, 63% and 50% higher, and mean maximum plasma concentrations (Cmax) of canagliflozin were 13%, 29% and 29% higher in subjects with mild, moderate and severe renal impairment, respectively; exposure in subjects with ESRD was similar to those with normal renal function and may be related to clearance via hemodialysis in these patientsCitation48. Despite the higher systemic exposure to canagliflozin in subjects with moderate and severe renal impairment, 24-hour UGE decreased with declining renal function, consistent with the dependency of UGE on GFRCitation48.
Renal effects of canagliflozin
Overview
The canagliflozin clinical development program included placebo- and active-controlled studies that enrolled ∼10,000 patients in studies lasting 52 or 104 weeks, as well as in an ongoing long-term outcomes studyCitation9–21. Assessments of kidney function and safety were performed across these studies. This review summarizes renal data from individual placebo- and active-controlled studies of canagliflozin, as well as from pooled populations derived from these studies, which included patients with varying kidney function status ().
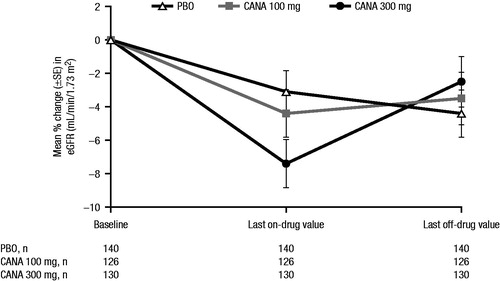
Across studies, treatment with canagliflozin was generally associated with early reductions in eGFR of ∼4 mL/min/1.73 m2 (ranging from approximately −2 to −6 mL/min/1.73 m2), along with commensurate increases in serum creatinine (ranging from approximately 1–15 μmol/L [∼0.01–0.2 mg/dL])Citation9–20. Treatment with canagliflozin was also associated with increases in BUN of approximately 1–4 mmol/L (∼3–11 mg/dL)Citation9–20. Across studies in various populations, the observed changes in eGFR were greatest at the first post-baseline visit (∼3–6 weeks) and generally stabilized or attenuated within 26 weeks ()Citation9–20. Across the Phase 3 development program, eGFR and creatinine levels were not universally measured after trial completion; however, among a subset of patients who had renal function measurements subsequent to discontinuation of study drug (n = 396), eGFR values increased toward baseline levels after discontinuation of canagliflozin treatment, demonstrating the reversibility of renal effects with canagliflozin ()Citation49. Notably, studies of other agents in this class have assessed post-completion, off-treatment creatinine levels, and have also found that acute reductions in eGFR were reversibleCitation50.
Figure 2. Mean eGFR over time in patients with T2DM as add-on to MET versus GLIM over 104 weeks, aged 55–80 years over 104 weeks, and with eGFR ≥30 and <50 mL/min/1.73 m2 over 52 weeks. eGFR, estimated glomerular filtration rate; T2DM, type 2 diabetes mellitus; MET, metformin; GLIM, glimepiride; PBO, placebo; CANA, canagliflozin. Add-on to MET vs GLIM data reprinted from American Diabetes Association, Diabetes Care, Copyright © 2015. Copyright and all rights reserved. Material from this publication has been used with the permission of American Diabetes AssociationCitation18. Older patients (55–80 years) data reprinted from Bode et al.Citation20. Copyright © 2015 John Wiley & Sons Ltd. All rights reserved. Data from patients with eGFR ≥30 to <50 mL/min/1.73 m2 reprinted from Yale et al.Citation19. Copyright © 2014 John Wiley & Sons Ltd. All rights reserved.
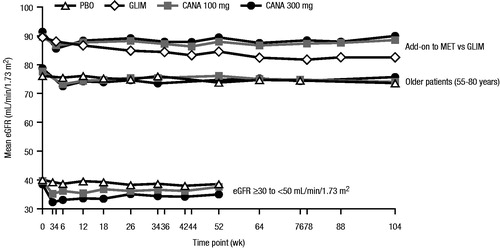
Figure 3. Percentage change in eGFR for patients who discontinued treatment* and had a post-treatment eGFR assessment >5 days after the last dose of study medicationCitation49. eGFR, estimated glomerular filtration rate; PBO, placebo; CANA, canagliflozin; SE, standard error. *Treatment discontinuation for any reason (not limited to changes in eGFR).
The changes in renal hemodynamics observed with SGLT2 inhibition would be expected to lead to reduced intraglomerular pressure and therefore a reduction in albuminuria as part of the pathway to renoprotection. Indeed, reductions in albumin-to-creatinine ratio (ACR) were observed in the subset of canagliflozin studies in which ACR was assessedCitation14,Citation16,Citation18,Citation19, suggesting the possibility of renoprotection.
Renal effects of canagliflozin in patients with normal renal function
Placebo-controlled studies
In the pooled, placebo-controlled population, larger reductions in eGFR and increases in serum creatinine and BUN were seen with canagliflozin 100 and 300 mg compared with placeboCitation46. Maximal reductions in eGFR were seen across groups from baseline to Week 6, with a return toward baseline through Week 26; mean changes from baseline to Week 26 were −2.1, −3.1 and −1.2 mL/min/1.73 m2 with canagliflozin 100 and 300 mg and placebo, respectivelyCitation46. The proportion of patients with clinically meaningful reductions in renal function (i.e. >50% reduction in eGFR) was small and not different across groups; only 2/2238 (<0.1%) patients (1/624 [0.2%] with placebo and 1/805 [0.1%] with canagliflozin 300 mg) had a >50% reduction in eGFR at any time post-baseline, and neither of these patients had an eGFR reduction >50% at Week 26Citation4 Citation6.
Active-controlled studies
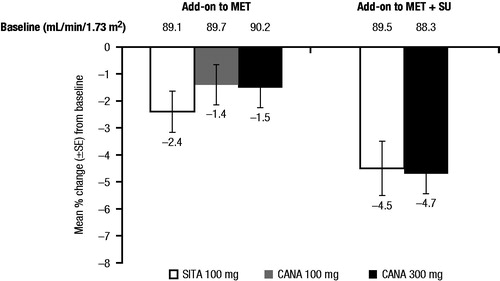
As add-on to metformin versus glimepiride, reductions in eGFR were seen at 4 weeks with canagliflozin 100 and 300 mg compared with glimepiride, consistent with the known acute effect of SGLT2 inhibitors (mean changes of −3.0, −5.8 and −1.2 mL/min/1.73 m2, respectively). Importantly, eGFR levels continued to decline during subsequent follow-up in the glimepiride-treated participants, but appeared to attenuate in those receiving canagliflozin. As a result, mean eGFR reductions at 52 weeks were smaller with canagliflozin 100 and 300 mg compared with glimepiride (least squares [LS] mean changes of −1.6, −2.6 and −4.6 mL/min/1.73 m2, respectively; differences [95% confidence interval (CI)] vs glimepiride of 2.9 [1.4, 4.4] and 1.9 [0.4, 3.5] mL/min/1.73 m2, respectively)Citation14; these differences persisted or increased over 104 weeks (differences [95% CI] vs glimepiride of 3.6 [2.1, 5.2] and 1.5 [−0.03, 3.1] mL/min/1.73 m2, respectively; )Citation18. By 52 weeks, reductions in eGFR with canagliflozin were generally similar to those seen with sitagliptin as add-on to metformin and as add-on to metformin plus sulfonylurea ()Citation11,Citation15.
Figure 4. Percentage change in eGFR at Week 52 in active-controlled studies of CANA versus SITACitation11,Citation15. eGFR, estimated glomerular filtration rate; CANA, canagliflozin; SITA, sitagliptin; SE, standard error; MET, metformin; SU, sulfonylurea.
Older patients
Older patients have a greater prevalence of CKD and, therefore, they may be more sensitive to renal changes induced by canagliflozin. In a placebo-controlled, Phase 3 study of canagliflozin in older patients with T2DM (55–80 years), changes in eGFR were similar to those seen in other placebo-controlled studies over 26 and 104 weeksCitation17,Citation20. The largest changes in eGFR and serum creatinine were observed at Week 6 and remained stable or returned toward baseline over 104 weeks (). At Week 26, mean changes in eGFR from baseline were −2.5, −4.0 and −1.4 mL/min/1.73 m2 with canagliflozin 100 and 300 mg and placebo, respectivelyCitation17. Mean changes in eGFR at Week 104 (−3.1, −3.1 and −3.1 mL/min/1.73 m2 with canagliflozin 100 and 300 mg and placebo, respectively) were similar to those seen at Week 26Citation20.
In a subgroup analysis of patients from the pooled, placebo-controlled population by age (<65 vs ≥65 years), eGFR reductions were slightly larger in older (average baseline eGFR, ∼76–77 mL/min/1.73 m2) versus younger patients (average baseline eGFR, ∼90–91 mL/min/1.73 m2)Citation51. In both age groups, transient decreases in eGFR were observed with canagliflozin relative to placebo over the first 3–6 weeks of treatment; however, eGFR stabilized or attenuated over the 26-week treatment period. Over 26 weeks, in the canagliflozin 100 and 300 mg and placebo groups, respectively, LS mean changes in eGFR were −2.3, −3.5 and −2.4 mL/min/1.73 m2 in patients aged <65 years (differences [95% CI] vs placebo of 0.06 [−1.3, 1.4] and −1.1 [−2.4, 0.3] mL/min/1.73 m2, respectively), and −2.2, −2.7 and −1.0 mL/min/1.73 m2 (differences [95% CI] vs placebo of −1.2 [−3.3, 1.0] and −1.7 [−3.9, 0.5] mL/min/1.73 m2, respectively) in patients aged ≥65 yearsCitation51.
Effects of canagliflozin in patients with T2DM and Stage 3 CKD
Overall efficacy
Canagliflozin was associated with reductions in HbA1c and body weight compared with placebo in patients with Stage 3 CKDCitation16,Citation19,Citation45, which were generally smaller than those seen in patients with normal or mildly impaired renal functionCitation9–15,Citation17. In a pooled analysis that included data from patients with Stage 3 CKD (baseline eGFR ≥30 and <60 mL/min/1.73 m2) from four placebo-controlled, Phase 3 studies, mean changes in HbA1c from baseline were −0.38% and −0.47% with canagliflozin 100 and 300 mg versus placebo, respectivelyCitation45. Higher percentages of patients with T2DM and Stage 3 CKD achieved HbA1c <7.0% with either canagliflozin 100 or 300 mg compared with placebo, indicating clinically meaningful glycemic improvementsCitation16,Citation19,Citation45. In contrast, the BP-lowering effect appeared similar to that in people with preserved kidney function and may be related, in part, to renal mechanisms separate from increased UGE (e.g., sensitivity to the mild osmotic diuresis)Citation19; relative to placebo, systolic BP was reduced by −2.8 and −4.4 mmHg with canagliflozin 100 and 300 mg, respectivelyCitation45. Canagliflozin was generally well tolerated in patients with Stage 3 CKD, with a safety profile similar to that seen in other canagliflozin Phase 3 studies ()Citation16,Citation19,Citation45.
Table 3. Summary of safety findings in the pooled, moderate renal impairment population*,Citation45.
Renal effects
In the Phase 3 study of canagliflozin in patients with T2DM and moderate renal impairment (baseline eGFR ≥30 and <50 mL/min/1.73 m2)Citation16,Citation19, early transient reductions in eGFR were observed with canagliflozin 100 and 300 mg and placebo that were similar in magnitude (albeit larger on a percentage basis) to those in the general T2DM population and stabilized over time (). At Week 52, mean changes in eGFR were −2.1, −4.0 and −1.6 mL/min/1.73 m2 with canagliflozin 100 and 300 mg and placebo, respectivelyCitation19. Canagliflozin 100 and 300 mg compared with placebo were associated with commensurate increases in serum creatinine (LS mean changes of 7.7, 15.8 and 6.2 μmol/L [0.08, 0.17 and 0.06 mg/dL], respectively) and in BUN (LS mean changes of 0.86, 1.35 and 0.04 mmol/L [2.7, 3.8 and 0.5 mg/dL], respectively) at Week 52Citation1 Citation9.
In the pooled, Stage 3 CKD population (baseline eGFR ≥30 and <60 mL/min/1.73 m2), reductions in eGFR at Week 26 were larger with canagliflozin 100 and 300 mg compared with placebo (mean changes of −1.7, −2.2 and 0.7 mL/min/1.73 m2, respectively). Results were also analyzed in subgroups of patients with Stage 3A CKD (eGFR ≥45 and <60 mL/min/1.73 m2; mean baseline eGFR, 53.3 mL/min/1.73 m2) and Stage 3B CKD (eGFR ≥30 and <45 mL/min/1.73 m2; mean baseline eGFR, 38.2 mL/min/1.73 m2)Citation45. In the Stage 3A CKD subgroup, eGFR reductions at Week 26 were −1.4, −2.3 and 1.0 mL/min/1.73 m2 with canagliflozin 100 and 300 mg and placebo, respectively; eGFR reductions at Week 26 were −2.4, −2.1 and 0.01 mL/min/1.73 m2, respectively, in the Stage 3B CKD subgroupCitation45. In the overall Stage 3 CKD population and the Stage 3A and 3B CKD subgroups, a small initial decline in eGFR was seen with canagliflozin, followed by a return toward baseline, which is consistent with the trend observed in patients with normal renal function in other Phase 3 studies of canagliflozin. The proportion of patients with larger reductions in renal function was also small and similar across groups; few patients had eGFR reductions >50% from baseline (5/332 [1.5%], 3/352 [0.9%] and 0/367 [0%] with canagliflozin 100 and 300 mg and placebo, respectively), and only 1/352 (0.3%) in the canagliflozin 100 mg group and none in the canagliflozin 300 mg or placebo groups experienced eGFR reductions >50% at the last post-baseline time point. Milder degrees of eGFR reduction (i.e., >30% reduction from baseline and <80 mL/min/1.73 m2) were more common in patients treated with canagliflozin 100 and 300 mg compared with placebo at any post-baseline assessment (31/332 [9.3%], 43/352 [12.2%] and 18/367 [4.9%], respectively)Citation49; fewer patients (10/332 [3.0%], 14/352 [4.0%] and 12/367 [3.3%], respectively) met these criteria at the last post-baseline assessment.
Effects of canagliflozin on the albumin-to-creatinine ratio
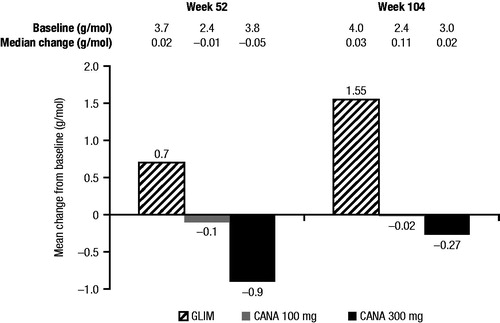
Canagliflozin provided reductions in ACR in the subset of studies that assessed changes in ACRCitation14,Citation16,Citation18,Citation19. In the active-controlled study of canagliflozin as add-on to metformin versus glimepiride, which enrolled patients with normal or mildly impaired renal function, reductions in ACR were seen with canagliflozin 100 and 300 mg, whereas an increase was seen with glimepiride at Week 52Citation1 Citation4 and Week 104Citation18 (). This result strongly suggests that the albuminuria-lowering effects are glucose-independent. In the study of patients with moderate renal impairment (eGFR ≥30 and <50 mL/min/1.73 m2), reductions in urinary albumin excretion and ACR were observed at Weeks 26 and 52 with both canagliflozin doses relative to placeboCitation16,Citation19.
Figure 5. Change in ACR in the study of CANA versus GLIM as add-on to metformin over 104 weeksCitation14,Citation18. ACR, albumin-to-creatinine ratio; CANA, canagliflozin; GLIM, glimepiride.
Renal-related adverse events
The incidence of renal-related AEs (e.g., blood creatinine increased, renal impairment and GFR decreased) reported by investigators during the studies was low and similar across groups among the pooled, placebo-controlled population (0.6%, 1.7% and 0.6% with canagliflozin 100 and 300 mg and placebo, respectively; )Citation46. In the pooled population of patients with Stage 3 CKD, the incidence of investigator-reported, renal-related AEs was higher with canagliflozin 100 and 300 mg compared with placebo (8.9%, 9.3% and 3.7%, respectively; )Citation45. In both pooled populations, the most common problems described by investigators included elevated blood creatinine, renal impairment and decreased GFR. Few renal-related AEs were deemed serious or led to study discontinuation.
Table 4. Renal-related adverse events in patients with and without renal impairment*.
Other safety findings
Canagliflozin is generally well tolerated; however, an increase in AEs related to SGLT2 inhibition (e.g., genital mycotic infections and urinary tract infections) with canagliflozin has been observed across studiesCitation9–21. These risks appear to be similar in people with T2DM who were treated with canagliflozin and had either normal kidney function or CKD. Findings related to other safety topics of interest (i.e., changes in potassium, volume depletion–related AEs, bone fractures and cardiovascular safety) from canagliflozin studies in patients with and without renal impairment are described in the following sections.
Potassium
Small changes in serum electrolyte levels (e.g., potassium, magnesium and phosphate) have been previously reported in patients with T2DM, especially in patients with reduced eGFR and in patients treated with canagliflozin 300 mgCitation52,Citation53. In addition, comorbid hypertension is common in patients with T2DM, and treatment with ACE inhibitors, ARBs and/or potassium-sparing diuretics can interfere with potassium excretion, particularly in patients with reduced eGFRCitation53. Thus, it is important to understand the potential effects of changes in renal function on the risk of potassium elevations that are associated with canagliflozin treatment.
In a pooled analysis based on renal function, mean baseline potassium levels were the same across groups in patients with eGFR ≥60 mL/min/1.73 m2 (4.3 mmol/L for all) and eGFR ≥45 and <60 mL/min/1.73 m2 (4.5 mmol/L for all). The proportions of patients who had potassium levels meeting outlier criteria (>upper limit of normal [ULN; 5.4 mmol/L] and >15% increase from baseline) at any time post-baseline with canagliflozin 100 and 300 mg and placebo were 4.5%, 6.8% and 4.7%, respectively, for those with eGFR ≥60 mL/min/1.73 m2; for those with eGFR ≥45 and <60 mL/min/1.73 m2, 5.2%, 9.1% and 5.5% met outlier criteria for potassium, respectivelyCitation53. Episodes where potassium elevations were considered to be clinically significant enough to be reported as AEs were less frequent overall. Such AEs were reported in 0.8%, 0.7% and 0.2% of patients with eGFR ≥60 mL/min/1.73 m2 and 1.4%, 2.1% and 1.5% of patients with eGFR ≥45 and <60 mL/min/1.73 m2 who were treated with canagliflozin 100 and 300 mg and placebo, respectivelyCitation53.
Volume depletion–related AEs
Treatment with canagliflozin may be associated with a natriuretic effect as well as an osmotic diuretic effect due to glucosuria. Therefore, data on AEs related to volume depletion (e.g., hypotension, postural dizziness, hypovolemia, dehydration, syncope and decreased urine output) were specifically collected in the clinical trials of canagliflozinCitation46. In the pooled, placebo-controlled population, the incidence of AEs related to volume depletion was low (<2%) across treatment groups, and most were mild or moderate in intensityCitation46. The incidence of volume depletion–related AEs was higher with canagliflozin 100 and 300 mg compared with placebo in the pooled population of patients with T2DM and Stage 3 CKD (5.0%, 8.5% and 2.6%, respectively)Citation45. Select populations (including patients with eGFR <60 mL/min/1.73 m2, patients ≥75 years of age and patients taking loop diuretics) are at increased risk for AEs related to volume depletion and should be monitored accordinglyCitation52.
Bone fractures
Patients with T2DM are at an increased risk for bone fractures compared with individuals without T2DM who have comparable bone mineral densityCitation54,Citation55. Specifically, appendicular and vertebral fracture rates are higher in patients with T2DM than in other populations with comparable bone mineral densityCitation56–58.
Canagliflozin was not associated with notable differences in bone mineral density over 104 weeksCitation59. In a previous analysis of a broader population, which was undertaken for the purpose of regulatory submission, canagliflozin was associated with an increased risk of fractures attributable to an excess number of fractures in the CANagliflozin cardioVascular Assessment Study (CANVAS), where participants were older and had prior history/risk of cardiovascular disease, lower baseline eGFR and higher baseline diuretic use; fractures in this population were imbalanced during the first 26 weeks of treatmentCitation59. Therefore, the effects of canagliflozin on fractures in patients with T2DM and CKD are of interest. In the study of canagliflozin in patients with T2DM and eGFR ≥30 and <50 mL/min/1.73 m2, the incidence of fracture AEs was low, with no imbalance across groups at Week 52 (1.1%, 1.1% and 2.2% with canagliflozin 100 and 300 mg and placebo, respectively)Citation19. In the pooled, placebo-controlled studies, the incidence of fracture AEs was 0.7%, 0.6% and 0.3% with canagliflozin 100 and 300 mg and placebo, respectively, over 26 weeksCitation59. The incidence of fractures was similar with canagliflozin and placebo or active comparator in patients with T2DM from the non-CANVAS studiesCitation59. In CANVAS, the incidence rates of fractures were 1.6, 1.6 and 1.1 per 100 patient-years of exposure with canagliflozin 100 and 300 mg and placebo, respectively, and most fracture AEs generally occurred early during treatment, with no differences in the incidence through Week 26. Further data on fracture incidence will be collected in ongoing studies.
Cardiovascular safety
Patients with T2DM are at increased risk for cardiovascular eventsCitation60, particularly if they have comorbid CKDCitation61. The ongoing CANVAS study will contribute to the evaluation of the long-term cardiovascular safety of canagliflozin compared with placebo in patients with T2DM who have an elevated risk for cardiovascular diseaseCitation47. In a prespecified interim meta-analysis of adjudicated major cardiovascular events in Phase 2 and 3 clinical studies of canagliflozin, including CANVAS, undertaken for the purposes of regulatory submissionCitation62, the hazard ratio for the composite primary endpoint of time to an event of cardiovascular death, non-fatal stroke, non-fatal myocardial infarction or unstable angina requiring hospitalization for canagliflozin (both doses pooled) compared with combined active and placebo comparators was 0.91 (95% CI: 0.68, 1.22), suggesting no increased cardiovascular risk associated with canagliflozinCitation59; rates of cardiovascular events were similar with both canagliflozin doses.
In addition to the lack of increased cardiovascular risk associated with canagliflozin, it has been hypothesized that canagliflozin may have an overall favorable effect on cardiovascular disease and progression to ESRD in patients with T2DM and impaired renal function. The effects of canagliflozin treatment on cardiovascular disease and progression of diabetic nephropathy will be evaluated in two ongoing trials, including CANVAS-R (ClinicalTrials.gov identifier: NCT01989754) and the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE; NCT02065791).
Current regulatory approvals of canagliflozin in CKD
Due to the diminished efficacy of canagliflozin in patients with Stage 3B CKD (eGFR ≥30 and <45 mL/min/1.73 m2) and the need for more data on the balance between efficacy and safety in patients with Stage 3 CKD, canagliflozin is currently only indicated for glycemic control in patients with Stage 3A CKD (eGFR ≥45 and <60 mL/min/1.73 m2)Citation52,Citation59. In the United States and Europe, patients with Stage 3A CKD are limited to receiving canagliflozin 100 mgCitation52. In Europe, patients must have baseline eGFR ≥60 mL/min/1.73 m2 to initiate canagliflozin therapy. Patients whose eGFR falls persistently <60 mL/min/1.73 m2 can continue treatment with canagliflozin 100 mg; however, if eGFR falls persistently <45 mL/min/1.73 m2, treatment must be discontinuedCitation59. Canagliflozin is not currently recommended for patients with eGFR <45 mL/min/1.73 m2.
Results from ongoing studies that are enrolling patients with Stage 3B CKD, including CANVAS, CANVAS-R and CREDENCE, will provide more information on the safety of canagliflozin in patients at high risk for cardiovascular disease and in patients with impaired renal function, as well as information on the potential cardiovascular and renal protective effects of canagliflozin.
Summary
The renal safety findings from the canagliflozin clinical program summarized in this review indicate that canagliflozin is generally well tolerated in patients with varying baseline kidney function status. While the majority of patients treated with canagliflozin had small initial reductions in eGFR with commensurate increases in serum creatinine that occurred shortly after initiation of treatment and were consistent with the known hemodynamic changes induced by SGLT2 inhibitors, these changes were generally transient and stabilized or attenuated over time. Reductions in ACR were also seen with canagliflozin treatment, possibly due to reduced intraglomerular pressure, raising the possibility of long-term renal protection and reductions in the risk of kidney failure. These results, along with reductions in systolic BP, suggest that canagliflozin may have an overall favorable effect on cardiovascular disease risk and the progression of CKD. Effects on renal parameters with canagliflozin treatment were similar on an absolute basis in patients with normal renal function and those with moderate renal impairment across Phase 3 studies. Changes in renal function parameters were also similar regardless of age, despite the fact that older patients tended to have lower baseline eGFR. In addition, smaller but meaningful reductions in HbA1c and body weight were observed in patients with reduced kidney function, whereas BP-lowering effects appeared to be preserved. Taken together, the current data suggest that canagliflozin may have renal and/or cardiovascular protective effects in T2DM, and large outcome studies are currently underway to evaluate these potential effects of canagliflozin treatment.
Transparency
Declaration of funding
This review article reports data from previously published studies funded by Janssen Research & Development, LLC. The authors prepared the manuscript with medical writing assistance funded by Janssen Global Services, LLC.
Declaration of financial other/relationships
V.P. has disclosed that he is supported by a Senior Research Fellowship from the National Health and Medical Research Council of Australia; has served on advisory boards and/or spoken at scientific meetings sponsored by Janssen, Baxter, AbbVie, Astellas, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, AstraZeneca, Eli Lilly, Merck and GlaxoSmithKline; and has a policy of honoraria going to his employer. M.J. has disclosed that she is supported by a Career Development Fellowship from the National Health and Medical Research Council of Australia and the National Heart Foundation, has received speaker’s fees from Amgen and Roche, and funding for a clinical trial from Gambro; and serves on steering committees for trials funded by Janssen; all honoraria are contributed directly to clinical research programs. U.V. and G.M. have disclosed that they are full-time employees of Janssen Research & Development, LLC.
CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors gratefully acknowledge Professor Carol Pollock of The University of Sydney for her helpful suggestions on the manuscript. Medical writing support was provided by Cherie Koch, PhD, of MedErgy, and was funded by Janssen Global Services, LLC.
Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation.
References
- Bailey RA, Wang Y, Zhu V, Rupnow MF. Chronic kidney disease in US adults with type 2 diabetes: an updated national estimate of prevalence based on Kidney Disease: Improving Global Outcomes (KDIGO) staging. BMC Res Notes 2014;7:415
- Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012;380:1662-73
- United States Renal Data System. 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2014. Available at: http://www.usrds.org/adr.aspx [Last accessed 27 August 2015]
- Perkovic V, Heerspink HL, Chalmers J, et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int 2013;83:517-23
- Zoungas S, Chalmers J, Neal B, et al. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med 2014;371:1392-406
- Jabbour SA. SGLT2 inhibitors to control glycemia in type 2 diabetes mellitus: a new approach to an old problem. Postgrad Med 2014;126:111-17
- Liang Y, Arakawa K, Ueta K, et al. Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS One 2012;7:e30555
- Sha S, Devineni D, Ghosh A, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab 2011;13:669-72
- Stenlöf K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 2013;15:372-82
- Stenlöf K, Cefalu WT, Kim KA, et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: findings from the 52-week CANTATA-M study. Curr Med Res Opin 2014;30:163-75
- Lavalle-González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 2013;56:2582-92
- Wilding JP, Charpentier G, Hollander P, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract 2013;67:1267-82
- Forst T, Guthrie R, Goldenberg R, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab 2014;16:467-77
- Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013;382:941-50
- Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week, randomized trial. Diabetes Care 2013;36:2508-15
- Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 2013;15:463-73
- Bode B, Stenlöf K, Sullivan D, et al. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: a randomized trial. Hosp Pract 2013;41:72-84
- Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, Phase 3 study. Diabetes Care 2015;38:355-64
- Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab 2014;16:1016-27
- Bode B, Stenlöf K, Harris S, et al. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55 to 80 years with type 2 diabetes. Diabetes Obes Metab 2015;17:294-303
- Neal B, Perkovic V, de Zeeuw D, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium glucose co-transporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care 2015;38:403-11
- Devineni D, Curtin CR, Polidori D, et al. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol 2013;53:601-10
- Abdul-Ghani MA, Norton L, Defronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev 2011;32:515-31
- Ortola FV, Ballermann BJ, Anderson S, et al. Elevated plasma atrial natriuretic peptide levels in diabetic rats. Potential mediator of hyperfiltration. J Clin Invest 1987;80:670-4
- Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587-97
- Vallon V, Rose M, Gerasimova M, et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 2013;304:F156-67
- Vallon V, Richter K, Blantz RC, et al. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol 1999;10:2569-76
- Thomson SC, Rieg T, Miracle C, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol 2012;302:R75-83
- Vallon V, Gerasimova M, Rose MA, et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 2014;306:F194-204
- Kojima N, Williams JM, Slaughter TN, et al. Renoprotective effects of combined SGLT2 and ACE inhibitor therapy in diabetic Dahl S rats. Physiol Rep 2015;3. [Epub ahead of print]. doi: 10.14814/phy2.12436
- Malatiali S, Francis I, Barac-Nieto M. Phlorizin prevents glomerular hyperfiltration but not hypertrophy in diabetic rats. Exp Diabetes Res 2008;2008:305403
- Kojima N, Williams JM, Takahashi T, et al. Effects of a new SGLT2 inhibitor, luseogliflozin, on diabetic nephropathy in T2DN rats. J Pharmacol Exp Ther 2013;345:464-72
- Gangadharan KM, Gross S, Mudaliar H, et al. Inhibition of kidney proximal tubular glucose reabsorption does not prevent against diabetic nephropathy in type 1 diabetic eNOS knockout mice. PLoS One 2014;9:e108994
- Gembardt F, Bartaun C, Jarzebska N, et al. The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Am J Physiol Renal Physiol 2014;307:F317-25
- Nagata T, Fukuzawa T, Takeda M, et al. Tofogliflozin, a novel sodium-glucose co-transporter 2 inhibitor, improves renal and pancreatic function in db/db mice. Br J Pharmacol 2013;170:519-31
- Christiansen JS, Gammelgaard J, Frandsen M, Parving HH. Increased kidney size, glomerular filtration rate and renal plasma flow in short-term insulin-dependent diabetics. Diabetologia 1981;20:451-6
- Lamos EM, Younk LM, Davis SN. Empagliflozin, a sodium glucose co-transporter 2 inhibitor, in the treatment of type 1 diabetes. Expert Opin Investig Drugs 2014;23:875-82
- Musso G, Gambino R, Cassader M, Pagano G. A novel approach to control hyperglycemia in type 2 diabetes: sodium glucose co-transport (SGLT) inhibitors. Systematic review and meta-analysis of randomized trials. Ann Med 2012;44:375-93
- Jungmann E. Prevention and treatment of diabetic nephropathy in older patients. Drugs Aging 2003;20:419-35
- Thomas MC. Renal effects of dapagliflozin in patients with type 2 diabetes. Ther Adv Endocrinol Metab 2014;5:53-61
- Brown NJ, Vaughan DE. Angiotensin-converting enzyme inhibitors. Circulation 1998;97:1411-20
- Strippoli GF, Craig M, Deeks JJ, et al. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ 2004;329:828
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53
- Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ 2013;185:949-57
- Yamout HM, Perkovic V, Davies M, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes and stage 3 nephropathy. Am J Nephrol 2014;40:64-74
- Usiskin K, Kline I, Fung A, et al. Safety and tolerability of canagliflozin in patients with type 2 diabetes: pooled analysis of phase 3 study results. Postgrad Med 2014;126:16-34
- Neal B, Perkovic V, de Zeeuw D, et al. Rationale, design, and baseline characteristics of the canagliflozin cardiovascular assessment study (CANVAS) – a randomized placebo-controlled trial. Am Heart J 2013;166:217-23
- Devineni D, Vaccaro N, Curtin CR, et al. The effect of renal and hepatic impairment on the pharmacokinetics of canagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Ther 2015;37:610-28
- Janssen Research & Development, LLC. Endocrinologic and Metabolic Drugs Advisory Committee January 10, 2013. Canagliflozin as an adjunctive treatment to diet and exercise alone or co-administered with other antihyperglycemic agents to improve glycemic control in adults with type 2 diabetes mellitus. JNJ-28431754 (Canagliflozin). NDA 204042. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM334551.pdf [Last accessed 27 August 2015]
- Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2014;2:369-84
- Sinclair A, Bode B, Harris S, et al. Efficacy and safety of canagliflozin compared with placebo in older patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. BMC Endocr Disord 2014;14:37
- Invokana® (canagliflozin) tablets, for oral use [package insert]. Titusville, NJ: Janssen Pharmaceuticals, 2014
- Weir MR, Kline I, Xie J, et al. Effect of canagliflozin on serum electrolytes in patients with type 2 diabetes in relation to estimated glomerular filtration rate (eGFR). Curr Med Res Opin 2014;30:1759-68
- Nicodemus KK, Folsom AR, Iowa Women's Health Study. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care 2001;24:1192-7
- Schwartz AV, Sellmeyer DE, Ensrud KE, et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab 2001;86:32-8
- Mishima T, Motoyama K, Imanishi Y, et al. Decreased cortical thickness, as estimated by a newly developed ultrasound device, as a risk for vertebral fracture in type 2 diabetes mellitus patients with eGFR of less than 60 mL/min/1.73 m2. Osteoporos Int 2015;26:229-36
- Inaba M, Okuno S, Kumeda Y, et al. Increased incidence of vertebral fracture in older female hemodialyzed patients with type 2 diabetes mellitus. Calcif Tissue Int 2005;76:256-60
- Yamamoto M, Yamaguchi T, Yamauchi M, et al. Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res 2009;24:702-9
- Invokana® (canagliflozin) tablets, for oral use [summary of product characteristics]. Beerse, Belgium: Janssen-Cilag International NV, 2015
- Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes 2008;26:77-82
- Van Buren PN, Toto R. Current update in the management of diabetic nephropathy. Curr Diabetes Rev 2013;9:62-77
- Food and Drug Administration. FDA Briefing Document. Invokana (Canagliflozin) Tablets. Endocrinologic and Metabolic Drugs Advisory Committee Meeting. January 10, 2013. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM334550.pdf [Last accessed 16 August 2013]
