Abstract
Objective:
Nonsteroidal anti-inflammatory drugs (NSAIDs) such as meloxicam are commonly used to treat osteoarthritis (OA) but are associated with potentially serious dose-related adverse events (AEs). SoluMatrix meloxicam has been developed with the goal of enabling effective treatment at low doses. This phase 3 study evaluated the efficacy and safety of low-dose SoluMatrix meloxicam capsules 5 mg and 10 mg administered once daily for 12 weeks in patients with OA-related pain.
Research design and methods:
This randomized, double-blind study enrolled patients ≥40 years of age with confirmed hip or knee OA (Kellgren–Lawrence grade II–III) who were chronic users of NSAIDs and/or acetaminophen for OA pain and had Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale mean scores ≥40 mm. Eligible patients experienced an OA pain flare (defined as a ≥15 mm increase in the WOMAC pain subscale score) following discontinuation of NSAIDs/acetaminophen. Patients were randomized to receive once-daily SoluMatrix meloxicam 5 mg or 10 mg, or placebo for 12 weeks.
ClinicalTrials.gov identifier: NCT01787188.
Main outcome measures:
The primary outcome measure was the mean change from baseline in WOMAC pain subscale score at week 12.
Results:
Low-dose SoluMatrix meloxicam 5 mg (−36.52 [2.49]; P = 0.0005) and 10 mg (−34.41 [2.68]; P = 0.0059) once-daily treatment significantly reduced the mean (standard error) WOMAC pain subscale score from baseline at week 12 compared with placebo (−25.68 [2.64]). Patients treated with SoluMatrix meloxicam 5 mg or 10 mg reported significantly greater improvements in total WOMAC score and in WOMAC stiffness and function subscale scores at 12 weeks compared with placebo. The most common AEs in the combined low-dose SoluMatrix meloxicam group were headache, diarrhea, nausea, osteoarthritis, and urinary tract infection.
Conclusions:
Low-dose SoluMatrix meloxicam may have a potential role as a new therapeutic option for the management of OA-related pain.
Introduction
Meloxicam is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam class with greater selectivity for cyclooxygenase (COX-2) compared with COX-1 at therapeutic doses. Reducing the dose of meloxicam results in an increased COX-1 sparing effectCitation1–4. Meloxicam is among the most frequently prescribed NSAIDs and is indicated for the relief of the signs and symptoms of osteoarthritis (OA), rheumatoid arthritis, and pauciarticular- or polyarticular-course juvenile rheumatoid arthritis in patients 2 years of age and olderCitation3,Citation5. Although meloxicam has been generally well tolerated, similar to other NSAIDs, it has been associated with an increased risk of serious cardiovascular (CV) and gastrointestinal (GI) adverse events (AEs)Citation6–13. Related to these concerns, the US Food and Drug Administration (FDA) issued a Public Health Advisory in 2005 requiring standard labeling for NSAIDs, including COX-2 selective agents, recommending that healthcare professionals should use NSAIDs at the lowest effective dose for the shortest duration, consistent with individual patient treatment goalsCitation14. In addition, in 2015, the FDA strengthened the language in the boxed warning regarding the increased risk of CV thrombotic events such as myocardial infarction and stroke for the existing prescription and over-the-counter non-aspirin NSAIDsCitation15. Professional medical organizations and health authorities outside of the US have made similar recommendations.
SoluMatrix meloxicam has been developed using SoluMatrix Fine Particle Technology with the aim of enabling effective treatment at lower doses than commercially available oral meloxicam drug products. This technology considerably reduces particle size, altering the pharmacokinetic properties of the NSAID-containing drug product and promoting rapid absorptionCitation16–19. Due to the dose-related risks for serious GI, CV, and renal AEs associated with NSAIDs, an oral NSAID formulation effective at low doses could provide clinically meaningful benefit in patients who require NSAID treatment. In a phase 1 study, SoluMatrix meloxicam 10 mg capsules demonstrated rapid meloxicam absorption with comparable peak plasma levels and an earlier time-to-peak plasma level compared with meloxicam 15 mg tablets (Mobic). Consistent with the reduction in meloxicam dosage, low-dose SoluMatrix meloxicam capsules provided a lower overall systemic exposure compared with conventional meloxicam 15 mg tablets under fasting conditionsCitation16,Citation19,Citation20.
We report the results of a phase 3 study that evaluated the efficacy and safety of investigational low-dose SoluMatrix meloxicam 5 mg and 10 mg capsules administered once daily for 12 weeks in patients with pain due to OA of the knee or hip.
Patients and methods
Patients
This study enrolled men and women with clinically and radiographically confirmed hip or knee OA (Kellgren–Lawrence grade II–IIICitation21) who were ≥40 years of age with a body weight ≥45 kg and a body mass index (BMI) ≤40 kg/m2. Patients were required to be chronic users of NSAIDs and/or acetaminophen for OA pain with a documented pain flare (≥15 mm increase in the Western Ontario and McMaster Universities Osteoarthritis Index [WOMAC] pain subscale score) following discontinuation of analgesic therapy. All enrolled patients had WOMAC Index pain subscale mean scores ≥40 mm at baseline. Participating patients provided signed written informed consent; the study was approved by an Institutional Review Board and adhered to the ethical principles of the International Conference on Harmonisation guidelines for Good Clinical Practice and the Declaration of HelsinkiCitation22,Citation23. Women of childbearing potential were excluded if pregnant or lactating and were required to use a medically acceptable method of birth control. Key exclusion criteria included a history of an allergy to NSAIDs or acetaminophen; regular use of opioid or opioid combination products to control OA pain; history or current diagnosis of peptic or gastric ulcers or GI bleeding; presence of a clinically significant unstable medical condition including cardiac disease; a history of alcohol or drug abuse; concomitant painful condition or history of major surgery in the target joint (e.g., joint replacement) that could confound or interfere with the evaluation of efficacy; or aspartate aminotransferase (AST) or alanine aminotransferase (ALT) ≥3 times upper limit of normal (ULN) at screening. The intent-to-treat (ITT) population was the primary population for the efficacy analysis and consisted of all patients who were successfully screened, randomized, and received at least one dose of the trial drug. The safety population was the population for all safety analyses and consisted of all patients who received at least one dose of the trial drug.
Study design and procedure
This was a phase 3, multicenter, randomized, double-blind, double-dummy, placebo-controlled, fixed-dose, parallel-group study (ClinicalTrials.gov identifier: NCT01787188). At the baseline visit, eligible patients were randomized to receive one of the three study drugs in a double-dummy fashion: (1) SoluMatrix meloxicam 5 mg capsule and a matching 10 mg placebo capsule once daily; (2) SoluMatrix meloxicam 10 mg capsule and a matching 5 mg placebo capsule once daily; or (3) matching 5 mg placebo capsule and matching 10 mg placebo capsule once daily. Patients were instructed to take study medication with water in the morning throughout the 12-week treatment period. Safety and efficacy assessments were performed at weeks 2, 6, and 12 or at the time of study withdrawal. Two telephone assessments occurred on a single day within 7 days of completion of the week 2 visit. Patients returned for a follow-up visit approximately 1 week after the last dose of the study drug. For rescue analgesia, patients were allowed one acetaminophen tablet (500 mg) every 4–6 hours as needed, up to 3000 mg daily. Patients were discouraged from taking rescue medication within 12 hours prior to any visit and were prohibited from receiving rescue medication 6 hours before any visit. The timing of daily rescue medication usage was electronically monitored and automatically compiled by the Medication Event Monitoring System (MEMS, WestRock Switzerland Ltd). The MEMS electronically recorded the date and time of each bottle opening. Patients were instructed to open the bottle only when rescue medication was needed and to promptly close the bottle after a dose was removed. Rescue medication use was also assessed on the drug accountability log as the number of acetaminophen tablets dispensed minus the number returned.
There were no changes to the protocol that altered patient eligibility criteria, protocol assessments, or prospectively defined analyses.
Efficacy assessments
The protocol-defined primary efficacy endpoint was the change from baseline in the WOMAC pain subscale score at week 12. Secondary efficacy endpoints included change from baseline in the WOMAC pain subscale score at weeks 2 and 6; change from baseline in the total WOMAC and in the WOMAC function and stiffness subscale scores at weeks 2, 6, and 12; and the average WOMAC pain, function, and stiffness subscale scores and average total WOMAC over the entire 12-week period; proportion of patients with a ≥30% or ≥50% reduction in pain intensity as assessed by the WOMAC pain subscale score at weeks 2, 6, and 12; a continuous responder analysis using the WOMAC pain subscale scores at week 12; Patient Global Impression of Change at week 12/ET; modified Outcome Measures in Rheumatology–Osteoarthritis Research Society International (OMERACT-OARSI) responder rates at week 12; and an exploratory analysis of change in pain intensity following dosing in the target joint within 1 week of the week 2 visit.
The WOMAC pain subscale assessment consists of five questions that were completed by the patients at screening and at baseline prior to randomization by asking them to recall their symptoms over the 24 hours prior to questioning. The total WOMAC scale consisting of pain, function, and stiffness subscale scores was administered at baseline (after randomization but before the study drug was dispensed), and throughout the dosing period at weeks 2, 6, and 12/ET.
Patient Global Impression of Change was assessed at week 12/ET by the patient by rating the change (7-point categorical scale) in overall status since beginning treatment with study drug by selecting one of the following responses: “Very much improved”, “Much improved”, “Minimally improved”, “No change”, “Minimally worse”, “Much worse”, or “Very much worse”. In an analysis of Patient Global Impression of Change, the proportion of patients with responses of “Very much improved” or “Much improved” vs “Much worse” or “Very much worse” in the active treatment groups were compared with those in the placebo group.
A continuous responder analysis was performed that included any patient achieving a reduction in WOMAC pain subscale. Patients who discontinued treatment were defined as nonresponders. For analysis of the proportion of patients with ≥30% and ≥50% reduction in WOMAC pain subscale score, WOMAC pain scores from ET visits were assigned to the closest visit with missing data. An additional responder analysis was based on modified OMERACT-OARSI criteria. The modified version of the OMERACT-OARSI criteria considered any Patient Global Impression of Change categorical response of “Minimally improved”, “Much improved”, or “Very much improved” to represent at least 20% improvement on a continuous scaleCitation24.
As an exploratory analysis of change in pain intensity in the target joint, on a single day following 2 weeks of dosing, patients rated their pain intensity using a numerical pain rating scale (NPRS) prior to receiving the daily dose of study medication and then at 2 hours ± 15 minutes postdose. The NPRS rated pain as: 0 (no pain) to 10 (worst pain imaginable).
Safety and tolerability assessments
Safety and tolerability were assessed as follows: AEs recorded at each study visit from the time the patient signed the informed consent form through the follow-up visit or week 12/ET visit. Other assessments included vital signs, physical examination, electrocardiograms (ECGs), and clinical laboratory testing. Abnormal physical examination findings were recorded as AEs.
Statistical analysis
Descriptive statistics included number of patients, mean, standard deviation (SD), minimum, maximum, frequency, and percentages. All statistical tests were two-sided unless otherwise indicated and a significance level of 0.05 was used for the primary efficacy analysis. The differences in least-squares (LS) means were used to evaluate the treatment effect between each active treatment arm and placebo. Least-squares means are obtained from the statistical models and are adjusted for covariates. A sequential test procedure was applied to address the multiple comparisons between each of the two SoluMatrix meloxicam dosing regimens (5 mg and 10 mg) vs placebo for the primary endpoint. If the test comparing SoluMatrix meloxicam 10 mg vs placebo produced a result that was statistically significant (P < 0.05), SoluMatrix meloxicam 5 mg was then compared with placebo at a significance level of 0.05. If the test comparing SoluMatrix meloxicam 10 mg vs placebo did not produce a statistically significant result, then comparison of SoluMatrix meloxicam 5 mg vs placebo was automatically considered nonsignificant. A restricted maximum likelihood-based mixed-model repeated measures (MMRM) analysis was conducted as the primary efficacy analysis using the ITT population. This included treatment as the main effect, investigative site and gender as blocking factors, and the baseline WOMAC pain subscale score as covariate. For secondary efficacy parameters, nominal P values were calculated comparing active treatment groups with placebo. Sample size was calculated to provide a minimum of 90% power to detect a minimal difference of 10.75 mm (effect size = 0.398) in WOMAC pain subscale between the two active treatment groups vs placebo using a two-sided, two-sample t-test at α = 0.05.
A sensitivity analysis of the primary efficacy MMRM was performed using the per protocol (PP) population. The PP population was a subset of the ITT population of patients who completed all 12 weeks of treatment, had a 12-week WOMAC pain score, and who did not incur a major protocol deviation that would challenge the validity of their data. Additional sensitivity analyses based on the ITT population were performed to assess the robustness of the results of the primary analysis and to test the assumptions of the MMRM model. The penalized MMRM model, which penalized patients who dropped out early, was performed to investigate the impact of early treatment discontinuation on the overall study results. Penalization was performed by multiplying the final reported WOMAC pain subscale score by the week at which study withdrawal occurred and then dividing this value by the total study length (12 weeks), reducing the WOMAC score based on when the patient was discontinued from the study. The pattern-mixture model was performed to confirm an MMRM assumption that missing data were “missing at random” (the missingness of the data does not depend on the missing value after conditioning on the observed data [such as prior WOMAC pain subscale scores and baseline covariates]). The Silverman Integrated Rank analysis was used to create a combined endpoint incorporating total rescue medication usage and WOMAC pain subscale scores at week 12Citation25. Total rescue medication use and WOMAC pain subscale scores were ranked separately and then combined to form a summated percentage difference of the mean rank. This variable was analyzed using an analysis of covariance (ANCOVA) model with treatment as the main effect, investigative site and gender as blocking factors, and with the baseline WOMAC pain subscale score as a covariate.
Odds ratios for patients reporting ‘Very much improved’ or ‘Much improved’ vs ‘Much worse’ or ‘Very much worse’ for the Patient Global Impression of Change were calculated, and the active treatment groups and placebo were compared using the Cochran–Mantel–Haenszel row means score test. Comparison of WOMAC function and subscale scores at weeks 2, 6, and 12 was performed similarly to the primary endpoint, using an MMRM analysis with treatment as the main effect. An ANCOVA model was used to compare average WOMAC subscale scores over the 12-week study.
Results
Disposition, demographics, and other baseline characteristics of patients
Of the 403 patients randomized, a majority (350, 86.8%) completed the 12-week study (). One patient randomized to the SoluMatrix meloxicam 5 mg group received SoluMatrix meloxicam 10 mg and was assigned to the 5 mg group for the ITT efficacy analysis but was analyzed as part of the SoluMatrix meloxicam 10 mg group for the analysis of safety. One patient withdrew prior to administration of study drug; hence, 402 patients were included in the ITT and safety populations. A summary of patient demographics and baseline characteristics is provided in . Most patients were female (65.9%) and white (78.6%). The patients were between 40 and 87 years of age, with a mean (SD) age of 60.7 (9.01) years and BMI of 30.9 (5.01) kg/m2. The majority of patients (51.7%) were ≥60 years of age. Most patients had OA of the knee (352 patients, 87.6%). The baseline mean WOMAC pain subscale score was nearly twice the minimum score required for study entry, indicating substantial OA pain among study participants. Mean (SD) total WOMAC score (68.91 [15.87]) and WOMAC function (67.69 [17.51]) and stiffness (70.71 [18.36]) subscale scores were similar across treatment groups ().
Figure 1. Patient disposition. aOne patient withdrew prior to administration of the study drug; hence, 402 patients were in the intent-to-treat and safety populations. bOne patient randomized to the SoluMatrix meloxicam 5 mg group received SoluMatrix meloxicam 10 mg and was recorded in the SoluMatrix meloxicam 10 mg group for analyses completed on the safety population, but remained in the 5 mg group for the analyses completed on the intent-to-treat population.
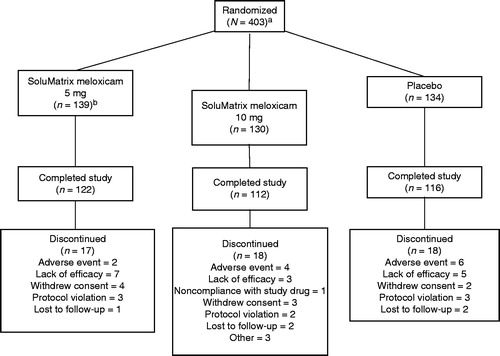
Table 1. Patient demographics and baseline characteristics.
Primary efficacy parameter
SoluMatrix meloxicam 5 mg (−36.52 [2.49]; P = 0.0005) and 10 mg (−34.41 [2.68]; P = 0.0059) treatment significantly reduced LS mean (standard error [SE]) WOMAC pain subscale score from baseline at week 12 compared with placebo (−25.68 [2.64]; ). The sensitivity analyses demonstrated similar treatment responses ().
Figure 2. Comparison of mean change from baseline in WOMAC pain subscale scores at weeks 2, 6, and 12 in patients with osteoarthritis. *P ≤ 0.024. †P = 0.1486. P values compared with placebo. All analyses are based on protocol-defined MMRM analysis (ITT population). ITT, intent-to-treat; LS, least squares; MMRM, mixed-model repeated measures; SE, standard error; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
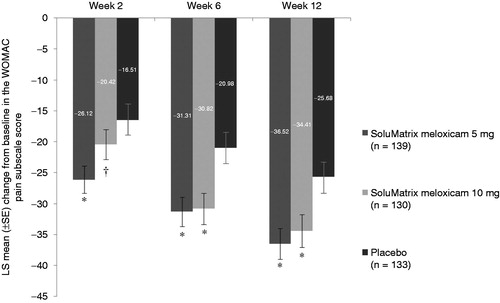
Table 2. Change in WOMAC pain subscale score from baseline comparison vs placebo at week 12 by MMRM and sensitivity analyses.
Additional WOMAC assessments
Changes from baseline in WOMAC pain subscale scores at weeks 2, 6, and 12 are provided in . Both SoluMatrix meloxicam 5 mg and 10 mg demonstrated statistically significant improvements from baseline in WOMAC pain subscale scores at week 6 compared with placebo. Patients treated with SoluMatrix meloxicam 5 mg (−10.44 [2.52]; P < 0.0001) and 10 mg (−7.82 [2.56]; P = 0.0024) reported significant LS mean (SE) differences from placebo in the average WOMAC pain subscale score changes from baseline over the 12-week treatment period.
Consistent with the trend for the WOMAC pain subscale, SoluMatrix meloxicam 5 mg and 10 mg treatment led to significant changes from baseline in the WOMAC function and stiffness subscale scores compared with placebo (). Patients in the SoluMatrix meloxicam 5 mg group experienced significant improvement in measures of function at weeks 2, 6, and 12; and those in the SoluMatrix meloxicam 10 mg group showed significant improvement at weeks 6 and 12 based on the difference in LS mean (SE) vs placebo for WOMAC function subscale scores (). Patients in both the 5 mg (−10.52 [2.65]; P < 0.0001) and 10 mg (−8.44 [2.68]; P = 0.0018) SoluMatrix meloxicam groups experienced statistically significant improvement from baseline in the LS mean (SE) WOMAC function subscale scores over the 12-week treatment period.
Figure 3. Comparison of mean change from baseline in the WOMAC (A) function and (B) stiffness subscale scores at weeks 2, 6, and 12 in patients with osteoarthritis. *P ≤ 0.0014. †P = 0.1065. ‡P ≤ 0.0379. P values compared with placebo. All analyses are based on protocol-defined MMRM analysis (ITT population). ITT, intent-to-treat; LS, least squares; MMRM, mixed-model repeated measures; SE, standard error; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
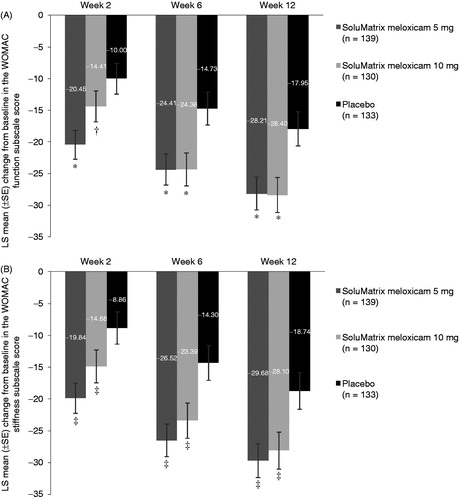
Similar improvement in the WOMAC stiffness subscale scores from baseline was reported by patients in the SoluMatrix meloxicam 5 mg and 10 mg treatment groups compared with placebo (). SoluMatrix meloxicam 5 mg (LS mean difference [SE]: −11.67 [2.75]; P < 0.0001) and 10 mg (−8.24 [2.80]; P = 0.0034) treatment groups reported significant reductions from baseline in the average WOMAC stiffness subscale score over 12 weeks compared with placebo.
Patients treated with SoluMatrix meloxicam 5 mg experienced significant improvement from baseline in the total WOMAC score (LS mean difference [SE]) that comprised measures of pain, stiffness, and function at week 2 (−10.34 [2.62]; P < 0.0001), week 6 (−9.83 [2.91]; P = 0.0008), and week 12 (−10.13 [3.15]; P = 0.0014) compared with placebo. Patients in the SoluMatrix meloxicam 10 mg group also experienced improvement compared with placebo based on change from baseline total WOMAC score at week 6 (LS mean difference [SE]: −9.42 [2.95]; P = 0.0015) and week 12 (−9.98 [3.20]; P = 0.0019), although the differences did not achieve statistical significance at week 2 (−4.39 [2.66]; P = 0.0997). However, statistically significant differences in the LS mean (SE) reductions from baseline in the average total WOMAC scores over the 12-week period compared with placebo were noted in both the SoluMatrix meloxicam 5 mg (−10.47 [2.59]; P < 0.0001) and 10 mg (−8.16 [2.63]; P = 0.0020) treatment groups.
Clinically important treatment response and Patient Global Impression of Change
A significantly higher proportion of patients reported a ≥30% reduction in WOMAC pain subscale score from baseline following treatment with SoluMatrix meloxicam 5 mg at weeks 2 (83 [61%]; P = 0.0002), 6 (88 [65.7%]; P = 0.0072), and 12 (97 [74.0%]; P = 0.0050) vs placebo (51 [38.6%]; 62 [49.2%]; 73 [57.5%], respectively); more patients in the SoluMatrix meloxicam 10 mg group experienced similar improvements at week 2 (60 [48.8%]; P = 0.1026), week 6 (83 [68.6%]; P = 0.0020), and week 12 (81 [68.1%]; P = 0.0863), which were statistically significant at week 6. In the SoluMatrix meloxicam 5 mg treatment group, a higher proportion of patients experienced a ≥50% reduction in WOMAC pain subscale score at weeks 2 (54 [39.7%]; P = 0.0151), 6 (69 [51.5%]; P = 0.0031), and 12 (78 [59.5%]; P = 0.0003 vs placebo); patients in the SoluMatrix meloxicam 10 mg group experienced statistically significant improvements at week 6 (58 [47.9%]; P = 0.0194) and week 12 (68 [57.1%]; P = 0.0016) compared with placebo (34 [25.8%]; 42 [33.3%]; 47 [37.0%], respectively).
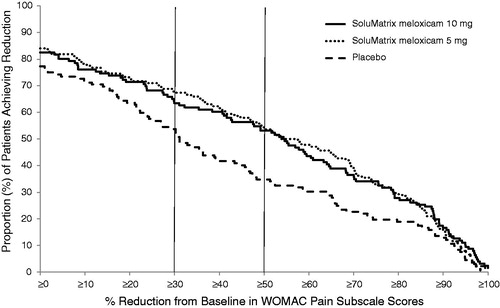
In a continuous responder analysis of patients achieving varying levels of pain reduction measured by WOMAC pain subscale ranging from 0% to 100% on a continuous scale, nearly 65% and more than 50% of patients in both the SoluMatrix meloxicam 5 mg and 10 mg groups achieved ≥30% and ≥50% reductions, respectively, in pain intensity by week 12 ().
Figure 4. Continuous responder analysis of the WOMAC pain subscale scores at week 12 in patients with osteoarthritis.
In the modified OMERACT-OARSI analysis, which utilized the categorical Patient Global Impression of Change as part of the definition of a responder, a significantly higher proportion of patients were considered responders at week 12 in the SoluMatrix meloxicam 5 mg (104/137 patients [75.9%]; P = 0.0390) and 10 mg (98/124 patients [79.0%]; P = 0.0096) treatment groups compared with placebo (83/129 patients; 64.3%).
A predefined analysis of patient satisfaction (Patient Global Impression of Change) allowed comparison of the numbers of patients experiencing substantial improvement or substantial worsening in each treatment arm compared with placebo. More patients in the SoluMatrix meloxicam 5 mg (50.0% [68/136] vs 3.7% [5/136]; P = 0.1378) or 10 mg (52.8% [66/125] vs 2.4% [3/125]; P = 0.0416) treatment groups at week 12 were considered “Very much improved” or “Much improved” vs “Much worse” or “Very much worse” compared with placebo (40.0% [52/130] vs 6.9% [9/130]), although the analysis was statistically significant only for the SoluMatrix meloxicam 10 mg treatment group.
Exploratory efficacy analysis
In a protocol-specified exploratory analysis, OA pain was assessed using the 11 point NPRS score before and 2 hours after each patient’s daily morning dose of study medication on a single day following the week 2 visit. Patients in the low-dose SoluMatrix meloxicam treatment groups reported a greater LS mean (SE) percentage reduction (5 mg, −33.44% [3.546%]; P = 0.0294; 10 mg, −30.54% [3.774%]; P = 0.1357) in the NPRS score compared with placebo (−24.32% [3.721%]), which was statistically significant in the SoluMatrix meloxicam 5 mg treatment group.
Rescue medication use
SoluMatrix meloxicam 5 mg (326.2 mg [41.36]; P = 0.0046) and 10 mg (313.6 mg [45.50]; P = 0.0024) treatment led to less rescue medication use compared with placebo (LS mean [SE]: 464.1 mg [43.73]) in an analysis of average daily rescue medication use among patients who received rescue medication at any time during the trial. Similarly, patients in the SoluMatrix meloxicam 5 mg (LS mean [SE] 25.3 [2.16] days; P = 0.0007) and 10 mg (23.5 [2.34] days; P < 0.0001 vs placebo) treatment groups used rescue medication significantly less frequently compared with those in the placebo group (33.9 [2.36] days) in an analysis of number of days that rescue medication was taken, as recorded by MEMS technology.
A post hoc analysis of rescue medication use in all patients at various time intervals (>midnight-to-6 AM, >6 AM-to-noon, >noon-to-6 PM, and >6 PM-to-midnight) demonstrated that a generally lower percentage of patients in the SoluMatrix meloxicam 5 mg and 10 mg treatment groups required rescue medication compared with placebo during each of the time intervals, and the pattern of use within each group was generally consistent across the >6 AM-to-noon, >noon-to-6 PM, and >6 PM-to-midnight time intervals. Significant differences in the proportion of patients with rescue medication events were recorded during the >6 AM-to-noon interval for the SoluMatrix meloxicam 5 mg (67.6% [94/139]; P = 0.0241) and 10 mg (66.9% [87/130]; P = 0.0191) treatment groups compared with placebo (79.7% [106/133]) and the >6 PM-to-midnight interval for the 10 mg treatment group (66.9% [87/130]; P = 0.0281) compared with placebo (78.9% [105/133]).
Safety analysis
The summary of the most frequent treatment-emergent AEs reported by ≥1% of patients in any treatment group is provided in . The most common AEs in the combined SoluMatrix meloxicam group were headache (7/269, 2.6%), diarrhea (7/269, 2.6%), nausea (6/269, 2.2%), OA (5/269, 1.9%), and urinary tract infection (5/269, 1.9%). The majority of AEs were considered mild to moderate in intensity. Three patients (0.7%) experienced severe AEs. In the placebo group, 1 (0.8%) patient experienced gastroenteritis and 1 (0.8%) experienced migraine; and in the SoluMatrix meloxicam 10 mg group, 1 patient (0.8%) experienced an increase in hepatic enzymes, although this increase was considered unrelated to trial drug. No deaths or serious AEs (SAEs) occurred during the study period. A smaller proportion of patients in the combined SoluMatrix meloxicam group (6 patients [2.2%]) withdrew from the study due to an AE compared with the placebo group (6 patients [4.5%]).
Table 3. Summary of most frequent treatment-emergent AEs (≥1% of patients in any group).
Vital signs and clinical laboratory analyses
Vital sign measures (mean systolic and diastolic blood pressure, heart rate, respiratory rate, and body temperature measurements), hematology laboratory values, urinalysis laboratory values, and ECG findings were stable over the course of the study and similar across treatment groups. The baseline mean clinical chemistry values were similar across treatment groups and within normal limits for most patients. Changes in mean chemistry values from baseline were small and similar across treatment groups, although some notable changes occurred at weeks 6 and 12 in both the SoluMatrix meloxicam 5 mg and 10 mg treatment groups compared with placebo, including slight changes in mean ALT (SoluMatrix meloxicam 5 mg: +2.2 U/L; 10 mg: +1.5 U/L; placebo: 0.0 U/L) AST (SoluMatrix meloxicam 5 mg: +0.9 U/L; 10 mg: +0.6 U/L; placebo: −0.6 U/L), alkaline phosphatase (SoluMatrix meloxicam 5 mg: −0.8 U/L; 10 mg: −1.6 U/L; placebo: −0.4 U/L) and blood urea nitrogen (SoluMatrix meloxicam 5 mg: +0.05 mmol/L; 10 mg: +0.5 mmol/L; placebo: −0.28 mmol/L) levels among patients treated with low-dose meloxicam at week 12. Laboratory findings of potential clinical concern are presented in .
Table 4. Proportion of patients with clinical laboratory assessments of potential concern.
Discussion
Low-dose SoluMatrix meloxicam was developed using SoluMatrix Fine Particle Technology to provide efficacy using lower doses than reference oral NSAID drug products. This objective is aligned with recommendations by the FDA and professional organizations that clinicians considering prescribing these agents for their patients should use the lowest effective dose for the shortest duration. This phase 3, 12-week placebo-controlled study of SoluMatrix meloxicam was undertaken to establish efficacy using dosing regimens that included the lowest daily NSAID dose among meloxicam drug products.
The patient demographics and baseline characteristics were typical of a population of patients with hip or knee OACitation26. The majority of these patients were obese, most (66%) were female, and 51.7% were 60 years of age or older. The severity of OA pain at baseline was significant; the mean baseline WOMAC pain subscale score was nearly twice the threshold score for study entry.
The efficacy of SoluMatrix meloxicam (5 mg and 10 mg) in the treatment of OA pain was demonstrated for the primary efficacy endpoint as well as various secondary efficacy measures. Both doses of SoluMatrix meloxicam met the primary endpoint with significant improvements in WOMAC pain subscale scores at 12 weeks compared with placebo. Significant improvements in WOMAC pain subscale scores from baseline were apparent in the SoluMatrix meloxicam 5 mg group as early as 2 weeks following the start of treatment. Patients treated with SoluMatrix meloxicam 5 mg and 10 mg experienced significantly greater improvements in total WOMAC score as well as WOMAC stiffness and function subscale scores at 12 weeks following the start of treatment compared with placebo. More patients reported substantial improvement in PGIC among SoluMatrix meloxicam recipients compared with placebo. The robustness of SoluMatrix meloxicam efficacy was also demonstrated in sensitivity analyses that included more conservative measurements of treatment efficacy including the penalized MMRM and Silverman Integrated Rank Analysis.
For WOMAC-based efficacy measures, no clear dose–response relationship was observed for SoluMatrix meloxicam 5 mg vs 10 mg; however, a dose response was observed for a number of other efficacy measures including PGIC, the proportion of ‘responders’ based on modified OMERACT-OARSI criteria, and rescue medication use. There were no differences in the baseline characteristics, including the severity and distribution of WOMAC pain subscores at baseline and at 12 weeks among the SoluMatrix meloxicam treatment groups. In previous studies of meloxicam 7.5 mg and 15 mg – although both dosing regimens demonstrated efficacy – a dose response for global efficacy was not consistently observed, similar to the findings in the current studyCitation27.
A phase 1 study demonstrated rapid meloxicam absorption following administration of SoluMatrix meloxicam in fasting conditions with a median time to maximum plasma concentration of 2 hours for SoluMatrix meloxicam capsules compared with 4 hours for meloxicam tabletsCitation20. In an exploratory analysis in this study, patient improvement measured by the 11-point NPRS at 2 hours after dosing, which corresponds to the previously observed maximum meloxicam plasma concentration, suggested an association between efficacy and drug absorption.
Among patients who used acetaminophen rescue analgesia, patients in the placebo group used significantly more rescue medication than those in either SoluMatrix meloxicam group, which may have contributed to the significant placebo effect observed in this study. An advantage of allowing rescue medication in the trial is the use of these data as a measure of drug efficacy in OA-related pain managementCitation28–30. A reduction in rescue medication use in the SoluMatrix meloxicam groups suggests that both doses are effective in the management of pain in OA patients. The Silverman Integrated Rank Analysis, which took into account rescue medication use and WOMAC pain subscale score, demonstrated significant improvement for both the SoluMatrix meloxicam 5 mg and 10 mg treatment groups at week 12.
SoluMatrix meloxicam 5 mg and 10 mg were generally well tolerated. The percentage and types of AEs reported were as expected and typical for this study population of patients with OA treated with NSAIDsCitation31–33. The majority of reported AEs were mild to moderate in intensity. A severe AE, an increase in hepatic enzymes, was reported in a single patient treated with low-dose meloxicam; however, this AE was considered unrelated to the study drug. Although some of the clinically significant changes in alkaline phosphatase, ALT, AST, bilirubin, blood urea nitrogen, creatinine, glucose, and potassium were associated with treatment, none were considered serious. No SAEs (requiring hospitalization; considered a “significant hazard” by the investigator) were reported by patients in any treatment group, and no deaths occurred during this study.
In a previous study in patients with hip or knee OA dosed for 12 weeks, meloxicam 15 mg (−4.5; P ≤ 0.001) and 7.5 mg (−3.4; P < 0.05) demonstrated significant differences in change in WOMAC pain subscale measured by Likert scale (range 0–20) compared with placebo (−2.2)Citation34. In the same study, reductions in the patients’ overall assessment of pain using a 100 mm Visual Analog Scale were observed for meloxicam 15 mg (−28.4; P ≤ 0.005) and 7.5 mg (−27.9; P ≤ 0.005) compared with placebo (−18.7). Although a direct comparison is not feasible, the magnitude of the treatment effect observed in the present study for SoluMatrix meloxicam 5 mg or 10 mg compares favorably with conventional meloxicam doses of 7.5 mg and 15 mgCitation34.
Potential limitations in this study include the high prevalence of rescue medication usage in all treatment groups (despite clear demonstration of efficacy for both treatment arms), the absence of an active comparator, and the relatively short (12-week) study period.
A 52-week open-label study of SoluMatrix meloxicam has been completed and will further clarify the safety profile of this new drug product. SoluMatrix meloxicam 5 mg and 10 mg are potentially promising alternatives to other currently approved meloxicam doses. In view of the greater COX-1 sparing effect of meloxicam at lower doses, SoluMatrix meloxicam may potentially represent an alternative to NSAID administration with a proton pump inhibitorCitation1,Citation35, as recommended by an OA treatment algorithm published in 2014 by the task force of the European Society for Clinical and Economic Aspects of Osteoporosis and OsteoarthritisCitation36. The results from this study support a potential role for low-dose SoluMatrix meloxicam as a new low-dose therapeutic option for the management of OA-related pain.
Transparency
Declaration of funding
This study was sponsored by Iroko Pharmaceuticals, LLC.
Declaration of financial/other relationships
R.A. has disclosed that he is a consultant to Pfizer, Teva Pharmaceutical Industries Ltd, Petah Tikva, Oletec, Novartis, and Johnson & Johnson; and consultant and member of the speaker’s bureau for Fening Pharmaceuticals and Iroko Pharmaceuticals LLC. M.H. has disclosed that he is a consultant for Bioiberica SA, Eli Lilly and Co., EMD Serono, Iroko Pharmaceuticals LLC, Novartis Pharma AG, Pfizer, Rottapharm Biotech, and Theralogix LLC. A.G. has disclosed that he is a stock shareholder of AbbVie, Amgen, Bristol-Myers Squibb, GlaxoSmithKline plc, Johnson & Johnson, Pfizer, and Regeneron; consultant for AbbVie, Amgen, Antares, AstraZeneca, Horizon, Iroko Pharmaceuticals, LLC, Pfizer, and Takeda; and speaker for AbbVie, Amgen, Antares, Celgene Corporation, Iroko Pharmaceuticals LLC, and Pfizer. M.J. has disclosed that he is an employee of Summit Analytical. C.Y. has disclosed that he is a stock shareholder and an employee of Iroko Pharmaceuticals, LLC.
CMRO peer reviewers on this manuscript received an honorarium from CMRO for their review work. Peer reviewer 1 has disclosed that he has been a consultant to or on the advisory board of Servier, Novartis, Negma, Eli Lilly, Wyeth, Amgen, GlaxoSmithKline, Roche, Merck, Nycomed, NPS, Theramex, and UCB. He has also received fees for speaking at invited lectures from Merck Sharp and Dohme, Elli Lilly, Rottapharm, IBSA, Genevrier, Novartis, Servier, Roche, GlaxoSmithKline, Teijin, Teva, Ebewee Pharma, Zodiac, Analis, Theramex, Nycomed, Novo-Nordisk, and Nolver; and has received grant support from Bristol Myers Squibb, Merck Sharp & Dohme, Rottapharm, Teva, Eli Lilly, Novartis, Roche, GlaxoSmithKline, Amgen, and Servier. Peer reviewer 2 has disclosed that he has received sponsorship funding from Servier, Novartis, and IBSA; has been the recipient of research/grant funding from Servier, Novartis, IBSA, and Rottapharm; and is a consultant/advisor to Servier and Rottapharm.
Acknowledgments
The authors thank the following individuals: Melissa Ortiz Alvidrez, Heidi Miracle, Olaolu Imasogie, Daniel Solorio, Alexis Gomez, Claire Sheridan, Melanie Lauterio, and the investigators and patients who participated in this study. Editorial support provided by Jill See PhD and Cole Brown MD of AlphaBioCom was funded by Iroko Pharmaceuticals LLC.
Prior presentations: Altman R, Hochberg M, Gibofsky A, et al. Lower-dose SoluMatrix meloxicam: efficacy and safety in a phase 3 study in adults with osteoarthritis pain. Presented at: the European League Against Rheumatism annual meeting. 11–14 June 2014, Paris, France. Published in: Ann Rheum Dis 2014;73(Suppl 2):250-1. Altman R, Hochberg M, Gibofsky A et al. Low-dose SoluMatrix meloxicam demonstrates low systemic exposure and efficacy in patients with osteoarthritis pain in clinical studies. Presented at: 2015 World Congress on Osteoarthritis (OARSI), 30 April–3 May 2015, Seattle, WA, USA. Published in: Osteoarthritis Cartilage;2015;23(Suppl 2):A324-5.
Notes
*SoluMatrix is a registered trademark of iCeutica Pty Ltd and is licensed to Iroko.
*SoluMatrix is a registered trademark of iCeutica Pty Ltd and is licensed to Iroko.
†SoluMatrix Fine Particle Technology is a trademark of iCeutica Inc., and the technology is licensed to Iroko for exclusive use in NSAIDs.
‡Mobic is a trademark of Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT, USA
References
- Tegeder I, Lotsch J, Krebs S, et al. Comparison of inhibitory effects of meloxicam and diclofenac on human thromboxane biosynthesis after single doses and at steady state. Clin Pharmacol Therapeut 1999;65:533-44
- Van Hecken A, Schwartz JI, Depre M, et al. Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1 in healthy volunteers. J Clin Pharmacol 2000;40:1109-20
- Brune K, Patrignani P. New insights into the use of currently available non-steroidal anti-inflammatory drugs. J Pain Res 2015;8:105-18
- Albengres E, Urien S, Barre J, et al. Clinical pharmacology of oxicams: new insights into the mechanisms of their dose-dependent toxicity. Int J Tissue React 1993;15:125-34
- Bartholomew M. Top 200 Drugs of 2012. Pharmacy Times, 2013. Available at: http://www.pharmacytimes.com/publications/issue/2013/july2013/top-200-drugs-of-2012 [Last accessed 25 September 2015]
- Brater DC. Anti-inflammatory agents and renal function. Semin Arthritis Rheum 2002;32(3 Suppl 1):33-42
- Coxib and traditional NSAID Trialists' (CNT) Collaboration. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013;382:769–79
- Graham DJ, Campen D, Hui R, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case–control study. Lancet 2005;365:475-81
- Lewis SC, Langman MJ, Laporte JR, et al. Dose–response relationships between individual nonaspirin nonsteroidal anti-inflammatory drugs (NANSAIDs) and serious upper gastrointestinal bleeding: a meta-analysis based on individual patient data. Br J Clin Pharmacol 2002;54:320-6
- McGettigan P, Henry D. Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med 2011;8:e1001098
- Schmidt M, Christiansen CF, Mehnert F, et al. Non-steroidal anti-inflammatory drug use and risk of atrial fibrillation or flutter: population based case–control study. BMJ (Clinical research ed) 2011;343:d3450
- Whelton A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med 1999;106:13-24s
- Distel M, Mueller C, Bluhmki E, Fries J. Safety of meloxicam: a global analysis of clinical trials. Br J Rheumatol 1996;35(Suppl 1):68-77
- Public Health Advisory – FDA Announces Important Changes and Additional Warnings for COX-2 Selective and Non-Selective Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). 2005. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm150314.htm [Last accessed 25 September 2015]
- FDA Drug Safety Communication: FDA strengthens warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) can cause heart attacks or strokes. 2015. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm451800.htm 07/17/2015 [Last accessed 14 July 2015]
- Olugemo K, Parenti D, Young C. [Abstract AB0869] Pharmacokinetics and safety of lower-dose SoluMatrix meloxicam in healthy adults. Ann Rheum Dis 2014;73(Suppl 2):1089
- Desjardins PJ, Olugemo K, Solorio D, Young CL. Pharmacokinetic properties and tolerability of low-dose SoluMatrix diclofenac. Clin Ther 2015;37:448-61
- Olugemo K, Solorio D, Sheridan C, Young CL. Pharmacokinetics and safety of low-dose submicron indomethacin 20 and 40 mg compared with indomethacin 50 mg. Postgraduate Med 2015;127:223-31
- Altman R, Hochberg M, Gibofsky A, et al. Low-dose SoluMatrix meloxicam demonstrates low systemic exposure and efficacy in patients with osteoarthritis pain in clinical studies. Osteoarthritis Cartilage 2015;23(Suppl 2):A324-5
- Full Prescribing Information for VIVLODEX. 2015. Iroko Pharmaceuticals, LLC. Available at: https://www.vivlodex.com/wp-content/uploads/2015/10/vivlodex-prescribing-information.pdf. [Last accessed 4 November 2015]. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207233s000lbl.pdf. [Last accessed 17 November 2015]
- Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494-502
- Baber N. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use (ICH). Br J Clin Pharmacol 1994;37:401-4
- World Medical Association. Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997;277:925-6
- Pham T, van der Heijde D, Lassere M, et al. Outcome variables for osteoarthritis clinical trials: The OMERACT-OARSI set of responder criteria. J Rheumatol 2003;30:1648-54
- Silverman DG, O’Connor TZ, Brull SJ. Integrated assessment of pain scores and rescue morphine use during studies of analgesic efficacy. Anesth Analg 1993;77:168-70
- Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clinics Geriatr Med 2010;26:355-69
- Reginster JY, Distel M, Bluhmki E. A double-blind, three-week study to compare the efficacy and safety of meloxicam 7.5 mg and meloxicam 15 mg in patients with rheumatoid arthritis. Br J Rheumatol 1996;35(Suppl 1):17-21
- Hosie J, Distel M, Bluhmki E. Meloxicam in osteoarthritis: a 6-month, double-blind comparison with diclofenac sodium. Br J Rheumatol 1996;35(Suppl 1):39-43
- Hochberg MC, Martel-Pelletier J, Monfort J, et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis 2015; doi:10.1136/annrheumdis-2014-206792
- Provenza JR, Shinjo SK, Silva JM, et al. Combined glucosamine and chondroitin sulfate, once or three times daily, provides clinically relevant analgesia in knee osteoarthritis. Clin Rheumatol 2014;34:1455-62
- Dahlberg LE, Holme I, Hoye K, Ringertz B. A randomized, multicentre, double-blind, parallel-group study to assess the adverse event-related discontinuation rate with celecoxib and diclofenac in elderly patients with osteoarthritis. Scand J Rheumatol 2009;38:133-43
- Fleischmann R, Tannenbaum H, Patel NP, et al. Long-term retention on treatment with lumiracoxib 100 mg once or twice daily compared with celecoxib 200 mg once daily: a randomised controlled trial in patients with osteoarthritis. BMC Musculoskel Disord 2008;9:32
- Saag K, van der Heijde D, Fisher C, et al. Rofecoxib, a new cyclooxygenase 2 inhibitor, shows sustained efficacy, comparable with other nonsteroidal anti-inflammatory drugs: a 6-week and a 1-year trial in patients with osteoarthritis. Osteoarthritis Studies Group. Arch Fam Med 2000;9:1124-34
- Yocum D, Fleischmann R, Dalgin P, et al. Safety and efficacy of meloxicam in the treatment of osteoarthritis: a 12-week, double-blind, multiple-dose, placebo-controlled trial. The Meloxicam Osteoarthritis Investigators. Arch Intern Med 2000;160:2947-54
- Churchill L, Graham AG, Shih CK, et al. Selective inhibition of human cyclo-oxygenase-2 by meloxicam. Inflammopharmacology 1996;4:125-35
- Bruyère O, Cooper C, Pelletier JP, et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum 2014;44:253-63
