Abstract
Objective:
Nonsteroidal anti-inflammatory drugs (NSAIDs) are standard therapy for osteoarthritis (OA). Topically applied NSAIDs reduce systemic exposure compared with oral NSAIDS, and European guidelines recommend their use. The NSAID diclofenac is available in a range of topical formulations. Diclofenac 1% gel and 1.5% four times daily and 2% twice daily (BID) solutions are approved to reduce pain from OA of the knee(s). The objective of this study was to investigate the efficacy and safety of diclofenac sodium 2% topical solution BID versus vehicle control solution for treating pain associated with OA of the knee.
Research design and methods:
A phase II, 4 week, randomized, double-blind, parallel-group, two-arm, vehicle-controlled study compared pain relief with diclofenac sodium 2% topical solution versus control (vehicle only) in patients aged 40 to 85 years with radiographically confirmed primary OA of the knee.
Clinical trial registration:
ClinicalTrials.gov identifier NCT01119898.
Main outcome measures:
The primary efficacy outcome was change from baseline to the final visit in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale. Secondary outcomes included additional WOMAC subscales and patient global assessment of OA. Treatment-emergent adverse events (TEAEs), skin irritation, and vital signs were assessed and collected throughout the study.
Results:
Of 260 patients randomized, 259 received ≥1 dose of study drug. Significantly greater reductions in least-squares mean (standard error) WOMAC pain scores were observed for diclofenac-treated (−4.4 [0.4]) versus vehicle-treated patients (−3.4 [0.4]) at the final visit (p = 0.040). The most commonly reported TEAEs were administration site conditions. The vehicle-treated group experienced slightly more TEAEs than the active treatment group (38.8% vs. 31.5%). No serious adverse events were reported.
Conclusions:
Administration of diclofenac sodium 2% topical solution BID resulted in significantly greater improvement in pain reduction in patients with OA of the knee versus vehicle control and was generally well tolerated.
Introduction
Osteoarthritis (OA) is a joint disease with a multifactorial etiology that leads to eventual loss of joint function and associated pain, stiffness, decreased mobility, and in some cases, disability. OA is characterized as a painful, inflammatory arthritis of the cartilageCitation1, with contributory genetic, immune, mechanical and other inflammatory mediated processes. Clinical evidence has demonstrated abnormalities of the synovium, synovial fluid, and subchondral bone, and inflammatory changes have been consistently noted in involved joints, especially with more advanced diseaseCitation2. OA specifically of the knee is associated with a large burden of both acute and chronic pain, decreased mobility, and reduced quality of lifeCitation3–5.
The estimated lifetime risk of OA of the knee is 13.8%, ranging from 9.6% for non-obese males to 23.9% for obese females. About 9.3% of the US population will be diagnosed with symptomatic knee OA by the age of 60. The estimated median age at knee OA diagnosis is 55 yearsCitation6. Nearly one in two people may develop symptomatic knee OA by 85 years of age, with lifetime risk highest among obese individuals (two in three)Citation7.
Nonsteroidal anti-inflammatory drugs (NSAIDs) exhibit both anti-inflammatory and analgesic activity, in part due to inhibition of prostaglandin biosynthesisCitation8. NSAIDs are one of the most frequently used therapies for managing the symptoms of OACitation9–11. However, oral NSAIDs are associated with an increased risk of cardiovascular, gastrointestinal, and renal toxicityCitation9,Citation12.
Topically administered NSAIDs reduce the amount of systemic exposure compared with oral NSAIDSCitation13,Citation14, thus generally reducing the incidence of gastrointestinal and other associated adverse events (AEs)Citation15–17. This is an important consideration for patients with comorbidities, such as the elderly, or for those patients with difficulty swallowing oral medicationsCitation18–20. In addition, the topical administration route is beneficial in providing pain relief while minimizing toxicity in patients with knee OA taking anticoagulants, and in those with renal and/or hepatic dysfunction, heart failure, or severe respiratory disease, as well as other pathological issues.
European guidelines have recommended the use of topical NSAIDs as a treatment option in OA for many years and, recently, after the approval of several topical NSAIDs by the US Food and Drug Administration, US guidelines are now increasingly recommending the use of topical NSAIDs as an alternative to oral agents in the treatment of OA when symptoms are limited to a small number of jointsCitation9.
Diclofenac is an NSAID whose mechanism of action (like that of other NSAIDs) is not completely understood but which may be related to prostaglandin synthetase or cyclooxygenase inhibition. A variety of topical diclofenac formulations are now available. These include diclofenac sodium gels and solutions, lotion, lecithin or epolamine gel, patch, or plaster, delivering a range of dosesCitation21–23. Topical NSAID diclofenac gel 1% and solution 1.5% four times daily (QID) have been shown to provide safe, localized treatment of OA painCitation11,Citation12,Citation24–26.
Diclofenac sodium 2% topical solution (Pennsaid 2%, Horizon Pharma USA Inc., Deerfield, IL, USA) is a novel formulation approved in January 2014 for reducing the pain associated with OA of the knee. Diclofenac sodium 2% (40.4 mg/2 mL twice daily [BID]) topical solution BID provides a similar daily dose to diclofenac sodium 1.5% (19.3 mg/40 drops QID) topical solution QID and provides a similar daily dose to oral diclofenac sodium (75 mg BID), with significantly lower systemic exposureCitation17.
The objective of this study was to investigate the efficacy and safety of diclofenac sodium 2% topical solution BID in patients with OA of the knee.
Patients and methods
Study design and participants
This was a phase II, 4 week, randomized, double-blind, parallel-group, two-arm, vehicle-controlled study. Patients eligible for inclusion were aged 40 to 85 years; had radiographically confirmed primary OA of the knee within 1 year prior to screening consistent with Grade 2 or 3 on the Kellgren–Lawrence grading scale; and were receiving stable analgesic therapy (i.e. at least 3 days per week for the previous month with an oral or topical NSAID or acetaminophen). Enrolled patients underwent a 3–14 day medication washout period prior to their first study treatment application and were required to demonstrate a ‘moderate flare’ (defined as a baseline minimum pain score of at least 5 using an 11 point numeric rating scale [NRS] and at least a 2 unit worsening from the screening pain score) of pain in at least one knee following washout of the pain medication.
Exclusion criteria were secondary OA (defined as OA resulting from a specific cause such as an injury or an effect of obesity, genetics, inactivity, or other disease) or symptomatic chondrocalcinosis (determined via radiographic diagnosis with symptoms being present) of the study knee; study drug contraindicated or known sensitivity to diclofenac or other NSAIDs; other severe medical condition; any abnormality that could confound interpretation of the safety results; uncontrolled diabetes; documented history of alcohol or drug abuse within 1 year prior to the screening visit; and breastfeeding females. Patients were also excluded if they had major surgery or previous damage to the study knee at any time, or minor knee surgery (e.g. any surgery other than major surgery including, but not limited to, cartilage repair, collateral ligament repair, or arthroscopic debridement) to the study knee within 1 year prior to screening; corticosteroid injection into the primary study knee within 90 days of screening or into any other joint within 30 days of screening, or topical corticosteroid use on the study knee. Additional exclusion criteria included intra-articular viscosupplementation in the primary study knee in the 6 months prior to screening; prior use of a stable opioid analgesic; any other painful or disabling conditions affecting the knee or leg, or disabling condition of the hands or skin disorder with current involvement of the hands or the knee(s); referral to an orthopedic surgeon for consideration of, or been advised to have, knee replacement or knee reconstruction surgery, or had advanced OA of the knee such that all cartilage was eroded (i.e. radiographic examination showed that the joint space was eliminated in both lateral and medial tibia/femoral compartments); or regular headaches requiring acetaminophen.
Treatment
Patients were randomly assigned by an interactive Web-based system in a 1:1 ratio to receive diclofenac sodium 2% topical solution or control (vehicle only) BID for 4 weeks. A balanced randomized block assignment () was utilized to ensure that an equal number of patients were assigned to each treatment group.
Table 1. Demographics and baseline disease characteristics*.
The first application of the study drug (approximately 2 mL, i.e. two pumps of the bottle per knee every 12 ± 2 hours for 4 weeks) was supervised at the study center, with subsequent applications self-administered on an outpatient basis. Patients were trained via a detailed instruction booklet on how to administer the study drug. When the contralateral knee was painful at any point during the study (including at baseline), patients could initiate treatment with the same regimen and then continue until the end of the study. Study drug dose and frequency and the 4 week study duration were selected based on data from the diclofenac sodium 1.5% solution development program and previously published studiesCitation12.
Patients were allowed acetaminophen (paracetamol) as rescue medication on an as-needed basis (up to 1950 mg/day) during the treatment period, except for the 3 days prior to each clinic visit. Reasons for withdrawal were recorded at each visit. Study medication compliance was assessed based on patient-reported administration of study medication daily for each dose using an interactive voice response system (IVRS), and by calculating the difference between dispensed and returned study drug bottle weights.
Efficacy assessments
The primary efficacy outcome was the change from baseline to the final visit in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC LK 3.1) pain subscale. Secondary outcomes included WOMAC physical function and stiffness subscales; patient global assessment (PGA) of OA status; knee pain intensity measured at midday, evening, and over the previous 24 hours using an NRS; cumulative proportion of responders analysis: proportion of patients with no response to 100% reduction in average NRS pain intensity ratings during the last 24 hours at the final visit (using the combined last observation carried forward [LOCF]/baseline observation carried forward [BOCF] imputation method) versus baseline; and use of rescue analgesia.
Using an IVRS, patients were instructed to rate their knee pain intensity daily for both knees (if eligible) using the NRS as follows: 1) knee pain at midday, reported at midday; 2) knee pain in the evening, reported in the evening; and 3) average knee pain over the last 24 hours, reported in the evening. In addition, patients reported the number of tablets of rescue medication taken in each 24 hour period, whether they took rescue medication within 4 hours of an IVRS pain assessment and whether each dose of study drug was applied.
Safety and tolerability assessments
Treatment-emergent AEs (TEAEs) and vital signs were assessed immediately after the first application of the study drug and throughout the 4 week study. Additional laboratory tests (chemistry, hematology, and urinalysis) were performed at screening and the final visit.
Study ethics
This study was approved by a central institutional review board and was conducted in compliance with applicable International Conference on Harmonisation guidelines for Good Clinical Practice and local regulations. Written informed consent was received from all enrolled patients prior to treatment. This study is registered with ClinicalTrials.gov (identifier: NCT01119898).
Statistical analysis
Analyses were conducted on the modified intent-to-treat (mITT) population, defined as all randomized patients who received at least one dose of study medication. Analyses of baseline characteristics were performed to identify randomization imbalances and covariates that were to be included in efficacy analyses. Data from continuous variables were compared using a two-sample t test; baseline PGA comparisons were made using the Wilcoxon rank-sum test; and all other categorical characteristics were compared using a chi-square test or Fisher’s exact test.
An analysis of covariance (ANCOVA) model was used for efficacy analyses, which compared least-squares (LS) mean change from baseline and standard errors (SEs) adjusted for significant cofactors. The following factors were included: baseline WOMAC pain subscale score; sex; age (categorized as <65 and ≥65 years); body mass index (<30 and ≥30 kg/m2); and contralateral knee treated (yes/no). The WOMAC was only used to rate the pain intensity of the study knee, even if both knees were treated. A chi-square test compared the proportion of patients requiring rescue medication, and the number of days of rescue medication use was compared between groups using an analysis of variance (ANOVA) method.
All statistical analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC, USA). Since previous findings have indicated that the diclofenac sodium 2% and 1.5% topical solutions are bioequivalent at the recommended dosesCitation17, this 4 week study was undertaken for statistical confirmation of previous efficacy findings from the diclofenac 1.5% topical solution 4 weekCitation12 and 12 week studiesCitation24,Citation27. In previous studies, the mean changes from baseline in WOMAC pain scores for the diclofenac 1.5% topical solution and vehicle control were approximately −6.0 and −4.7, respectively, with standard deviations (SDs) of approximately 4.5. Based on these estimates, the proposed sample size of 260 patients (130 patients per treatment group) provided 80% power at a significance level of 0.10 to detect a difference between diclofenac sodium 2% topical solution and vehicle control. Therefore, statistical tests were two-sided with a significance level of α = 0.10. Methods of imputing missing efficacy values for patients who discontinued from the study early (used for week 2 and week 4 results analyses) included LOCF, BOCF, and combined LOCF/BOCF.
Results
Patient disposition and demographics
The study was performed at 23 sites in the United States. Of the 260 patients randomized, 259 received at least one dose of the study drug (mITT population) and 239 completed the study. Numerical completion rates were higher for patients who received diclofenac sodium 2% topical solution compared with vehicle control (93.8% and 90.7%, respectively). Reasons for early study withdrawal (eight patients withdrew in the diclofenac sodium group and 12 patients in the vehicle control group) included AEs, patient withdrawal of consent, lack of efficacy, protocol noncompliance, withdrawal of patient by sponsor (one patient did not qualify during the protocol-specified screening period), and other. There were no significant differences between treatment groups in demographics or baseline characteristics ().
Dosing compliance
Approximately 95% of patients self-reported >96% compliance by week 4; however, calculation of the difference between dispensed and returned study drug bottle weights showed that many patients administered lower than expected average doses in both treatment groups using the study drug dispensing system. The mean compliance rates based on weight were similar among the diclofenac and vehicle control groups (77% and 73%, respectively) at week 4.
Efficacy
Significantly greater reductions in LS mean (SE) WOMAC pain scores were observed for patients receiving diclofenac sodium 2% topical solution (−4.4 [0.4]) compared with vehicle control (−3.4 [0.4]) at the final visit (p = 0.040) (). Significant improvement with active treatment over control was also observed for WOMAC physical function scores (p = 0.061), WOMAC joint stiffness scores (p = 0.097), and PGA scores (p = 0.085) ().
Table 2. Efficacy outcomes at week 4 (LOCF/BOCF)*.
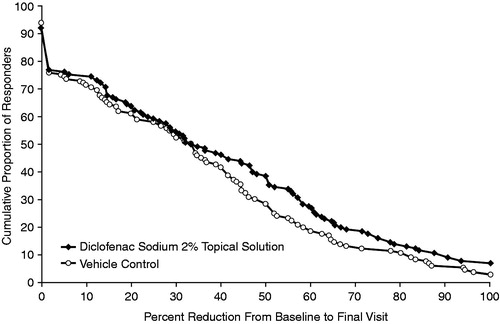
No significant differences between diclofenac sodium 2% topical solution and vehicle control were observed in mean NRS pain intensity scores at midday, evening, or over the previous 24 hours (respective LS means [SE]: midday: −2.9 [0.2] vs. −2.4 [0.2], p = 0.143; evening: −3.1 [0.2] vs. −2.7 [0.2], p = 0.179; over the last 24 hours: −2.9 [0.2] vs. −2.4 [0.2], p = 0.128) (). A higher proportion of patients receiving diclofenac sodium 2% topical solution responded by either ≥30% or ≥50% in NRS average pain intensity reduction over the last 24 hours (change from baseline to week 4) compared with vehicle control ().
Figure 1. Daily NRS pain intensity: average daily pain during the last 24 hours at final visit, reported in the evening (last observation carried forward/baseline observation carried forward). NRS, numeric rating scale.
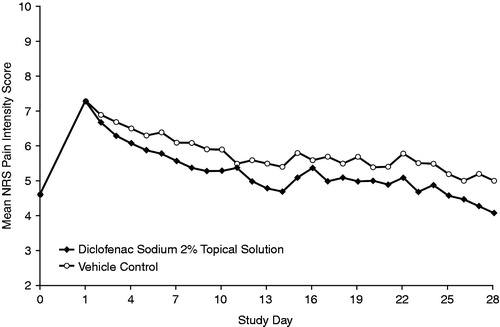
Figure 2. Cumulative proportion of responders from no response to 100% response using reduction in average numeric rating scale pain intensity ratings over the last 24 hours at final visit in the two treatment groups as measure of response (last observation carried forward/baseline observation carried forward).
Pain intensity in patients receiving diclofenac sodium 2% topical solution, as assessed by NRS and measured over the 12 hour dosing period from the midday dose to evening dose, demonstrated that responders who had a pain reduction ≥30% showed no statistically significant difference between the midday and evening mean levels of pain (p = 0.424). This finding was also similar for responders who had a pain reduction ≥50% (p = 0.383). Thus, these results indicate that pain management provided by the diclofenac sodium 2% topical solution regimen was maintained throughout the 12 hour dosing interval among responders.
During weeks 2–4, significantly fewer patients in the diclofenac active treatment group were using rescue medication compared with the vehicle control group (52.3% vs. 63.6%; p = 0.067 [chi-square test]). Overall, the mean number of days that rescue medication was used throughout the study was statistically significantly lower with diclofenac versus the vehicle control group (mean [SD]: 6.9 [7.4] vs. 8.8 [8.2] days; p = 0.054 [ANOVA LS means]). Results were similar irrespective of the imputation method used for missing data.
Efficacy covariate assessment
OA involvement in the contralateral knee was the only co-factor found to have a favorable impact on the efficacy analyses. The percentage of patients with contralateral knees treated was greater in the vehicle control group than in the diclofenac sodium 2% topical solution group (69.0% vs. 63.0%, respectively).
Those in whom the contralateral knee was treated with diclofenac sodium 2% topical solution showed greater pain subscale score reduction (LS mean change: −4.5) than those who did not treat the contralateral knee with diclofenac sodium 2% topical solution (LS mean change: −3.3; p = 0.025). As with pain scores, active treatment of the contralateral knee was associated with a statistically significantly greater improvement in physical function (LS mean change using LOCF/BOCF: −14.1 vs. −10.4 [p = 0.042] in those who treated their contralateral knee vs. those who did not). Active treatment of the contralateral knee also improved stiffness outcome in the study knee (LS mean change: −1.7 vs. −1.2 [p = 0.043] when comparing patients with treated vs. untreated contralateral knees, respectively).
Safety and tolerability
The most commonly reported TEAEs were administration site conditions, which included application site dryness, exfoliation, erythema, pruritus, and pain (). The vehicle control group experienced slightly more TEAEs than the active treatment group receiving diclofenac sodium 2% topical solution. Furthermore, the incidences of administration site reactions deemed by the investigator to be related or possibly related to the study drug were lower in the active treatment group than those reported in the vehicle control group (33% vs. 58%; p = 0.07) () – predominately due to erythema (p < 0.01) and pruritus (p < 0.001). No serious AEs were reported.
Table 3. Most common* TEAEs determined to be related or possibly related to study drug (n = 259).
Discussion
This phase II, 4 week, randomized, vehicle-controlled study evaluated the efficacy and safety of the NSAID diclofenac sodium 2% topical solution in the symptomatic treatment of OA of the knee. Efficacy findings demonstrated that for the primary endpoint (i.e. WOMAC pain subscale score from baseline to the final report of the pain subscale score), patients reported significantly greater reductions in WOMAC pain scores with diclofenac sodium 2% topical solution versus vehicle control (p = 0.040). Moreover, improvement in the secondary outcomes was also directionally and statistically significantly greater in the active treatment group versus the vehicle control group as assessed under the study protocol (α = 0.10): physical function (p = 0.061), PGA scores (p = 0.085), and stiffness (p = 0.097). An analysis of NRS scores with the active treatment in two responder groups (≥30% and ≥50% reduction in NRS score) indicated no significant differences in mean NRS pain intensity scores at midday, evening, or over the previous 24 hours, suggesting that the BID regimen maintained pain relief throughout the 12 hour dosing interval. In general, the active treatment group had a higher proportion of responders across the range of response levels shown compared with control.
Regarding the safety and tolerability findings, the most commonly reported TEAEs were administration site conditions. Incidence of TEAEs (including administration site reactions) was slightly lower with active treatment versus vehicle control, and no serious AEs were reported. It is currently unknown why the vehicle control resulted in more site conditions; however, a potential hypothesis is that diclofenac sodium may have prevented some of the erythema and inflammation associated with vehicle administration.
Diclofenac sodium gel 1% and 1.5% and 2% topical solution formulations are all approved in the United States for the treatment of knee OACitation11. The efficacy data from this study compared favorably with data from other studies of 1.5% and 1% diclofenac sodium and other topical formulations in treatment of pain for knee OACitation12,Citation24,Citation28–30. In a similarly designed study with diclofenac 1.5% solution conducted by Bookman et al.Citation12, the mean change in WOMAC pain score compared with vehicle control over 4 weeks was 1.4, which was comparable but slightly greater than the results found in this study. However, the baseline pain scores in our patient population were significantly higher than those reported in the Bookman et al. studyCitation12, and we controlled for contralateral knee treatment. In a 4 week, randomized, double-blind study comparing another topical NSAID (eltenac gel, structurally similar to diclofenac) with oral diclofenac and placebo gel for control of pain in patients with OA of the knee, eltenac gel was as effective as oral diclofenac and better tolerated over 4 weeks, albeit with a higher incidence of local skin reactions versus placeboCitation31. Greater efficacy versus placebo was also shown in another 4 week, double-blind, placebo-controlled study of eltenac gel 1% (3 g three times daily) compared with placebo gel in 237 patients with OA of the knee; however, statistically greater efficacy for eltenac gel 1% over placebo was only shown in a subgroup of patients with higher baseline OA severity, and the study did not achieve its primary endpointCitation32.
A 12 week, five-arm, randomized, double-blind study demonstrated that the efficacy of diclofenac 1.5% topical solution was greater than topical placebo and dimethyl sulfoxide (DMSO) vehicle for WOMAC pain reduction (−1.3) and as effective as oral diclofenac in 775 patients with knee OACitation27. Tolerability of the topical formulation was better than the oral formulation, and fewer digestive system and laboratory abnormalities were observed with the diclofenac 1.5% solution compared with oral diclofenacCitation27. Our data suggest that the diclofenac sodium 2% topical solution has similar efficacy compared with the diclofenac 1.5% solution, and potentially greater patient convenience than this and other topical diclofenac preparations (less frequent dosing and ease of application)Citation33.
The safety data reported here were comparable to data from studies of diclofenac sodium topical 1% and 1.5% gel/solutionsCitation12,Citation24,Citation25,Citation28–30,Citation34. Other studies have shown topical diclofenac sodium to have equivalent symptom relief to the oral formulation, with lower incidence of systemic side effectsCitation14,Citation35,Citation36. In a pooled safety analysis of data from two 12 week, randomized, double-blind, controlled studies in patients with radiographically confirmed symptomatic knee OA (n = 927) comparing diclofenac topical solution with oral diclofenac, the overall incidence of AEs was similar in the two treatment groups; however, the incidence of application site reactions (predominantly dry skin) was significantly higher with the topical formulation, whereas greater gastrointestinal and cardiovascular events, increases in liver enzymes and creatinine, and greater decreases in creatinine clearance and hemoglobin were reported with the oral formulationCitation35. Similar findings were seen when topical and oral ibuprofen formulations were compared in a large equivalence study (the TOIB study) in 282 older patients (aged ≥50 years) with chronic knee pain, using data from a randomized controlled trial and a patient preference studyCitation36. This study found that efficacy and tolerability were similar with the two treatments but with a slightly increased incidence of minor AEs with the oral formulationCitation36.
With topical diclofenac formulations, benefits have been observed related to the lower frequency BID dosing regimen versus the QID regimen, particularly with regard to patient complianceCitation33. In a subjective survey-based study comparing perceptions and preferences of diclofenac sodium 2% topical solution BID with diclofenac sodium gel (Voltaren Gel) QID in 24 healthy volunteers, several characteristics and subjective responses were rated significantly better for diclofenac sodium 2% topical solution, and more patients preferred or highly preferred diclofenac sodium 2% topical solution versus diclofenac sodium gelCitation33.
Although there are no studies demonstrating patient preferences for a less frequent topical treatment in knee OA specifically, studies have shown links between lower regimen complexity or treatment burden and treatment satisfaction/improved adherenceCitation37.
We reported variable and suboptimal study drug container weight-based compliance (77%) with both active and control treatments based on typical expectation (89%)Citation27. This may have indicated insufficient patient application technique despite care taken in providing technique education.
A key limitation of this trial, as well as other knee OA trials, is that of a significant placebo response; however, the level of placebo response seen in this study was larger than placebo responses cited in a meta-analysis of OA trials, including 122 patients with knee OACitation38. This may have been due to the flare design of our study or a publication bias of reporting smaller placebo-effect studies. Despite the high placebo effect reported in this study, there was still a significant reduction in the primary outcome (p < 0.05) and other secondary outcomes. We also reported a lower level of rescue medication use in the active treatment group compared with the control group. Another limitation is that the duration of this trial was 4 weeks compared with the standard of 12 weeks for a phase III study. The intent of this phase II study was to demonstrate benefits consistent with those previously observed with a different dosing interval of the same formulation, and this was achieved.
Conclusions
In this 4 week study, diclofenac sodium 2% topical solution with a BID dosing regimen demonstrated significantly greater improvement in pain reduction compared with control. Physical function, stiffness, and overall status (of knee OA) also demonstrated statistically significant improvements. Diclofenac sodium 2% topical solution was generally well tolerated, with a safety profile similar to that previously reported for diclofenac sodium 1.5% topical solution. This study provides support for the effectiveness and tolerability of diclofenac sodium 2% topical solution when applied BID for treating the pain of knee OA.
Clinical implications
Topically administered NSAIDs appear to have several advantages in the treatment of OA of the knee. Compared with oral NSAIDS, topical NSAIDs reduce the amount of systemic exposure. Consequently, topically applied NSAIDs reduce the incidence of AEs typically associated with increased systemic exposure to these agents when administered orally, e.g., gastrointestinal AEs. Moreover, topical administration of NSAIDs is also beneficial for patients who have comorbidities, difficulty swallowing oral medications such as the elderly, or who are otherwise intolerant of oral NSAIDs, and it minimizes toxicity in patients with knee OA who are taking anticoagulants, and those with renal and/or hepatic dysfunction, heart failure, severe respiratory disease, or other pathological issues. In the present study, administration of diclofenac sodium 2% topical solution BID resulted in a significantly greater improvement in pain reduction in patients with OA of the knee versus vehicle control while generally being well tolerated in this patient population.
Transparency
Declaration of funding
This manuscript was supported by Horizon Pharma USA Inc. This study was supported by Mallinckrodt Pharmaceuticals.
Author contributions: L.T.W. was an investigator in the trial, contributed significantly to the data analysis plan, interpretation of results, and writing of the manuscript. J.D.K. contributed significantly to the data analysis plan and interpretation of results. R.J.H. contributed significantly to the data analysis plan, interpretation of results, and writing of the manuscript.
Declaration of financial/other relationships
L.T.W. has disclosed that he has no significant relationships with or financial interests in any commercial companies related to this study or article. J.D.K. has disclosed that he is a current employee of Horizon Pharma USA Inc. and holds stock in Horizon Pharmaceuticals. R.J.H. has disclosed that he has received consulting fees from Cadence Pharmaceuticals Inc., Horizon Pharma USA Inc., Hospira, Novartis, and Pozen Pharmaceuticals Inc., and is a previous employee of Searle/Pfizer. He has no financial interests in any of these companies.
CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Mary Hines and Alan J. Klopp PhD of inScience Communications, Springer Healthcare provided medical writing support funded by Horizon Pharma USA Inc.
Previous presentation: Data from this study were published as an abstract and poster at the 33rd Annual Scientific Meeting of the American Pain Society (APS), 30 April–3 May 2014, Tampa, FL, USA (Poster #484).
Notes
*Pennsaid is a registered trademark of Horizon Pharma USA Inc., Deerfield, IL, USA
*Voltaren Gel is a registered trademark of Novartis Consumer Health Inc., Parsippany, NJ, USA
References
- Benito MJ, Veale DJ, FitzGerald O, et al. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis 2005;64:1263-7
- Guilak F, Fermor B, Keefe FJ, et al. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res 2004;423:17-26
- Reid MC, Shengelia R, Parker SJ. Pharmacologic management of osteoarthritis-related pain in older adults: a review shows that many drug therapies provide small-to-modest pain relief. HSS J 2012;8:159-64
- Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1323-30
- Lapane KL, Yang S, Driban JB, et al. Effects of prescription nonsteroidal antiinflammatory drugs on symptoms and disease progression among patients with knee osteoarthritis. Arthritis Rheum 2015;67:724-32
- Losina E, Weinstein AM, Reichmann WM, et al. Lifetime risk and age at diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care Res 2013;65:703-11
- Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum 2008;59:1207-13
- Vane JR, Botting RM. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am J Med 1998;104:2-8S; discussion 21-2S
- Balmaceda CM. Evolving guidelines in the use of topical nonsteroidal anti-inflammatory drugs in the treatment of osteoarthritis. BMC Musculoskelet Disord 2014;15:27
- Wagenitz A, Mueller EA, Frentzel A, Cambon N. Comparative efficacy and tolerability of two sustained-release formulations of diclofenac: results of a double-blind, randomised study in patients with osteoarthritis and a reappraisal of diclofenac’s use in this patient population. Curr Med Res Opin 2007;23:1957-66
- Barthel HR, Axford-Gatley RA. Topical nonsteroidal anti-inflammatory drugs for osteoarthritis. Postgrad Med 2010;122:98-106
- Bookman AA, Williams KS, Shainhouse JZ. Effect of a topical diclofenac solution for relieving symptoms of primary osteoarthritis of the knee: a randomized controlled trial. CMAJ 2004;171:333-8
- Baraf HS, Gloth FM, Barthel HR, et al. Safety and efficacy of topical diclofenac sodium gel for knee osteoarthritis in elderly and younger patients: pooled data from three randomized, double-blind, parallel-group, placebo-controlled, multicentre trials. Drugs Aging 2011;28:27-40
- Ozguney I. An alternative topical treatment of osteoarthritis of the knee with cutaneous diclofenac solution. Expert Opin Pharmacother 2008;9:1805-16
- Evans JM, McMahon AD, McGilchrist MM, et al. Topical non-steroidal anti-inflammatory drugs and admission to hospital for upper gastrointestinal bleeding and perforation: a record linkage case-control study. BMJ 1995;311:22-6
- Fuller P, Roth S. Diclofenac sodium topical solution with dimethyl sulfoxide, a viable alternative to oral nonsteroidal anti-inflammatories in osteoarthritis: review of current evidence. J Multidiscip Healthc 2011;4:223-31
- Holt RJ, Taiwo T, Kent JD. Bioequivalence of diclofenac sodium 2% and 1.5% topical solutions relative to oral diclofenac sodium in healthy volunteers. Postgrad Med 2015;127:581–90
- Argoff CE, Gloth FM. Topical nonsteroidal anti-inflammatory drugs for management of osteoarthritis in long-term care patients. Ther Clin Risk Manag 2011;7:393-9
- Altman RD, Barthel HR. Topical therapies for osteoarthritis. Drugs 2011;71:1259-79
- Baraf HS, Gold MS, Petruschke RA, Wieman MS. Tolerability of topical diclofenac sodium 1% gel for osteoarthritis in seniors and patients with comorbidities. Am J Geriatr Pharmacother 2012;10:47-60
- Banning M. Topical diclofenac: clinical effectiveness and current uses in osteoarthritis of the knee and soft tissue injuries. Expert Opin Pharmacother 2008;9:2921-9
- McCarberg BH, Argoff CE. Topical diclofenac epolamine patch 1.3% for treatment of acute pain caused by soft tissue injury. Int J Clin Pract 2010;64:1546-53
- Rainsford KD, Kean WF, Ehrlich GE. Review of the pharmaceutical properties and clinical effects of the topical NSAID formulation, diclofenac epolamine. Curr Med Res Opin 2008;24:2967-92
- Roth SH, Shainhouse JZ. Efficacy and safety of a topical diclofenac solution (Pennsaid) in the treatment of primary osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled clinical trial. Arch Intern Med 2004;164:2017-23
- Roth SH, Fuller P. Diclofenac sodium topical solution 1.5% w/w with dimethyl sulfoxide compared with placebo for the treatment of osteoarthritis: pooled safety results. Postgrad Med 2011;123:180-8
- Roth SH, Fuller P. Pooled safety analysis of diclofenac sodium topical solution 1.5% (w/w) in the treatment of osteoarthritis in patients aged 75 years or older. Clin Interv Aging 2012;7:127-37
- Simon LS, Grierson LM, Naseer Z, et al. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis. Pain 2009;143:238-45
- Barthel HR, Haselwood D, Longley S III, et al. Randomized controlled trial of diclofenac sodium gel in knee osteoarthritis. Semin Arthritis Rheum 2009;39:203-12
- Baraf HS, Gold MS, Clark MB, Altman RD. Safety and efficacy of topical diclofenac sodium 1% gel in knee osteoarthritis: a randomized controlled trial. Phys Sportsmed 2010;38:19-28
- Baer PA, Thomas LM, Shainhouse Z. Treatment of osteoarthritis of the knee with a topical diclofenac solution: a randomised controlled, 6-week trial [ISRCTN53366886]. BMC Musculoskelet Disord 2005;6:44
- Sandelin J, Harilainen A, Crone H, et al. Local NSAID gel (eltenac) in the treatment of osteoarthritis of the knee. A double blind study comparing eltenac with oral diclofenac and placebo gel. Scand J Rheumatol 1997;26:287-92
- Ottillinger B, Gomor B, Michel BA, et al. Efficacy and safety of eltenac gel in the treatment of knee osteoarthritis. Osteoarthritis Cartilage 2001;9:273-80
- Galer BS. A comparative subjective assessment study of Pennsaid® and Voltaren Gel®, two topical formulations of diclofenac sodium. Pain Pract 2011;11:252-60
- Peniston JH, Gold MS, Alwine LK. An open-label, long-term safety and tolerability trial of diclofenac sodium 1% gel in patients with knee osteoarthritis. Phys Sportsmed 2011;39:31-8
- Roth SH, Fuller P. Diclofenac topical solution compared with oral diclofenac: a pooled safety analysis. J Pain Res 2011;4:159-67
- Underwood M, Ashby D, Carnes D, et al. Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study. Health Technol Assess 2008;12:iii-iv, ix-155
- Barbosa CD, Balp M, Kulich K, et al. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Preference Adherence 2012;6:39-48
- Zhang W, Robertson J, Jones AC, et al. The placebo effect and its determinants in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis 2008;67:1716-23
