Abstract
As we reach the deadline for the United Nations fourth Millennium Development Goal to reduce child mortality, many inequalities in vaccine access still exist, particularly for children in developing countries. Here we discuss some of the barriers to vaccine access in these countries, as well as some of the innovative approaches that could address these. Finally, we discuss the need to create a global environment conducive to innovation directed at low-resource settings, aimed to ultimately increase vaccine coverage.
Introduction
Immunization is one of the most important health interventions for children. In the first 5 years of life, a large cause of childhood mortality is attributable to vaccine-preventable infections such as pneumonia and diarrhea, particularly for children in developing countriesCitation1. This led the United Nations to develop its fourth Millennium Development Goal (MDG4) to cut mortality rates in children aged under 5 years by two thirds between 1990 and 2015Citation2. Gavi (The Vaccine Alliance) was created as a partnership between the public and private sector to facilitate this by making immunization available for all through: (1) increasing access to new and underused vaccines in developing economies; and (2) ensuring that vaccination programs are sustainableCitation3,Citation4. By providing purchasing capacity and logistics expertise, Gavi has improved access to life-saving vaccines. Yet despite these efforts, in the past 3 years average immunization coverage rates have stagnated as funding falls and political will plateausCitation5. Global coverage against Haemophilus influenzae type b (Hib) was estimated to be only 52% at the end of 2013, and 16% of children in countries supported by Gavi still did not receive a full course of a diphtheria, tetanus, pertussis (DTP)-containing vaccineCitation6, even though pentavalent vaccine covering DTP, hepatitis B and Hib can increase coverage against these diseases by combining five vaccines in oneCitation7.
As no single approach has been proven to be 100% effective in improving vaccination coverage rates, a multi-component strategy will be crucial to maximize the chances of success.
Innovative vaccine technologies could help to reach more children
The reach of vaccination programs in developing countries must be increased, particularly in sub-Saharan Africa and Southern Asia, where four out of every five deaths in children under 5 years occur. Children in sub-Saharan Africa are more than 15 times more likely to die before the age of 5 years than children in developed regions, and more than half of these deaths are due to diseases that are preventable and treatable through simple, affordable interventionsCitation1.
While innovations in the immunization field and vaccine delivery could help, so far only two specifically targeting developing countries have been introduced globally. These are vaccine vial monitorsCitation8 and autodisable (AD) syringes, both of which were conceived of over two decades agoCitation8. The development of these innovations by the Program for Appropriate Technology in Health (PATH) was mandated by the World Health Organization (WHO), enabled by catalytic funding from organizations such as the Bill and Melinda Gates Foundation and the United States Agency for International Development. Otherwise, there has been little incentive for the private sector to innovate under pressure to make vaccines available as cheaply as possible. This is despite increasing research and development costs and a shift in focus towards developing vaccines that are more complex and, therefore, often more costly to produce. Currently, the UNICEF Supply Division does not allocate funding specifically to procure innovative products, and international aid donors do not see value in spending their limited funds supporting the purchase of more expensive vaccines, despite potential improvements in safety, delivery and, ultimately, coverage. This impacts on difficult-to-reach areas in developing countries where there is potentially a greater requirement for innovative products.
There are specific challenges encountered in difficult-to-reach areas
Challenges in vaccinating all children include logistical difficulties, a requirement for supplies to be carried manually in remote areasCitation9, limited access to health services or healthcare workersCitation10, and variability in the numbers of children attending immunization sessions. Where session sizes are small and multi-dose policy does not apply, healthcare workers may not wish to open a vial if all the doses are not going to be usedCitation11. Children in rural areas are therefore less likely to receive a full course of immunization; furthermore, political conflict, migration and corruption can all be barriers to vaccine accessCitation12–16.
An additional danger faced by children in developing and transitional countries is the burden of disease resulting from unsafe injections. Unsafe injections can cause a substantial proportion of new cases of hepatitis B, hepatitis C and HIVCitation17, and demonstrate the need for innovative technologies to reduce these risks. WHO advises that by 2020 all countries should use safety-engineered injection devices (where appropriate), including reuse-prevention syringesCitation18. Worryingly, even countries that introduce safety measures can slip back into unsafe practices. A recent example was brought to light in a Gavi report from July 2014 showing that Bosnia and Herzegovina discontinued its use of AD syringes and safety boxes after Gavi support ended, mainly due to a lack of instructions and practical skills regarding their useCitation19.
Supranational organizations are recognizing the requirement for more incentives to develop vaccines for resource-limited settings
WHO is developing a ‘total system cost-effectiveness framework’ which will help countries to assess the full costs of vaccination per dose delivered, rather than price per dose purchased, factoring in the full benefits of different methods, such as improved safety, and variables such as wastage, personnel training and vaccine administration timeCitation20. This should demonstrate the benefits of new technologies and provide incentives for industry to evaluate, develop and bring these technologies to market. Gavi is also creating a new strategic plan, one of the objectives of which will be to incentivize the development of immunization-related technologiesCitation21.
Innovative approaches could have a significant impact on the future of immunization programs in developing countries
New vaccination technologies could provide major contributions to global health and immunization programs by simplifying the vaccination process. These technologies could be particularly relevant for developing countries, provided they can access them. New technologies in early development include vaccines available in patch form and presentations that do not require a cold chainCitation22,Citation23.
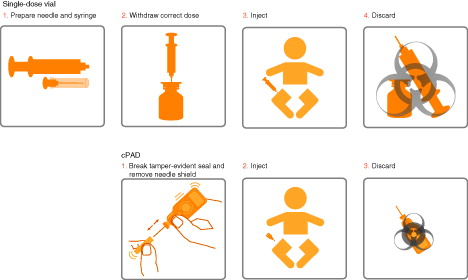
Another new technology is the all-in-one, compact, pre-filled, autodisable (cPAD) injection system. The cPAD is a single dose of vaccine contained in a lightweight plastic container, with a pre-attached needle. It was developed by PATH in response to WHO and other organizations recognizing a great need for easy-to-use, single-use, AD syringes that reduce the risk of blood-borne infectionsCitation24. This technology is expected to increase vaccine access in difficult-to-reach areas, as well as encouraging manufacturers to bring innovation to developing countries; Gavi has expressed optimism that it is likely to be successful in an increasingly complex immunization worldCitation25. Due to the all-in-one design of the cPAD, fewer manipulations are required than single- or multi-dose vials (). This reduces administration timeCitation26,Citation27 while potentially reducing handling errorsCitation28 and increasing accurate dose administrationCitation29. Importantly, the AD format reduces the likelihood of cross-contamination and missed vaccination opportunitiesCitation26. The lightweight and compact design also provides benefits in transport and storage logistics, with similar or smaller requirements for cold-chain volume compared to current WHO pre-qualified pentavalent vaccines in single-dose vialsCitation30,Citation31 and reduced medical waste compared to single- and multi-dose pentavalent vaccinesCitation26. These advantages could facilitate immunization outreach programsCitation27–29,Citation32–34.
Investigation into the application of controlled temperature chain (CTC) represents another promising avenue for improving immunization programs in developing countries. The requirement for cold chain storage can represent a major challenge to vaccine delivery, particularly in countries where the cold chain infrastructure and required electrical supply are not always availableCitation35,Citation36. With this in mind, CTC provides an alternative approach where vaccine doses are stored at a defined temperature outside the standard 2–8 °C for a specific duration and temperature, dependent on the vaccine’s heat-stability profileCitation37. As such, several studies have demonstrated the potential utility of CTC for certain vaccinesCitation38–40. WHO has now produced advice and guidance for program decision-makers and managers pertaining to specific vaccines. For use in CTC, meningitis A vaccine is currently licensed and WHO pre-qualified (i.e. meeting standards for safety, efficacy, manufacturing and ability to meet specific program needs)Citation41,Citation42. More vaccines are planned to be added to CTC, for example pneumococcus, tetanus toxoidCitation43, vaccines to improve campaigns in epidemic settings (cholera), and to successfully deliver a birth dose for hepatitis BCitation44.
More work is needed to support innovation in developing markets
The global community must focus on frameworks that allow countries to access new technologies, as well as providing incentives for manufacturers and allowing innovation. We believe that there are a number of changes that could be made to support and work together to meet these aims. Guidance from WHO and other global stakeholders on the mid- and long-term needs in these markets should be formalized to help manufacturers develop vaccines and technologies that address the issues. In addition, factoring in not only cost per vaccine dose purchased but also the overall impact on the system involved in procurement decisions (cost, safety, logistics, ease-of-use, healthcare worker time savings and impact on coverage), or offering procurement commitments for innovative technologies, would allow countries access to these technologies. It would also allow manufacturers to make a case to prioritize such development for resource-limited settings. For example, a recent study compared the cost of vaccine in cPAD format to single- or multi-dose vials and considered coverage as well as costs such as purchase/wastage of vaccine and safe injection equipment, storage, transport and distribution, healthcare staff, medical waste management and start-up activities. For all presentations, the largest cost was vaccine, including wastage rates. Whilst vaccine in cPAD was more costly in Cambodia (compared to the currently used single-dose vials), cPAD offered cost advantages in Ghana (compared to multi-dose vials) and Peru (compared to single-dose vials), and provided advantages in dry storage volume savings and reduced medical wasteCitation34.
Global stakeholders need to work together to provide guidance and simplify regulatory approval of new vaccine containers, which can cause a substantial delay in the approval of new products.
Final thoughts
With vaccine-preventable diseases representing a major cause of childhood mortalityCitation1, efforts continue to be focused on increasing vaccination coverage to meet the MDG4 goalCitation2, particularly in difficult-to-reach areas where remote locations, political conflict, migration and corruption are barriers to vaccine access. Several approaches show promise, from innovative, simpler to administer vaccines and incentives for their development to investigation into the viability of CTC.
However, with no increase in average immunization coverage rates in the past 3 yearsCitation5, more work is required. Collaboration between global stakeholders to employ a combination of all available strategies will be crucial in order to increase the likelihood of success.
Transparency
Declaration of funding
This work was supported by Janssen Infectious Diseases and Vaccines.
Author contributions: The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. We take full responsibility for the scope, direction, content of, and editorial decisions relating to the manuscript, were involved at all stages of development and have approved the submitted manuscript.
Declaration of financial/other relationships
O.P. and P.I.d.P. have disclosed that they are employees of Janssen Infectious Diseases and Vaccines.
Acknowledgments
Medical writing assistance was provided by Laura George, on behalf of Complete HealthVizion, and was contracted and compensated by Janssen Infectious Diseases and Vaccines.
References
- Children: reducing mortality. Fact Sheet N°178. World Health Organization, 2014. Available at: http://www.who.int/mediacentre/factsheets/fs178/en/ [Last accessed 14 August 2015]
- Gaffey MF, Das JK, Bhutta ZA. Millennium Development Goals 4 and 5: past and future progress. Semin Fetal Neonatal Med 2015: published online Aug 12, doi: 10.1016/j.siny.2015.07.001
- Brugha R, Starling M, Walt G. GAVI, the first steps: lessons for the Global Fund. Lancet 2002;359:435-8
- Hinman AR, McKinlay MA. Immunization equity. Am J Prev Med 2015: published online Aug 14, doi: 10.1016/j.amepre.2015.04.018
- Press release: political support crucial to reach ‘the fifth child' with vaccines. UNICEF, 2013. Available at: http://www.unicef.org/media/media_68809.html [Last accessed 14 August 2015]
- Global immunization data. World Health Organization, UNICEF, 2014. Available at: http://www.who.int/immunization/monitoring_surveillance/global_immunization_data.pdf [Last accessed 14 August 2015]
- Bairwa M, Pilania M, Rajput M, et al. Pentavalent vaccine. A major breakthrough in India's Universal Immunization Program. Hum Vaccin Immunother 2012;8:1314-16
- VVM history and milestones. World Health Organization, 2015. Available at: http://www.who.int/immunization_standards/vaccine_quality/VVM_history_milestones.pdf?ua=1 [Last accessed 14 August 2015]
- Cold-chain: the last child, the last mile. UNICEF, 2014. Available at: http://www.unicef.org/supply/index_68352.html [Last accessed 14 August 2015]
- Finding the final fifth. Inequalities in immunisation. Save the Children, 2012. Available at: http://www.savethechildren.org.uk/sites/default/files/docs/Finding-the-Final-Fifth.pdf [Last accessed 14 August 2015]
- Stokes-Prindle C, Privor-Dumm L, Haidari L, et al. Coverage, cost, and safety impacts of primary container choice. International Vaccine Access Center. 2013. Available at: http://www.jhsph.edu/research/centers-and-institutes/ivac/projects/IVAC-PrimaryContainerBriefReport-2013.pdf [Last accessed 14 August 2015]
- Kassahun MB, Biks GA, Teferra AS. Level of immunization coverage and associated factors among children aged 12–23 months in Lay Armachiho District, North Gondar Zone, Northwest Ethiopia: a community based cross sectional study. BMC Res Notes 2015;8:239
- Hu Y, Li Q, Chen E, et al. Determinants of childhood immunization uptake among socio-economically disadvantaged migrants in East China. Int J Environ Res Public Health 2013;10:2845-56
- Kusuma YS, Kumari R, Pandav CS, Gupta SK. Migration and immunization: determinants of childhood immunization uptake among socioeconomically disadvantaged migrants in Delhi, India. Trop Med Int Health 2010;15:1326-32
- Holt E. Ukraine at risk of polio outbreak. Lancet 2013;381:2244
- Senessie C, Gage GN, von Elm E. Delays in childhood immunization in a conflict area: a study from Sierra Leone during civil war. Confl Health 2007;1:14
- Pépin J, Abou Chakra CN, Pépin E, et al. Evolution of the global burden of viral infections from unsafe medical injections, 2000–2010. PLoS One 2014;9:e99677
- WHO guideline on the use of safety-engineered syringes for intramuscular, intradermal and subcutaneous injections in health-care settings. World Health Organization, 2015. Available at: http://www.who.int/injection_safety/global-campaign/injection-safety_guidline.pdf [Last accessed 14 August 2015]
- Final evaluation of Gavi Alliance’s support to Bosnia and Herzegovina. Final Evaluation Report. July 31, 2014. Gavi, The Vaccine Alliance, 2014. Available at: http://www.gavi.org/Results/Evaluations/Gavi-support-to-Bosnia-and-Herzegovina-evaluation/ [Last accessed 14 August 2015]
- SAGE October 2014. Strategic Advisory Group of Experts on Immunization 21–23 October 2014. World Health Organization Department of Immunization, Vaccines and Biologicals (IVB), 2014. Available at: http://www.who.int/immunization/sage/meetings/2014/october/Yellow-bookSAGE2014_final.pdf [Last accessed 14 August 2015]
- Gavi Board meeting, 18–19 June 2014. Gavi, The Vaccine Alliance, 2014. Available at: http://www.gavi.org/about/governance/gavi-board/minutes/2014/18-june/ [Last accessed 14 August 2015]
- Alcock R, Cottingham MG, Rollier CS, et al. Long-term thermostabilization of live poxviral and adenoviral vaccine vectors at supraphysiological temperatures in carbohydrate glass. Sci Transl Med 2010;2:19ra12
- Pearson FE, McNeilly CL, Crichton ML, et al. Dry-coated live viral vector vaccines delivered by nanopatch microprojections retain long-term thermostability and induce transgene-specific T cell responses in mice. PLoS One 2013;8:e67888
- The radically simple Uniject™ injection system. PATH, 2014. Available at: http://path.org/projects/uniject.php [Last accessed 14 August 2015]
- Global Immunization News. 29 February 2012. World Health Organization, 2012. Available at: http://www.who.int/immunization/GIN_February_2012.pdf [Last accessed 14 August 2015]
- Colombini A, Guillermet E, Dicko HM, et al. Optimal approaches for the use of DTP-HepB-Hib vaccine in a compact prefilled auto disable (cPAD) syringe in resource-poor settings. 16th International Congress on Infectious Diseases, Cape Town, South Africa, 2–5 April 2014
- Pentavalent vaccine in the Uniject™ injection system. A time and motion study. PATH, 2014. Available at: http://www.path.org/publications/files/TS_pentavalent_vac.pdf [Last accessed 14 August 2015]
- Kasi SG, Prabhu SV, Sanjay S, et al. Prefilled syringes versus vials: impact on vaccination efficiency and patient safety in Indian private market. Pediatr Infect Dis 2013;5:181-6
- The Uniject injection system: multi-country experience and evidence. Briefing Summary. PATH, 2011. Available at: http://www.path.org/publications/files/RH_depo_subq_experience.pdf [Last accessed 14 August 2015]
- Kristensen D. Case study: the Uniject™ injection system. Global Vaccine and Immunization Research Forum. 2014. Available at: http://www.who.int/immunization/research/forums_and_initiatives/03_Kristensen_GVIRF14_Uniject.pdf [Last accessed 14 August 2015]
- Immunization standards. WHO prequalified vaccines. World Health Organization, 2014. Available at: http://www.who.int/immunization_standards/vaccine_quality/PQ_vaccine_list_en/en/ [Last accessed 14 August 2015]
- Sutanto A, Suarnawa IM, Nelson CM, et al. Home delivery of heat-stable vaccines in Indonesia: outreach immunization with a prefilled, single-use injection device. Bull World Health Organ 1999;77:119-26
- Stakeholder perceptions of pentavalent vaccine (DTwP-HepB-Hib) in the Uniject™ injection system. PATH, Peruvian Ministry of Health, World Health Organization, 2014. Available at: http://www.path.org/publications/files/TS_dtwp_hepb_hib.pdf [Last accessed 14 August 2015]
- Nogier C, Hanlon P, Wiedenmayer K, Maire N. Can a compact pre-filled auto-disable injection system (cPAD) save costs for DTP-HepB-Hib vaccine as compared with single-dose (SDV) and multi-dose vials (MDV)? Evidence from Cambodia, Ghana, and Peru. Drugs Real World Outcomes 2015;2:43-52
- Humphreys G. Vaccination: rattling the supply chain. Bull World Health Organ 2011;89:324-5
- Zaffran M, Vandelaer J, Kristensen D, et al. The imperative for stronger vaccine supply and logistics systems. Vaccine 2013;31(Suppl 2):B73-80
- McCarney S, Zaffran M. Controlled Temperature Chain – the new term for Out of the Cold Chain. 2009. Available at: http://www.technet-21.org/en/resources/documents/cold-chain-equipment/725-controlled-temperature-chain-the-new-term-for-out-of-the-cold-chain/file [Last accessed 14 August 2015]
- Ren Q, Xiong H, Li Y, et al. Evaluation of an outside-the-cold-chain vaccine delivery strategy in remote regions of western China. Public Health Rep 2009;124:745-50
- Halm A, Yalcouyé I, Kamissoko M, et al. Using oral polio vaccine beyond the cold chain: a feasibility study conducted during the national immunization campaign in Mali. Vaccine 2010;28:3467-72
- Hipgrave DB, Tran TN, Huong VM, et al. Immunogenicity of a locally produced hepatitis B vaccine with the birth dose stored outside the cold chain in rural Vietnam. Am J Trop Med Hyg 2006;74:255-60
- Use of the MenAfriVac™ (meningitis A vaccine) in a controlled temperature chain (CTC) during campaigns. Guidance for immunization programme decision-makers and managers. World Health Organization, 2014. Available at: http://apps.who.int/iris/bitstream/10665/86018/1/WHO_IVB_13.04_eng.pdf [Last accessed 14 August 2015]
- Meningococcal A conjugate 10 dose presentation. World Health Organization, 2013. Available at: http://www.who.int/immunization_standards/vaccine_quality/PQ_197_MenAconjugate_10dose_SII/en/ [Last accessed 14 August 2015]
- Juan-Giner A, Domicent C, Langendorf C, et al. A cluster randomized non-inferiority field trial on the immunogenicity and safety of tetanus toxoid vaccine kept in controlled temperature chain compared to cold chain. Vaccine 2014;32:6220-6
- The controlled temperature chain (CTC): frequently asked questions. World Health Organization Department of Immunization, Vaccines and Biologicals, 2015. Available at: http://www.who.int/immunization/programmes_systems/supply_chain/resources/Controlled-Temperature-Chain-FAQ.pdf [Last accessed 14 August 2015]