Abstract
The European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) treatment algorithm for knee osteoarthritis (OA) recommends symptomatic slow-acting drugs for osteoarthritis (SYSADOAs) first line for the medium to long term management of OA, due to their ability to control pain, improve function, and delay joint structural changes. Among SYSADOAs, glucosamine is probably the most widely used intervention. In the present review of glucosamine for knee OA, we have investigated whether the evidence is greater for the patented crystalline glucosamine sulfate (pCGS) preparation (Rottapharm/Meda) than for other glucosamine formulations. Glucosamine is actually widely available in many forms, as the prescription-grade pCGS preparation, generic and over-the-counter formulations of glucosamine sulfate (GS) and food supplements containing glucosamine hydrochloride (GH), which vary substantially in molecular form, pharmaceutical formulation and dose regimens. Only pCGS is given as a highly bioavailable once daily dose (1500 mg) with a proven pharmacological effect. pCGS consistently reaches the plasma levels of around 10 μM required to inhibit interleukin-1 induced expression of genes involved in the pathophysiology of joint inflammation and tissue destruction, compared with sub-therapeutic levels achieved with GH. It is evident, from careful consideration of the evidence base, that only the pCGS formulation of glucosamine reliably provides an effect size on pain that is higher than that of paracetamol and equivalent to that provided by non-steroidal anti-inflammatory drugs. In comparison, the effect size on pain of non-crystalline GS preparations and GH from randomized controlled trials is repeatedly demonstrated to be zero. In addition, there is evidence that chronic administration of pCGS has disease-modifying effects, with a reduction in the need for total joint replacement surgery lasting for at least 5 years after treatment cessation. Consequently, the pCGS preparation (Rottapharm/Meda) is the logical choice, with demonstrated medium-term control of pain and lasting impact on disease progression.
Introduction
Traditionally, the pharmacological management of osteoarthritis (OA) has focused on therapies that may improve or control symptoms, or at least provide rescue analgesia. More recently, the use of symptomatic slow-acting drugs for osteoarthritis (SYSADOAs), in particular prescription-grade glucosamine sulfate (GS) and chondroitin sulfate (CS), has been proposed as a first-line pharmacological treatment for slow-onset medium to long term control of symptoms in OACitation1. SYSADOAs have demonstrated symptomatic effects as well as potential disease-modifying effects, based upon reports of downregulation in the expression of several inflammatory and degenerative mediators resulting in an effect on pain and symptoms and also a slower degradation of the cartilage, hence preventing disease progressionCitation2. The clinical impact of this molecular mechanism has been observed as a reduction of pain and increased function, and radiological measurement of reduced joint space narrowing (JSN)Citation3,Citation4.
While multiple international evidence-based guidelines for OA management exist, agreement on the different treatment modalities is lackingCitation5–9. The main source of disagreement regarding the use of SYSADOAs derives from the fact that the regulatory status and, subsequently, the availability and labeling of these medications substantially differ in separate countries and regions of the worldCitation10. Glucosamine, in particular, is available as the high quality prescription-grade patented crystalline glucosamine sulfate (pCGS) formulation (Rottapharm/Meda)Citation11, generic and over-the-counter (OTC) formulations of GS and food supplements mostly containing the glucosamine hydrochloride (GH) salt. Glucosamine generics, OTC products and food/nutritional supplements vary substantially from pCGS in their molecular forms, pharmaceutical formulation and dose regimens. Only pCGS is given as a highly bioavailable once daily dose (1500 mg) with a proven pharmacological effectCitation12.
The European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO)Citation13,Citation14 has recently developed a treatment algorithm recommendation which helps the prescribing physician to prioritize interventions in the management of knee OA, based upon the available evidence applicable across Europe and internationallyCitation1. The ESCEO Task Force acknowledged the variance in efficacy demonstrated with various glucosamine formulations in clinical studies, and recommends: “Among SYSADOAs, prescription [crystalline] glucosamine sulfate should be differentiated from other glucosamine preparations”Citation1. In this review article on the use of glucosamine in the treatment of OA, we will explore the reasoning behind this distinction between glucosamine formulations by examination of the evidence base.
Mechanism of action
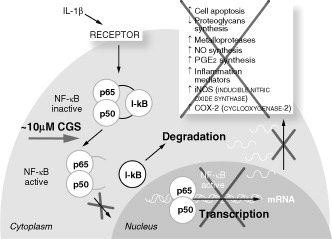
Glucosamine is a naturally occurring building block for complex long-chain glycosaminoglycans that are linked to a core protein in proteoglycan molecules (aggrecans), and form part of the cartilage matrix. When administered exogenously, glucosamine exerts specific pharmacological effects on osteoarthritic cartilage and chondrocytesCitation15,Citation16. Glucosamine affects gene expression of OA cartilage, and the anti-catabolic activities of glucosamine are responsible for its therapeutic effectsCitation17. Glucosamine is demonstrated in vitro to reduce prostaglandin E2 (PGE2) production and inhibit activation of the nuclear factor kappa B (NF-κB) pathway, thus inhibiting the cytokine intracellular signaling cascade in chondrocytes and synovial cells ()Citation2,Citation16–19. In OA, glucosamine induces reversal of the pro-inflammatory and joint-degenerating effects of interleukin-1 (IL-1)Citation16. Interleukin-1 beta (IL-1β) is a potent pro-inflammatory cytokine produced in high amounts in the OA joint, where it triggers the expression of inflammatory factors such as cyclooxygenase-2 (COX-2), inducible form of nitric oxide (iNOS), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNFα). IL-1β also induces cells to produce more IL-1β as well as matrix degradation factors, such as metalloproteinases (MMPs) and a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member TSs (ADAM-TSs). Most of these genes are under the transcriptional control of NF-κB. Glucosamine at clinically relevant concentrations reduces COX-2, iNOS, and microsomal prostaglandin E synthase-1 (mPGEs1) gene expression and PGE2 synthesis after IL-1β stimulation, suggesting that glucosamine can control the cascade triggered by inflammatory stimuliCitation20.
Figure 1. The mechanism of action of patented crystalline glucosamine sulfate: inhibition of interleukin-1-stimulated signaling pathway and gene expression. Adapted from Chiusaroli et al. 2011Citation2, Largo et al. 2003Citation18, and Gouze et al. 2002Citation19.
CGS, crystalline glucosamine sulfate; IL-1β, interleukin-1 beta; I-κB, a cellular protein that inhibits NF-κB; NF-κB, nuclear factor kappa B.
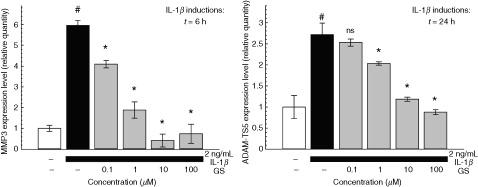
While these effects can be demonstrated in vitro with most glucosamine salts or preparations, pCGS is the only glucosamine formulation for which such effects can be confirmed at the concentrations that are actually achieved in biological fluids after administration at therapeutic doses in humans. Indeed, while it is unknown whether these in vitro effects are relevant to the therapeutic activities observed in clinical trials, pCGS inhibits IL-1-stimulated gene expression of joint degeneration mediators in human chondrocyte cells at concentrations in the range of 10 μM, similar to those found in the plasma or synovial fluid of knee OA patients after receiving pCGS at the therapeutic dose of 1500 mg once dailyCitation2. A dose-dependent effect of pCGS on IL-1β-induced gene expression of matrix degradation factors MMP-3 (stromelysin-1) and ADAM-TS5 (aggrecanase 2) was observed ()Citation2. Long-term oral administration of GS reduces the destruction of cartilage and upregulation of MMP-3 mRNA in in vitro modelsCitation21. Furthermore, studies in a human osteoarthritic explant model demonstrate that GS is a stronger inhibitor of gene expression than GH, when both are administered at 5 mM dosesCitation22.
Figure 2. The dose-dependent effect of patented crystalline glucosamine sulfate on the IL-1β-induced gene expression of joint degeneration mediators MMP-3 (stromelysin-1) and ADAM-TS5 (aggrecanase 2) in human chondrocytes is optimized at clinically relevant concentrations (∼10 μM). Reproduced from Chiusaroli et al. 2011Citation2, with permission granted under the Creative Commons Attribution License. #p<0.001 vs. untreated cells; *p<0.001 vs. IL-1β alone; ns, not significantly different from IL-1β alone.
Differences between GS and GH formulations may be important at both the pharmacokinetic and pharmacologic levels and may help to explain the divergent findings found in clinical trials with different glucosamine salts and formulations. Sulfate concentrations increase after administration of GS, which may overcome a deficiency in inorganic sulfur, caused by a low level of dietary proteins, essential for the synthesis of proteoglycans that are important for chondrocyte metabolismCitation23,Citation24. GS is effective in animal models of surgically induced OACitation25, and is demonstrated to improve OA histological changes with a 60% reduction in the synovitis score compared with controlsCitation26. However, surgically induced experimental OA may not reflect all aspects of spontaneous idiopathic OA in humans. When given chronically to STR/ort mice who develop spontaneous OA with age, in which the whole joint undergoes degenerative changes entirely similar to those described in human OACitation27, pCGS ameliorated the histological damage, the extent of lesion, and histomorphometry in this animal modelCitation2.
Pharmacokinetics
Pharmacokinetic studies demonstrate that a once daily dose of pCGS at 1500 mg leads to mean plasma concentration at steady state of 9 μM of glucosamine in healthy volunteersCitation28, i.e., in the 10 μM range shown to be effective in counteracting IL-1-induced gene expression, while administration of GH (500 mg tid) leads to steady state levels of only 1.2 μMCitation29. Peak plasma levels of glucosamine after single dose GH (1500 mg) are about one third of those measured after pCGS at the same dose (1500 mg), while GH dosing with the regimen used routinely in practice of 500 mg three times daily further lowers the peak levels by 50%, even at steady state ()Citation29. In a cross-over study, change from pCGS to GH resulted in a 50% decrease in peak plasma concentration and 75% reduction in total bioavailabilityCitation12, which might be explained by the differences in dosing regimen and pharmaceutical formulation. Notably, the poor bioavailability obtained with GH may go some way to explain the poor results obtained with this formulation in the NIH-supported GAIT study (Glucosamine/chondroitin Arthritis Intervention Trial), which failed to demonstrate any efficacy for GH versus placeboCitation30. Importantly, in OA patients, peak glucosamine concentrations at 7.17 μM (range 3.35 to 22.7) in the plasma and 4.34 μM (range 3.22 to 18.1) in the synovial fluid have been measured after once daily administration of pCGS (1500 mg)Citation28,Citation31.
Table 1. Pharmacokinetic parameters for pCGS (1500 mg qd) and glucosamine hydrochloride (1500 mg qd or 500 mg tid). Adapted from Persiani et al. 2005Citation28 and Jackson et al. 2010Citation29.
A lack of appropriate stabilization of GS is shown to impact on the active ingredient availability; moreover, the quality of non-pCGS glucosamine formulations may be sub-optimalCitation32. An investigation of 14 dietary supplements and OTC preparations of glucosamine found that only one contained the claimed amount of the active ingredient, while the others contained variable quantities ranging from 59 to 138% of the labeled doseCitation32. Thus, only the pCGS formulation remains stable and reliably delivers sufficient plasma concentrations of glucosamine in the range that has been shown to be pharmacologically effective in reducing the expression of IL-1-induced cartilage degradation enzymes in human chondrocyte culturesCitation2.
Efficacy
The current treatment of OA is based upon primary pain and loss of function control. Numerous studies of varying quality have been conducted to determine the effect of glucosamine on pain. A Cochrane review of 25 randomized controlled trials of all glucosamine formulations in 4963 OA patients, when limited to studies with adequate concealment, failed to show any benefit of glucosamine for painCitation33. However, when the trials using pCGS were analyzed in isolation, it was found to be superior to placebo for pain (standardized mean difference [SMD] −1.11; 95% confidence interval [CI] −1.66 to −0.57) and function (Lequesne index SMD −0.47; 95% CI −0.82 to −0.12). Conversely, analysis of those trials using a non-pCGS preparation of glucosamine failed to reach statistical significance for pain or functionCitation33.
Possible explanations for the difference in efficacy found between different glucosamine formulations have focused on the poor quality of some trials included in the meta-analyses and the potential risk of bias which may distort the results. The Cochrane review found superiority for the pCGS formulation on pain in OA, but with high heterogeneity between trials (I2 = 92%)Citation33. One solution is to focus only on the high quality trials of glucosamine. A subgroup analysis in the Cochrane review of three pivotal trials found pCGS to be significantly superior to placebo in terms of Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale score (SMD −0.17; 95% CI −0.32 to −0.01; p = 0.037), with zero heterogeneity between trialsCitation33.
Eriksen and colleagues performed a stratified meta-analysis to address the potential risk of bias due to unsatisfactory handling of the data, i.e. during randomization and concealment and statistical analysesCitation34. They found that only eight studies met the standard for ‘low risk of bias’. This analysis confirmed that the five studies with non-pCGS even with a ‘low risk of bias’ found a non-significant effect on pain reduction (0.02; 95% CI −0.08 to 0.12). In contrast, analysis of the three ‘low risk of bias’ studies with pCGS confirmed a reduction in pain with effect size of 0.27 (95% CI −0.43 to −0.12)Citation3,Citation4,Citation34,Citation35. This recent finding is in total agreement with an earlier analysis of the same three trials of pCGS judged to be of highest quality using the Jadad quality score for clinical trialsCitation36,Citation37. In the absence of industry bias, several other factors may explain the difference in efficacy observed between quality clinical trials of glucosamine preparations. The superiority of pCGS may be explained by the unique stabilized formulation of glucosamine, single once daily dosing regimen (1500 mg) and high bioavailability, reaching higher glucosamine concentration in the plasma, compared with other preparationsCitation12.
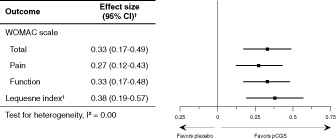
The impact of pCGS formulation on other symptom outcomes is demonstrated in further analysis of results from the pivotal three trials, with a significant effect size on WOMAC total score, WOMAC pain and function subscale scores, and Lequesne index, with a complete absence of heterogeneity ()Citation36.
Figure 3. Symptom outcomes for patented crystalline glucosamine sulfate formulation in knee osteoarthritis: pooled effect size from three pivotal trials. Adapted from Reginster 2007Citation36.
†Estimates and 95% confidence intervals (CIs) from fixed-model meta-analysis method using the pooled standard deviation in each study/outcomeCitation3,Citation4,Citation35: the data in the table have been depicted as a forest plot in the right-hand panel. ‡Not assessed in one studyCitation3.
The effect size for pCGS on pain may be considered as only moderate at 0.27. However, it is notable that pCGS has a greater effect on pain than that of paracetamol (with effect size of 0.14; 95% CI 0.05 to 0.22)Citation38, which may still be used as first-line rescue analgesia for OACitation1. In addition, the effect size of pCGS on pain over treatment periods ranging between 6 months and 3 years is equivalent to that achieved with oral non-selective or COX-2 selective non-steroidal anti-inflammatory drugs (NSAIDs), at 0.29 (95% CI 0.22 to 0.35) for much shorter treatment coursesCitation39, which are recommended as step 2 treatments in persistently symptomatic OA patientsCitation1. Thus, although it would be desirable to have interventions available in OA that provide more than a moderate effect, it is still a major finding that some interventions can indeed provide such a reliable and consistent efficacy pattern.
Safety
For all treatments, the balance of risk versus benefits must be considered prior to administration. Oral NSAIDs are recommended for intermittent or cyclical use due to concerns over gastrointestinal (GI) and cardiovascular adverse eventsCitation1. There is also accumulating evidence for an increased risk of GI adverse events with paracetamol use, with elevation in liver enzymesCitation38. Conversely, pCGS may be taken safely in the long term with an adverse event rate comparable with that of placeboCitation3,Citation4,Citation33,Citation35.
Disease-modifying effects
Evidence that long-term administration of pCGS over 3 years delays joint structure changes is provided by two trials, suggesting a potential benefit of pCGS beyond symptom control when used early in the treatment algorithm. Analysis of joint space width (JSW) at trial enrollment and after 3 years of treatment in the two trials of pCGS versus placebo demonstrates a reduction in JSN with pCGS. In one study, a significant difference in JSN of 0.33 mm (95% CI 0.12 to 0.54) was observed with pCGS versus placebo after 3 years (p = 0.003)Citation3. In the second study, pCGS treatment for 3 years was shown to completely prevent narrowing of the joint (JSN +0.04 mm; 95% CI −0.06 to 0.14; p = 0.001) ()Citation40. It should be noted that these trials used a radiological technique that is no longer considered state of the art today (although it was at the time the studies were performed). Nonetheless, post-hoc analysis of these findings demonstrate (i.e. demonstrates) that there was no bias determined by patient positioning or radiological view, thus confirming the validity of these resultsCitation41.
Table 2. Prevention of joint space narrowing in knee osteoarthritis with pCGS formulation over 3 years’ treatment. Reproduced from Bruyere et al. 2016Citation40, with permission granted under the Creative Commons Attribution License.
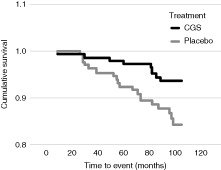
A lack of progression of JSN (determined at a threshold of 0.5 mm [>0.3–0.7 mm]) has demonstrated predictive value of >90% for not having joint replacement surgeryCitation42. In both studies, fewer patients treated with pCGS experienced predefined severe JSN (>0.5 mm) compared with patients treated with placebo: 15% vs. 30% (p = 0.013)Citation3 and 5% vs. 14% (p = 0.05)Citation4. Long-term follow-up of knee OA patients who had participated in the two 3 year trials of pCGS and received treatment for at least 12 months revealed in a post-hoc analysis that total joint replacement (TJR) had occurred in over twice as many patients from the placebo group (14.5%) in the 5 years of follow up compared with those patients formerly receiving pCGS (6.3%; p = 0.024), demonstrating a 57% reduction in risk of TJR with pCGS (relative risk 0.43; 95% CI 0.20 to 0.92)Citation43. Treatment with pCGS significantly delayed the need for TJR surgery (p = 0.026) ()Citation43.
Figure 4. Effect of prior patented crystalline glucosamine sulfate formulation on cumulative incidence of total joint replacement surgery for up to 5 years following treatment. Reproduced from Bruyère et al. 2008Citation43, with permission.
CGS, crystalline glucosamine sulfate.
Pharmacoeconomics
Evaluation of the cost-effectiveness of treatments and impact on healthcare budgets is increasingly important. Economic evaluation allows comparison of different strategies in terms of cost (intervention costs and disease costs) and consequences, e.g. quality-adjusted life years (QALYs). Six months’ treatment with pCGS is shown to be a highly cost-effective therapy compared with paracetamol and placebo in the treatment of knee OA, in terms of incremental cost-effectiveness ratio (ICER)Citation35,Citation44. Further, a systematic review and economic evaluation has determined the incremental cost per QALY gain for adding GS to current care over a lifetime horizon to be around £21,335Citation45. Sensitivity analysis determined that the cost-effectiveness of GS therapy was particularly dependent on the magnitude of the quality of life gain, the change in knee TJR probability, and the discount rate.
Continuous treatment with pCGS results in a reduction in intake of other concomitant medication for OA and in a reduction in healthcare consultations and examinations, as demonstrated in a long-term follow up of OA patientsCitation43. A subset of patients who had previously taken part in a randomized trial attended a follow-up clinic visit at which the total average cost of OA-related resources per year was calculated to have approximately halved among those that had received pCGS versus placebo (€292 versus €605; p = 0.024) (). The total cost of OA medications taken among the placebo group (including analgesics and NSAIDs) was almost double that of the pCGS group (€204 with placebo vs. €108 with pCGS); while the number of specialist, general practitioner (GP) and paramedic visits, and examinations (radiographs, gastroscopies and non-OA exams) were consistently higher among the placebo group compared with pCGS patientsCitation43.
Table 3. Use of health resources per patient per year among osteoarthritis patients who had received pCGS formulation 5 years previously versus placebo. Adapted from Bruyère et al. 2008Citation43.
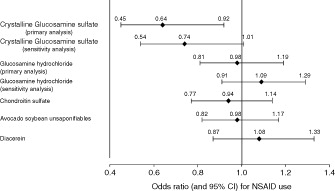
Further evidence for a reduction in the need for rescue pain analgesia achieved with continuous pCGS is provided by a recent study, which is representative of all OA patients in everyday life. The Pharmaco-Epidemiology of GonArthroSis (PEGASus) study was conducted by the French Health Authorities in collaboration with a panel of French rheumatologists and epidemiologists; the primary objective of the study was to assess the impact of SYSADOAs on the use of NSAIDsCitation46. Adults with knee and/or hip OA consulting a rheumatologist or GP for symptom flare were recruited into the PEGASus study and assigned to a SYSADOA treatment according to the physician’s or patient’s choice. During up to 24 months’ follow-up, SYSADOA switching, continuation or discontinuation was permitted. Among all SYSADOA treatments, including GH, CS, avocado soybean unsaponifiables, and diacerein, in the primary analysis only pCGS achieved a significant reduction in NSAID use of 36% (odds ratio [OR] 0.64; 95% CI 0.45 to 0.92) (). The reduction in NSAID use was even greater, approaching a 50% reduction, when patients who received >4 months of treatment with pCGS were considered alone (OR 0.52; 95% CI 0.28 to 0.95)Citation46. It may be expected that similar findings in support of the cost-effectiveness of pCGS will be found in other countries, despite the different financing of healthcare systems.
Figure 5. Odds ratio (with 95% confidence interval) for NSAID use with symptomatic slow-acting osteoarthritis drugs in the Pharmaco-Epidemiology of GonArthroSis (PEGASus) study. Reproduced from Rovati et al. 2016Citation46, with permission granted under the Creative Commons Attribution License.
CI, confidence interval; NSAID, non-steroidal anti-inflammatory drug (NSAID).
Discussion
The ESCEO treatment algorithm for knee OA recommends chronic SYSADOA treatment, specifically the patented crystalline glucosamine sulfate (pCGS) formulation and prescription CS, as first line therapy for slow-onset medium to long term control of symptomsCitation1. The ESCEO guidelines recognize that glucosamine is available in many forms, and yet not all formulations of glucosamine provide equivalent effects. Thus, the ESCEO Task Force recommends that the pCGS formulation should be differentiated from other glucosamine preparations due to a clear divergence in the evidence baseCitation1.
In this paper, we have set out the evidence for differentiation of pCGS from other glucosamine formulations. Publication of this review serves to educate and inform physicians as to this difference; however, we are aware that patient education is an essential element of successful disease management. The ESCEO algorithm, along with other guidelines, recommends a core set of initial measures that each knee OA patient should undergo, including information access and education, weight loss if overweight and an appropriate exercise programCitation1. The patient should be informed that while OA cannot as yet be cured, an improvement in symptoms and a control of disease progression may be obtained with the correct use of appropriate medications. Educating the patient on the difference between pCGS and the many other glucosamine formulations widely available will help to ensure treatment adherence to the correct preparation and maximize treatment outcomes.
Mechanistic studies support the role of pCGS as both a symptom- and structure-modifying agent in OA, via glucosamine-induced reversal of the pro-inflammatory and joint-degenerating effects of IL-1. Specifically, pCGS inhibits IL-1-induced expression of genes involved in the pathophysiology of joint inflammation and tissue destruction at a plasma concentration of 10 μM. Only the pCGS formulation given as a highly bioavailable once daily dose (1500 mg) reliably reaches the plasma level of around 10 μM required to deliver a therapeutic effect.
Randomized controlled trials and meta-analyses consistently show a moderate effect size on pain for pCGS of 0.27, which is higher than that of paracetamol (0.14), considered as the gold standard first-line rescue analgesic medication, and comparable with the effect size obtained for oral NSAIDs of 0.29. In comparison, the effect size on pain obtained with non-crystalline GS preparations and GH from randomized trials and meta-analyses is repeatedly demonstrated to be zeroCitation33.
Conclusion
The goals of treatment for OA are to reduce symptoms and ultimately slow disease progression, which may in turn reduce the impact of OA on the patient’s mobility and quality of life, and lead to a reduction in the need for rescue analgesia and joint replacement surgery in the long-term, with consequent reduction in healthcare resource needsCitation47. As well as a moderate effect on pain, there is evidence that chronic administration of pCGS can have disease-modifying effects, delaying joint structural changes and leading to a reduction in need for knee replacement surgery. Furthermore, real-life pharmacoeconomic studies demonstrate a long-term reduction in the need for pain analgesia and NSAIDs with pCGS therapy over 12 months, with significant reduction in costs associated with medication, healthcare consultations and examinations. Consequently, exposing patients to a non-pCGS glucosamine preparation (sulfate or hydrochloride salt) which is not expected to provide any clinical benefit may be considered as unethical and, from a socioeconomic point of view, a waste of economic resources both in terms of direct drug costs and increased utilization of healthcare systems. Thus, the pCGS formulation (1500 mg once daily) is the logical choice to maximize clinical benefit in OA patients with demonstrated medium-term control of pain and lasting impact on disease progression.
Transparency
Declaration of funding
This manuscript was written as a result of discussions during an ESCEO workshop meeting held in San Francisco, USA (7 November 2015), which was funded by the ESCEO asbl, Belgium.
Author contributions: All authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval of the version to be published.
Declaration of financial/other relationships
E.J.K. has disclosed that he has received travel sponsorship from Roche Poland and Berlin Chemie, and speaker and/or consultancy fees from Meda AB, Rottapharm, AbbVie, Berlin Chemie, Celgene, Egys, Merck Sharp and Dohme (MSD), Novartis, Pfizer, Roche Poland, Polfarma, UCB, and USP. V.K. has disclosed that he has no significant relationships with or financial interests in any commercial companies related to this study or article. S.S. has disclosed that he has received speaker fees from Meda AB. O.B. has disclosed that he has received grant support from IBSA, MSD, Nutraveris, Novartis, Pfizer, Rottapharm, Servier, and Theramex; lecture fees from IBSA, Rottapharm, Servier, and SMB. C.C. has disclosed that he has received consulting fees and honoraria from Alliance for Better Bone Health, Amgen, Eli Lilly, GlaxoSmithKline (GSK), Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda and UCB. J.-Y.R. has disclosed that he has received consulting fees from Servier, Novartis, Negma, Lilly, Wyeth, Amgen, GSK, Roche, Merckle, Nycomed-Takeda, NPS, IBSA-Genevrier, Theramex, UCB, Asahi Kasei, Endocyte; lecture fees from: MSD, Lilly, Rottapharm, IBSA, Genevrier, Novartis, Servier, Roche, GSK, Merckle, Teijin, Teva, Analis, Theramex, Nycomed, NovoNordisk, Ebewee Pharma, Zodiac, Danone, Will Pharma, Amgen; and grant support from Bristol Myers Squibb, MSD, Rottapharm, Teva, Roche, Amgen, Lilly, Novartis, GSK, Servier, Pfizer, Theramex, Danone, Organon, Therabel, Boehringer, Chiltern, Galapagos.
CMRO peer reviewers on this manuscript have received an honorarium from CMRO for their review work, but have no relevant financial or other relationships to disclose.
Acknowledgments
Editorial assistance in the preparation of this manuscript was provided by Lisa Buttle PhD of Medscript Ltd, which was funded by the ESCEO asbl, Belgium.
References
- Bruyere O, Cooper C, Pelletier JP, et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum 2014;44:253-63
- Chiusaroli R, Piepoli T, Zanelli T, et al. Experimental pharmacology of glucosamine sulfate. Int J Rheumatol 2011;2011:939265
- Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 2001;357:251-6
- Pavelka K, Gatterova J, Olejarova M, et al. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med 2002;162:2113-23
- Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62:1145-55
- Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res 2012;64:465-74
- Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg 2013;21:571-6
- McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014;22:363-88
- Reginster JY, Cooper C, Hochberg M, et al. Comments on the discordant recommendations for the use of symptomatic slow-acting drugs in knee osteoarthritis. Curr Med Res Opin 2015;31:1041-5
- Cutolo M, Berenbaum F, Hochberg M, et al. Commentary on recent therapeutic guidelines for osteoarthritis. Semin Arthritis Rheum 2014;44:611-17
- De Wan M, Volpi G, inventors; Rottapharm, assignee. Method of preparing mixed glucosamine salts. USA patent 5,847,107. 1998
- Altman RD. Glucosamine therapy for knee osteoarthritis: pharmacokinetic considerations. Expert Rev Clin Pharmacol 2009;2:359-71
- Marcucci G, Zimran A, Bembi B, et al. Gaucher disease and bone manifestations. Calcif Tissue Int 2014;95:477-94
- Kanis JA, Rizzoli R, Cooper C, Reginster JY. Challenges for the development of bone-forming agents in Europe. Calcif Tissue Int 2014;94:469-73
- Hamerman D. The biology of osteoarthritis. N Engl J Med 1989;320:1322-30
- Rovati LC, Girolami F, Persiani S. Crystalline glucosamine sulfate in the management of knee osteoarthritis: efficacy, safety, and pharmacokinetic properties. Ther Adv Musculoskelet Dis 2012;4:167-80
- Reginster JY, Neuprez A, Lecart MP, et al. Role of glucosamine in the treatment for osteoarthritis. Rheumatol Int 2012;32:2959-67
- Largo R, Alvarez-Soria MA, Diez-Ortego I, et al. Glucosamine inhibits IL-1beta-induced NFkappaB activation in human osteoarthritic chondrocytes. Osteoarthritis Cartilage 2003;11:290-8
- Gouze JN, Bianchi A, Becuwe P, et al. Glucosamine modulates IL-1-induced activation of rat chondrocytes at a receptor level, and by inhibiting the NF-kappa B pathway. FEBS Lett 2002;510:166-70
- Chan PS, Caron JP, Rosa GJ, Orth MW. Glucosamine and chondroitin sulfate regulate gene expression and synthesis of nitric oxide and prostaglandin E(2) in articular cartilage explants. Osteoarthritis Cartilage 2005;13:387-94
- Taniguchi S, Ryu J, Seki M, et al. Long-term oral administration of glucosamine or chondroitin sulfate reduces destruction of cartilage and up-regulation of MMP-3 mRNA in a model of spontaneous osteoarthritis in Hartley guinea pigs. J Orthop Res 2012;30:673-8
- Uitterlinden EJ, Jahr H, Koevoet JL, et al. Glucosamine decreases expression of anabolic and catabolic genes in human osteoarthritic cartilage explants. Osteoarthritis Cartilage 2006;14:250-7
- Hoffer LJ, Kaplan LN, Hamadeh MJ, et al. Sulfate could mediate the therapeutic effect of glucosamine sulfate. Metabolism 2001;50:767-70
- Cordoba F, Nimni ME. Chondroitin sulfate and other sulfate containing chondroprotective agents may exhibit their effects by overcoming a deficiency of sulfur amino acids. Osteoarthritis Cartilage 2003;11:228-30
- Altman RD, Cheung H. Glucosamine sulfate on cartilage: lapine study. Arthritis Rheum 2001;44:1535
- Wen ZH, Tang CC, Chang YC, et al. Glucosamine sulfate reduces experimental osteoarthritis and nociception in rats: association with changes of mitogen-activated protein kinase in chondrocytes. Osteoarthritis Cartilage 2010;18:1192-202
- Mason RM, Chambers MG, Flannelly J, et al. The STR/ort mouse and its use as a model of osteoarthritis. Osteoarthritis Cartilage 2001;9:85-91
- Persiani S, Roda E, Rovati LC, et al. Glucosamine oral bioavailability and plasma pharmacokinetics after increasing doses of crystalline glucosamine sulfate in man. Osteoarthritis Cartilage 2005;13:1041-9
- Jackson CG, Plaas AH, Sandy JD, et al. The human pharmacokinetics of oral ingestion of glucosamine and chondroitin sulfate taken separately or in combination. Osteoarthritis Cartilage 2010;18:297-302
- Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med 2006;354:795-808
- Persiani S, Rotini R, Trisolino G, et al. Synovial and plasma glucosamine concentrations in osteoarthritic patients following oral crystalline glucosamine sulphate at therapeutic dose. Osteoarthritis Cartilage 2007;15:764-72
- Russell AS, Aghazadeh-Habashi A, Jamali F. Active ingredient consistency of commercially available glucosamine sulfate products. J Rheumatol 2002;29:2407-9
- Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2009:CD002946
- Eriksen P, Bartels EM, Altman RD, et al. Risk of bias and brand explain the observed inconsistency in trials on glucosamine for symptomatic relief of osteoarthritis: a meta-analysis of placebo-controlled trials. Arthritis Care Res (Hoboken) 2014;66:1844-55
- Herrero-Beaumont G, Ivorra JA, Del Carmen Trabado M, et al. Glucosamine sulfate in the treatment of knee osteoarthritis symptoms: a randomized, double-blind, placebo-controlled study using acetaminophen as a side comparator. Arthritis Rheum 2007;56:555-67
- Reginster JY. The efficacy of glucosamine sulfate in osteoarthritis: financial and nonfinancial conflict of interest. Arthritis Rheum 2007;56:2105-10
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12
- Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 2010;18:476-99
- Bjordal JM, Klovning A, Ljunggren AE, Slordal L. Short-term efficacy of pharmacotherapeutic interventions in osteoarthritic knee pain: a meta-analysis of randomised placebo-controlled trials. Eur J Pain 2007;11:125-38
- Bruyere O, Altman RD, Reginster JY. Efficacy and safety of glucosamine sulfate in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin Arthritis Rheum 2016;45(4 Suppl):S12-17
- Pavelka K, Bruyere O, Rovati LC, et al. Relief in mild-to-moderate pain is not a confounder in joint space narrowing assessment of full extension knee radiographs in recent osteoarthritis structure-modifying drug trials. Osteoarthritis Cartilage 2003;11:730-7
- Altman RD, Abadie E, Avouac B, et al. Total joint replacement of hip or knee as an outcome measure for structure modifying trials in osteoarthritis. Osteoarthritis Cartilage 2005;13:13-19
- Bruyère O, Pavelka K, Rovati LC, et al. Total joint replacement after glucosamine sulphate treatment in knee osteoarthritis: results of a mean 8-year observation of patients from two previous 3-year, randomised, placebo-controlled trials. Osteoarthritis Cartilage 2008;16:254-60
- Scholtissen S, Bruyere O, Neuprez A, et al. Glucosamine sulphate in the treatment of knee osteoarthritis: cost-effectiveness comparison with paracetamol. Int J Clin Pract 2010;64:756-62
- Black C, Clar C, Henderson R, et al. The clinical effectiveness of glucosamine and chondroitin supplements in slowing or arresting progression of osteoarthritis of the knee: a systematic review and economic evaluation. Health Technol Assess 2009;13:1-148
- Rovati LC, Girolami F, D’Amato M, Giacovelli G. Effects of glucosamine sulfate on the use of rescue non-steroidal anti-inflammatory drugs in knee osteoarthritis: results from the Pharmaco-Epidemiology of GonArthroSis (PEGASus) study. Semin Arthritis Rheum 2016;45(4 Suppl):S34-41
- Cooper C, Adachi JD, Bardin T, et al. How to define responders in osteoarthritis. Curr Med Res Opin 2013;29:719-29
