Abstract
Creatine is one of the most popular nutritional ergogenic aids for athletes. Studies have consistently shown that creatine supplementation increases intramuscular creatine concentrations which may help explain the observed improvements in high intensity exercise performance leading to greater training adaptations. In addition to athletic and exercise improvement, research has shown that creatine supplementation may enhance post-exercise recovery, injury prevention, thermoregulation, rehabilitation, and concussion and/or spinal cord neuroprotection. Additionally, a number of clinical applications of creatine supplementation have been studied involving neurodegenerative diseases (e.g., muscular dystrophy, Parkinson’s, Huntington’s disease), diabetes, osteoarthritis, fibromyalgia, aging, brain and heart ischemia, adolescent depression, and pregnancy. These studies provide a large body of evidence that creatine can not only improve exercise performance, but can play a role in preventing and/or reducing the severity of injury, enhancing rehabilitation from injuries, and helping athletes tolerate heavy training loads. Additionally, researchers have identified a number of potentially beneficial clinical uses of creatine supplementation. These studies show that short and long-term supplementation (up to 30 g/day for 5 years) is safe and well-tolerated in healthy individuals and in a number of patient populations ranging from infants to the elderly. Moreover, significant health benefits may be provided by ensuring habitual low dietary creatine ingestion (e.g., 3 g/day) throughout the lifespan. The purpose of this review is to provide an update to the current literature regarding the role and safety of creatine supplementation in exercise, sport, and medicine and to update the position stand of International Society of Sports Nutrition (ISSN).
Background
Creatine is one of the most popular nutritional ergogenic aids for athletes. Studies have consistently shown that creatine supplementation increases intramuscular creatine concentrations, can improve exercise performance, and/or improve training adaptations. Research has indicated that creatine supplementation may enhance post-exercise recovery, injury prevention, thermoregulation, rehabilitation, and concussion and/or spinal cord neuroprotection. A number of clinical applications of creatine supplementation have also been studied involving neurodegenerative diseases (e.g., muscular dystrophy, Parkinson’s, Huntington’s disease), diabetes, osteoarthritis, fibromyalgia, aging, brain and heart ischemia, adolescent depression, and pregnancy. The purpose of this review is to provide an update to the current literature regarding the role and safety of creatine supplementation in exercise, sport, and medicine and to update the position stand of International Society of Sports Nutrition (ISSN) related to creatine supplementation.
Metabolic role
Creatine, a member of the guanidine phosphagen family, is a naturally occurring non-protein amino acid compound found primarily in red meat and seafood [Citation1–Citation4]. The majority of creatine is found in skeletal muscle (~95%) with small amounts also found in the brain and testes (~5%) [Citation5, Citation6]. About two thirds of intramuscular creatine is phosphocreatine (PCr) with the remaining being free creatine. The total creatine pool (PCr + Cr) in the muscle averages about 120 mmol/kg of dry muscle mass for a 70 kg individual [Citation7]. However, the upper limit of creatine storage appears to be about 160 mmol/kg of dry muscle mass in most individuals [Citation7, Citation8]. About 1–2% of intramuscular creatine is degraded into creatinine (metabolic byproduct) and excreted in the urine [Citation7, Citation9, Citation10]. Therefore, the body needs to replenish about 1–3 g of creatine per day to maintain normal (unsupplemented) creatine stores depending on muscle mass. About half of the daily need for creatine is obtained from the diet [Citation11]. For example, a pound of uncooked beef and salmon provides about 1–2 g of creatine [Citation9]. The remaining amount of creatine is synthesized primarily in the liver and kidneys from arginine and glycine by the enzyme arginine:glycine amidinotransferase (AGAT) to guanidinoacetate (GAA), which is then methylated by guanidinoacetate N-methyltransferase (GAMT) using S-adenosyl methionine to form creatine (see Fig. ) [Citation12].
Fig. 1 Chemical structure and biochemical pathway for creatine synthesis. From Kreider and Jung [Citation6]
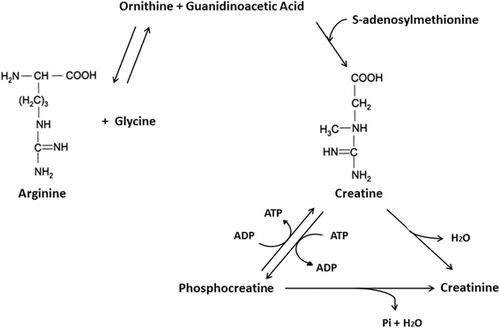
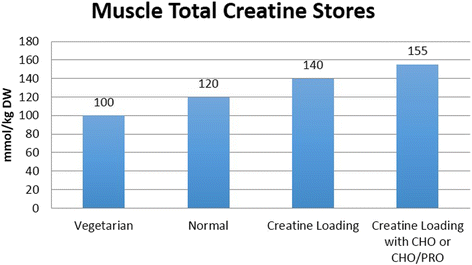
Some individuals have been found to have creatine synthesis deficiencies due to inborn errors in AGAT, GMAT and/or creatine transporter (CRTR) deficiencies and therefore must depend on dietary creatine intake in order to maintain normal muscle and brain concentrations of PCr and Cr [Citation13–Citation19]. Vegetarians have been reported to have lower intramuscular creatine stores (90–110 mmol/kg of dry muscle) and therefore may observe greater gains in muscle creatine content from creatine supplementation [Citation11, Citation13, Citation20, Citation21]. Conversely, larger athletes engaged in intense training may need to consume 5–10 g/day of creatine to maintain optimal or capacity whole body creatine stores [Citation22] and clinical populations may need to consume 10–30 g/day throughout their lifespan to offset creatine synthesis deficiencies and/or provide therapeutic benefit in various disease states [Citation13, Citation19, Citation23].
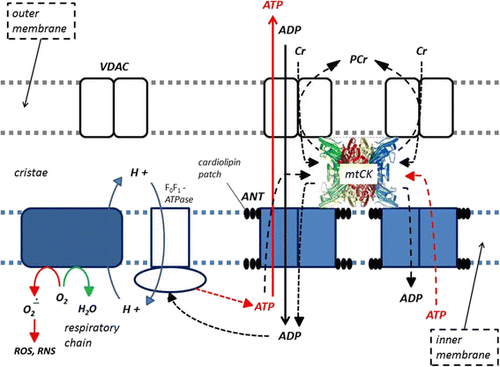
Phosphagens are prevalent in all species and play an important role in maintaining energy availability [Citation1, Citation2, Citation24, Citation25]. The primary metabolic role of creatine is to combine with a phosphoryl group (Pi) to form PCr through the enzymatic reaction of creatine kinase (CK). Wallimann and colleagues [Citation26–Citation28] suggested that the pleiotropic effects of Cr are mostly related to the functions of CK and PCr (i.e., CK/PCr system). As adenosine triphosphate (ATP) is degraded into adenosine diphosphate (ADP) and Pi to provide free energy for metabolic activity, the free energy released from the hydrolysis of PCr into Cr + Pi can be used as a buffer to resynthesize ATP [Citation24, Citation25]. This helps maintain ATP availability particularly during maximal effort anaerobic sprint-type exercise. The CK/PCr system also plays an important role in shuttling intracellular energy from the mitochondria into the cytosol (see Fig. ). The CK/PCr energy shuttle connects sites of ATP production (glycolysis and mitochondrial oxidative phosphorylation) with subcellular sites of ATP utilization (ATPases) [Citation24, Citation25, Citation27]. In this regard, creatine enters the cytosol through a CRTR [Citation16, Citation29–Citation31]. In the cytosol, creatine and associated cytosolic and glycolytic CK isoforms help maintain glycolytic ATP levels, the cytosolic ATP/ADP ratio, and cytosolic ATP-consumption [Citation27]. Additionally, creatine diffuses into the mitochondria and couples with ATP produced from oxidative phosphorylation and the adenine nucleotide translocator (ANT) via mitochondrial CK (see Fig. ). ATP and PCr can then diffuse back into the cytosol and help buffer energy needs. This coupling also reduces the formation of reactive oxygen species (ROS) and can therefore act as a direct and/or indirect antioxidant [Citation32–Citation35]. The CK/PCr energy shuttle thereby connects sites of ATP production (glycolysis and mitochondrial oxidative phosphorylation) with subcellular sites of ATP utilization (ATPases) in order to fuel energy metabolism [Citation24, Citation25, Citation27]. In this way, the CK/PCr system thereby serves as an important regulator of metabolism which may help explain the ergogenic and potential therapeutic health benefits of creatine supplementation [Citation4, Citation27, Citation33, Citation36–Citation45].
Fig. 2 Proposed creatine kinase/phosphocreatine (CK/PCr) energy shuttle. CRT = creatine transporter; ANT = adenine nucleotide translocator; ATP = adenine triphosphate; ADP = adenine diphosphate; OP = oxidative phosphorylation; mtCK = mitochondrial creatine kinase; G = glycolysis; CK-g = creatine kinase associated with glycolytic enzymes; CK-c = cytosolic creatine kinase; CK-a = creatine kinase associated with subcellular sites of ATP utilization; 1 – 4 sites of CK/ATP interaction. From Kreider and Jung [Citation6]
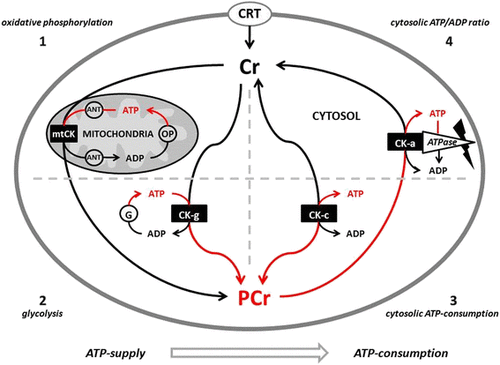
Fig. 3 Role of mitochondrial creatine kinase (mtCK) in high energy metabolite transport and cellular respiration. VDAC = voltage-dependent anion channel; ROS = reactive oxygen species; RNS = reactive nitrogen species; ANT = adenine nucleotide translocator; ATP = adenine triphosphate; ADP = adenine diphosphate; Cr = creatine; and, PCr = phosphocreatine. From Kreider and Jung [Citation6]
Supplementation protocols
In a normal diet that contains 1–2 g/day of creatine, muscle creatine stores are about 60–80% saturated. Therefore, dietary supplementation of creatine serves to increase muscle creatine and PCr by 20–40% (see Fig. .) [Citation7, Citation8, Citation10, Citation46–Citation48]. The most effective way to increase muscle creatine stores is to ingest 5 g of creatine monohydrate (or approximately 0.3 g/kg body weight) four times daily for 5–7 days [Citation7, Citation10]. However, higher levels of creatine supplementation for longer periods of time may be needed to increase brain concentrations of creatine, offset creatine synthesis deficiencies, or influence disease states [Citation13, Citation19, Citation23]. Once muscle creatine stores are fully saturated, creatine stores can generally be maintained by ingesting 3–5 g/day, although some studies indicate that larger athletes may need to ingest as much as 5–10 g/day in order to maintain creatine stores [Citation7, Citation8, Citation10, Citation46–Citation48]. Ingesting creatine with carbohydrate or carbohydrate and protein have been reported to more consistently promote greater creatine retention [Citation8, Citation22, Citation49, Citation50]. An alternative supplementation protocol is to ingest 3 g/day of creatine monohydrate for 28 days [Citation7]. However, this method would only result in a gradual increase in muscle creatine content compared to the more rapid loading method and may therefore have less effect on exercise performance and/or training adaptations until creatine stores are fully saturated. Research has shown that once creatine stores in the muscle are elevated, it generally takes 4–6 weeks for creatine stores to return to baseline [Citation7, Citation48, Citation51]. Additionally, it has been recommended that due to the health benefits of creatine, individuals should consume about 3 g/day of creatine in their diet particularly as one ages [Citation27]. No evidence has suggested that muscle creatine levels fall below baseline after cessation of creatine supplementation; therefore, the potential for long-term suppression of endogenous creatine synthesis does not appear to occur [Citation22, Citation52].
Fig. 4 Approximate muscle total creatine levels in mmol/kg dry weight muscle reported in the literature for vegetarians, individuals following a normal diet, and in response to creatine loading with or without carbohydrate (CHO) or CHO and protein (PRO). From Kreider and Jung [Citation6]
Bioavailability
The most commonly studied form of creatine in the literature is creatine monohydrate [Citation53]. The uptake of creatine involves the absorption of creatine into the blood and then uptake by the target tissue [Citation53]. Plasma levels of creatine typically peak at about 60 min after oral ingestion of creatine monohydrate [Citation7]. An initial rise in plasma creatine levels, followed by a reduction in plasma levels can be used to indirectly suggest increased uptake into the target tissue [Citation53]. However, the gold standards for measuring the effects of creatine supplementation on target tissues are through magnetic resonance spectroscopy (MRS), muscle biopsy, stable isotope tracer studies, and/or whole body creatine retention assessed by measuring the difference between creatine intake and urinary excretion of creatine [Citation53].
Creatine is stable in solid form but not in aqueous solution due to an intramolecular cyclization [Citation54]. Generally, creatine is converted to creatinine at higher rates the lower the pH and the higher the temperature. For example, research has shown that creatine is relatively stable in solution at neutral pH (7.5 or 6.5). However, after 3 days of storage at 25 °C, creatine degrades to creatinine (e.g., 4% at pH 5.5; 12% at pH 4.5; and 21% at pH 3.5) [Citation53, Citation55]. The degradation of creatine into creatinine over time is the main reason that creatine is sold in solid form. However, this does not mean that creatine is degraded into creatinine in vivo through the digestive process. In this regard, the degradation of creatine to creatinine can be reduced or halted be either lowering the pH under 2.5 or increasing the pH [Citation53]. A very low pH results in the protonation of the amide function of the creatine molecule, thereby preventing the intra-molecular cyclization [Citation53]. Therefore, the conversion of creatine to creatinine in the gastrointestinal tract is minimal regardless of transit time; absorption into the blood is nearly 100% [Citation10, Citation53, Citation56, Citation57].
The vast majority of studies assessing the efficacy of creatine supplementation on muscle phosphagen levels, whole body creatine retention, and/or performance have evaluated creatine monohydrate. Claims that different forms of creatine are degraded to a lesser degree than creatine monohydrate in vivo or result in a greater uptake to muscle are currently unfounded [Citation53]. Clinical evidence has not demonstrated that different forms of creatine such as creatine citrate [Citation50], creatine serum [Citation58], creatine ethyl ester [Citation59], buffered forms of creatine [Citation60], or creatine nitrate [Citation61] promote greater creatine retention than creatine monohydrate [Citation53].
Ergogenic value
Table presents the reported ergogenic benefits of creatine supplementation. A large body of evidence now indicates that creatine supplementation increases muscle availability of creatine and PCr and can therefore enhance acute exercise capacity and training adaptations in adolescents [Citation62–Citation66], younger adults [Citation61, Citation67–Citation77] and older individuals [Citation5, Citation40, Citation43, Citation78–Citation85]. These adaptations would allow an athlete to do more work over a series of sets or sprints leading to greater gains in strength, muscle mass, and/or performance due to an improvement in the quality of training. Table presents the types of sport events in which creatine supplementation has been reported to benefit. Creatine supplementation has primarily been recommended as an ergogenic aid for power/strength athletes to help them optimize training adaptations or athletes who need to sprint intermittently and recover during competition (e.g., American football, soccer, basketball, tennis, etc.). After creatine loading, performance of high intensity and/or repetitive exercise is generally increased by 10–20% depending on the magnitude of increase in muscle PCr [Citation46].
Table 1 Potential ergogenic benefits of creatine supplementation
Table 2 Examples of sport events that may be enhanced by creatine supplementation
Benefits have been reported in men and women, although the majority of studies have been conducted on men and some studies suggest that women may not see as much gain in strength and/or muscle mass during training in response to creatine supplementation [Citation20, Citation51, Citation64, Citation86–Citation90]. However, as will be described below, a number of other applications in sport may benefit athletes involved in high intensity intermittent and endurance events as well. In terms of performance, the International Society of Sports Nutrition (ISSN) has previously concluded in its position stand on creatine supplementation that creatine monohydrate is the most effective ergogenic nutritional supplement currently available to athletes in terms of increasing high-intensity exercise capacity and lean body mass during training [Citation5, Citation78]. Recent position stands by the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine on nutrition for athletic performance all drew similar conclusions [Citation91, Citation92]. Thus, a wide-spread consensus now exists in the scientific community that creatine supplementation can serve as an effective nutritional ergogenic aid that may benefit athletes involved in numerous sports as well as individuals involved in exercise training.
Prevalence of use in sport
Creatine is found in high amounts in the food supply and therefore its use is not banned by any sport organization although some organizations prohibit provision of some types of dietary supplements to athletes by their teams [Citation5, Citation53, Citation78, Citation91, Citation92]. In these instances, athletes can purchase and use creatine on their own without penalty or violation of their banned substance restrictions. Americans consume over four million kilograms (kg) a year of creatine with worldwide use much higher [Citation53]. The reported prevalence of creatine use among athletes and military personnel in survey-based studies has generally been reported to be about 15–40% [Citation93–Citation101], with use more common in male strength/power athletes. High school athletes have been reported to have similar prevalence of use of creatine [Citation95–Citation97, Citation102]. In 2014, the NCAA reported that creatine was among the most popular dietary supplements taken by their male athletes (e.g., baseball - 28.1%, basketball - 14.6%, football - 27.5%, golf - 13.0%, ice hockey - 29.4%, lacrosse - 25.3%, soccer 11.1%, swimming - 19.2%, tennis - 12.9%, track and field - 16.1%, wrestling - 28.5%) while female athletes reported a use rate of only 0.2 to 3.8% in various sports [Citation103]. Comparatively, these NCAA athletes reported relatively high alcohol (83%), tobacco (10–16%), and marijuana (22%) use along with minimal androgenic anabolic steroid use (0.4%). As will be noted below, no study has reported any adverse or ergolytic effect of short- or long-term creatine supplementation while numerous studies have reported performance and/or health benefits in athletes and individuals with various diseases. Therefore, the prevalence of alcohol, tobacco and drug use among NCAA athletes would seemingly be a much greater health concern than athletes taking creatine.
Other applications in sport and training
Recent research demonstrates a number of other applications of creatine supplementation that may benefit athletes involved in intense training and individuals who want to enhance training adaptations. For example, use of creatine during training may enhance recovery, reduce the risk of injury and/or help individuals recover from injuries at a faster rate. The following describes some applications of creatine in addition to serving as an ergogenic aid.
Enhanced recovery
Creatine supplementation can help athletes recover from intense training. For example, Green and coworkers [Citation8] reported that co-ingesting creatine (5 g) with large amounts of glucose (95 g) enhanced creatine and carbohydrate storage in muscle. Additionally, Steenge et al. [Citation49] reported that co-ingesting creatine (5 g) with 47–97 g of carbohydrate and 50 g of protein enhanced creatine retention. Nelson and colleagues [Citation104] reported that creatine loading prior to performing an exhaustive exercise bout and glycogen loading promoted greater glycogen restoration than just carbohydrate loading alone. Since glycogen replenishment is important to promoting recovery and preventing overtraining during intensified training periods [Citation78], creatine supplementation may help athletes who deplete large amounts of glycogen during training and/or performance to maintain optimal glycogen levels.
Evidence also suggests that creatine supplementation may reduce muscle damage and/or enhance recovery from intense exercise. For example, Cooke and associates [Citation105] evaluated the effects of creatine supplementation on muscle force recovery and muscle damage following intense exercise. They reported that participants supplemented with creatine had significantly greater isokinetic (+10%) and isometric (+21%) knee extension strength during recovery from exercise-induced muscle damage. Additionally, plasma CK levels were significantly lower (−84%) after 2, 3, 4, and 7 days of recovery in the creatine supplemented group compared to controls. The authors concluded that creatine improved the rate of recovery of knee extensor muscle function after injury. Santos and coworkers [Citation106] evaluated the effects of creatine loading in experienced marathon runners prior to performing a 30 km race on inflammatory markers and muscle soreness. The researchers reported that creatine loading attenuated the changes in CK (−19%), prostaglandin E2 (−61%), and tumor necrosis factor (TNF) alpha (−34%) and abolished the increase in lactate dehydrogenase (LDH) compared to controls. Similar findings were reported by Demince et al. [Citation107] who reported that creatine supplementation inhibited the increase of inflammatory markers (TNF-alpha and C-reactive protein) in response to intermittent anaerobic sprint exercise. Finally, Volek and colleagues [Citation77] evaluated the effects of creatine supplementation (0.3 g/kg/d) for 4 weeks during an intensified overreaching period followed by a 2 weeks taper. The researchers found that creatine supplementation was effective in maintaining muscular performance during the initial phase of high-volume resistance training overreaching that otherwise results in small performance decrements. These findings suggest that creatine supplementation can help athletes tolerate heavy increases in training volume. Therefore, there is strong evidence that creatine supplementation can help athletes enhance glycogen loading; experience less inflammation and/or muscle enzyme efflux following intense exercise; and tolerate high volumes of training and/or overreaching to a greater degree thereby promoting recovery.
Injury prevention
Several studies have reported that creatine supplementation during training and/or competition either has no effect or reduces the incidence of musculoskeletal injury, dehydration, and/or muscle cramping. For example, several initial studies on creatine supplementation provided 15–25 g/day of creatine monohydrate for 4 – 12 weeks in athletes engaged in heavy training with no reported side effects [Citation67, Citation77, Citation108–Citation110]. Kreider and colleagues [Citation109] reported that American collegiate football players ingesting 20 or 25 g/day of creatine monohydrate with a carbohydrate/protein supplement for 12 weeks during off season conditioning and spring football practice experienced greater gains in strength and muscle mass with no evidence of any adverse side effects. Additionally, in a study specifically designed to assess the safety of creatine supplementation, American collegiate football players ingesting about 16 g/day of creatine for 5 days and 5–10 g/day for 21 months had no clinically significant differences among creatine users and controls in markers of renal function, muscle and liver enzymes, markers of catabolism, electrolytes, blood lipids, red cell status, lymphocytes, urine volume, clinical urinalysis, or urine specific gravity [Citation22]. Meanwhile, creatine users experienced less incidence of cramping, heat illness/dehydration, muscle tightness, muscle strains/pulls, non-contact injuries, and total injuries/missed practices than those not taking creatine [Citation111].
Similar findings were reported by Greenwood and coworkers [Citation112] who examined injury rates during a 4 months American collegiate football season among creatine users (0.3 g/kg/day for 5 days, 0.03 g/kg/day for 4 months) and non-users. The researchers reported that creatine users experienced significantly less incidence of muscle cramping, heat illness/dehydration, muscle tightness, muscle strains, and total injuries compared to athletes who did not supplement their diet with creatine. Likewise, Cancela and associates [Citation113] reported that creatine supplementation (15 g/day x 7-d, 3 g/day x 49-d) during soccer training promoted weight gain but that those taking creating had no negative effects on blood and urinary clinical health markers. Finally, Schroder et al. [Citation114] evaluated the effects of ingesting creatine (5 g/day) for three competitive seasons in professional basketball players. The researchers found that long-term low-dose creatine monohydrate supplementation did not promote clinically significant changes in health markers or side effects. Thus, contrary to unsubstantiated reports, the peer-reviewed literature demonstrates that there is no evidence that: 1) creatine supplementation increases the anecdotally reported incidence of musculoskeletal injuries, dehydration, muscle cramping, gastrointestinal upset, renal dysfunction, etc.; or that 2) long-term creatine supplementation results in any clinically significant side effects among athletes during training or competition for up to 3 years. If anything, evidence reveals that athletes who take creatine during training and competition experience a lower incidence of injuries compared to athletes who do not supplement their diet with creatine.
Enhanced tolerance to exercise in the heat
Like carbohydrate, creatine monohydrate has osmotic properties that help retain a small amount of water. For example, initial studies reported that creatine loading promoted a short-term fluid retention (e.g., about 0.5 – 1.0 L) that was generally proportional to the acute weight gain observed [Citation22, Citation46]. For this reason, there was interest in determining if creatine supplementation may help hyper-hydrate an athlete and/or improve exercise tolerance when exercising in the heat [Citation76, Citation115–Citation126]. For example, Volek and colleagues [Citation76] evaluated the effects of creatine supplementation (0.3 g/kg/day for 7 days) on acute cardiovascular, renal, temperature, and fluid-regulatory hormonal responses to exercise for 35 min in the heat. The researchers reported that creatine supplementation augmented repeated sprint cycle performance in the heat without altering thermoregulatory responses. Kilduff and associates [Citation123] evaluated the effects of creatine supplementation (20 g/day for 7 days) prior to performing exercise to exhaustion at 63% of peak oxygen uptake in the heat (30.3 °C). The researchers reported that creatine supplementation increased intracellular water and reduced thermoregulatory and cardiovascular responses to prolonged exercise (e.g., heart rate, rectal temperature, sweat rate) thereby promoting hyper-hydration and a more efficient thermoregulatory response during prolonged exercise in the heat. Watson and colleagues [Citation117] reported that short-term creatine supplementation (21.6 g/day for 7 days) did not increase the incidence of symptoms or compromise hydration status or thermoregulation in dehydrated (−2%), trained men exercising in the heat. Similar findings were observed by several other groups [Citation118, Citation119, Citation127, Citation128] leading researchers to add creatine to glycerol as a highly effective hyper-hydrating strategy to help athletes better tolerate exercise in the heat [Citation116, Citation120–Citation122, Citation125, Citation126]. These findings provide strong evidence that creatine supplementation (with or without glycerol) may serve as an effective nutritional hyper-hydration strategy for athletes engaged in intense exercise in hot and humid environments thereby reducing risk to heat related-illness [Citation5, Citation129].
Enhanced rehabilitation from injury
Since creatine supplementation has been reported to promote gains in muscle mass and improved strength, there has been interest in examining the effects of creatine supplementation on muscle atrophy rates as a result of limb immobilization and/or during rehabilitation [Citation130]. For example, Hespel and coworkers [Citation131] examined the effects of creatine supplementation (20 g/day down to 5 g/day) on atrophy rates and rehabilitation outcomes in individuals who had their right leg casted for 2 weeks. During the 10 week rehabilitation phase, participants performed three sessions a week of knee extension rehabilitation. The researchers reported that individuals in the creatine group experienced greater changes in the cross-sectional area of muscle fiber (+10%) and peak strength (+25%) during the rehabilitation period. These changes were associated with greater changes in myogenic regulating factor 4 (MRF4) and myogenic protein expression. In a companion paper to this study, Op’t Eijnde et al. [Citation132] reported that creatine supplementation offset the decline in muscle GLUT4 protein content that occurs during immobilization and increased GLUT4 protein content during subsequent rehabilitation training in healthy subjects. Collectively, these findings suggest that creatine supplementation lessened the amount of muscle atrophy and detrimental effects on muscle associated with immobilization while promoting greater gains in strength during rehabilitation. Similarly, Jacobs and associates [Citation133] examined the effects of creatine supplementation (20 g/d for 7 days) on upper extremity work capacity in individuals with cervical-level spinal cord injury (SCI). Results revealed that peak oxygen uptake and ventilatory anaerobic threshold were increased following creatine supplementation. Conversely, Tyler et al. [Citation134] reported that creatine supplementation (20 g/day for 7 days, and 5 g/day thereafter) did not significantly affect strength or functional capacity in patients recovering from anterior cruciate ligament (ACL) surgery. Moreover, Perret and colleagues [Citation135] reported that creatine supplementation (20 g/day for 6 days) did not enhance 800 m wheelchair performance in trained SCI wheelchair athletes. While not all studies show benefit, there is evidence that creatine supplementation may help lessen muscle atrophy following immobilization and promote recovery during exercise-related rehabilitation in some populations. Thus, creatine supplementation may help athletes and individuals with clinical conditions recover from injuries.
Brain and spinal cord neuroprotection
The risk of concussions and/or SCI in athletes involved in contact sports has become an international concern among sports organizations and the public. It has been known for a long time that creatine supplementation possesses neuroprotective benefits [Citation29, Citation38, Citation40, Citation136]. For this reason, a number of studies have examined the effects of creatine supplementation on traumatic brain injury (TBI), cerebral ischemia, and SCI. For example, Sullivan et al. [Citation137] examined the effects of 5 days of creatine administration prior to a controlled TBI in rats and mice. The researchers found that creatine monohydrate ameliorated the extent of cortical damage by 36 to 50%. The protection appeared to be related to creatine-induced maintenance of neuronal mitochondrial bioenergetics. Therefore, the researchers concluded that creatine supplementation may be useful as a neuroprotective agent against acute and chronic neurodegenerative processes. In a similar study, Haussmann and associates [Citation138] investigated the effects of rats fed creatine (5 g/100 g dry food) before and after a moderate SCI. The researchers reported that creatine ingestion improved locomotor function tests and reduced the size of scar tissue after the SCI. The authors suggested that pretreatment of patients with creatine may provide neuroprotection in patients undergoing spinal surgery who are at risk to SCI. Similarly, Prass and colleagues [Citation139] reported findings that creatine administration reduced brain infarct size following an ischemic event by 40%.
Adcock et al. [Citation140] reported that neonatal rats fed 3 g/kg of creatine for 3 days observed a significant increase in the ratio of brain PCr to Pi and a 25% reduction in the volume of edemic brain tissue following cerebral hypoxic ischemia. The authors concluded that creatine supplementation appears to improve brain bioenergetics thereby helping minimize the impact of brain ischemia. Similarly, Zhu and colleagues [Citation141] reported that oral creatine administration resulted in a marked reduction in ischemic brain infarction size, neuronal cell death, and provided neuroprotection after cerebral ischemia in mice. The authors suggested that given the safety record of creatine, creatine might be considered as a novel therapeutic agent for inhibition of ischemic brain injury in humans. Allah et al. [Citation142] reported that creatine monohydrate supplementation for 10 weeks reduced the infarction size and improved learning/memory following neonatal hypoxia ischemia encephalopathy in female mice. The authors concluded that creatine supplementation has the potential to improve the neuro-function following neonatal brain damage. Finally, Rabchevsky and associates [Citation143] examined the efficacy of creatine-supplemented diets on hind limb functional recovery and tissue sparing in adult rats. Rats were fed a control diet or 2% creatine-supplemented chow for 4–5 weeks prior to and following SCI. Results revealed that creatine feeding significantly reduced loss of gray matter after SCI. These findings provide strong evidence that creatine supplementation may limit damage from concussions, TBI, and/or SCI [Citation33, Citation144].
Potential medical uses of creatine
Given the role of creatine in metabolism, performance, and training adaptations; a number of researchers have been investigating the potential therapeutic benefits of creatine supplementation in various clinical populations. The following highlights some of these applications.
Creatine synthesis deficiencies
Creatine deficiency syndromes are a group of inborn errors (e.g., AGAT deficiency, GAMT deficiency, and CRTR deficiency) that reduce or eliminate the ability to endogenously synthesize or effect transcellular creatine transport [Citation17]. Individuals with creatine synthesis deficiencies have low levels of creatine and PCr in the muscle and the brain. As a result, they often have clinical manifestations of muscle myopathies, gyrate atrophy, movement disorders, speech delay, autism, mental retardation, epilepsy, and/or developmental problems [Citation13, Citation17, Citation145]. For this reason, a number of studies have investigated the use of relatively high doses of creatine monohydrate supplementation (e.g., 0.3 – 0.8 g/kg/day equivalent to 21 – 56 g/day of creatine for a 70 kg person, or 1 – 2.7 times greater than the adult loading dose) throughout the lifespan as a means of treating children and adults with creatine synthesis deficiencies [Citation13, Citation17, Citation145–Citation149]. These studies generally show some improvement in clinical outcomes particularly for AGAT and GAMT with less consistent effects on CRTR deficiencies [Citation145].
For example, Battini et al. [Citation150] reported that a patient diagnosed at birth with AGAT deficiency who was treated with creatine supplementation beginning at 4 months of age experienced normal psychomotor development at 18 months compared to siblings who did not have the deficiency. Stockler-Ipsiroglu and coworkers [Citation151] evaluated the effects of creatine monohydrate supplementation (0.3 – 0.8 g/kg/day) in 48 children with GMAT deficiency with clinical manifestations of global developmental delay/intellectual disability (DD/ID) with speech/language delay and behavioral problems (n = 44), epilepsy (n = 35), or movement disorder (n = 13). The median age at treatment was 25.5 months, 39 months, and 11 years in patients with mild, moderate, and severe DD/ID, respectively. The researchers found that creatine supplementation increased brain creatine levels and improved or stabilized clinical symptoms. Moreover, four patients treated younger than 9 months had normal or almost normal developmental outcomes. Long-term creatine supplementation has also been used to treat patients with creatine deficiency-related gyrate atrophy [Citation152–Citation156]. These findings and others provide promise that high-dose creatine monohydrate supplementation may be an effective adjunctive therapy for children and adults with creatine synthesis deficiencies [Citation18, Citation145, Citation157–Citation159]. Additionally, these reports provide strong evidence regarding the long-term safety and tolerability of high-dose creatine supplementation in pediatric populations with creatine synthesis deficiencies, including infants less than 1 year of age [Citation157].
Neurodegenerative diseases
A number of studies have investigated the short and long-term therapeutic benefit of creatine supplementation in children and adults with various neuromuscular diseases like muscular dystrophies [Citation160–Citation165], Huntington’s disease [Citation23, Citation166–Citation171]; Parkinson disease [Citation23, Citation40, Citation166, Citation172–Citation174]; mitochondria-related diseases [Citation29, Citation175–Citation177]; and, amyotrophic lateral sclerosis or Lou Gehrig’s Disease [Citation166, Citation178–Citation184]. These studies have provided some evidence that creatine supplementation may improve exercise capacity and/or clinical outcomes in these patient populations. However, Bender and colleagues [Citation23] recently reported results of several large clinical trials evaluating the effects of creatine supplementation in patients with Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS). A total of 1,687 patients took an average of 9.5 g/day of creatine for a total of 5,480 patient years. Results revealed no clinical benefit on patient outcomes in patients with PD or ALS. However, there was some evidence that creatine supplementation slowed down progression of brain atrophy in patients with HD (although clinical markers were unaffected). Whether creatine supplementation may have a role in mediating other clinical markers in these patient populations and/or whether individual patients may respond more positively to creatine supplementation than others, remain to be determined. Nevertheless, these studies show that creatine supplementation has been used to treat children and adults with neurodegenerative conditions and is apparently safe and well-tolerated when taking up to 30 g/day for 5 years in these populations.
Ischemic heart disease
Creatine and phosphocreatine play an important role in maintaining myocardial bioenergetics during ischemic events [Citation33]. For this reason, there has been interest in assessing the role of creatine or phosphocreatine in reducing arrhythmias and/or improving heart function during ischemia [Citation185–Citation194]. In a recent review, Balestrino and colleagues [Citation33] concluded that phosphocreatine administration, primarily as an addition to cardioplegic solutions, has been used to treat myocardial ischemia and prevent ischemia-induced arrhythmia and improve cardiac function with some success. They suggested that creatine supplementation may protect the heart during an ischemic event. Thus, prophylactic creatine supplementation may be beneficial for patients at risk for myocardial ischemia and/or stroke.
Aging
A growing collection of evidence supports that creatine supplementation may improve health status as individuals age [Citation41, Citation43–Citation45, Citation195]. In this regard, creatine supplementation has been reported to help lower cholesterol and triglyceride levels [Citation67, Citation196]; reduce fat accumulation in the liver [Citation197]; reduce homocysteine levels [Citation198]; serve as an antioxidant [Citation199–Citation202]; enhance glycemic control [Citation132, Citation203–Citation205]; slow tumor growth in some types of cancers [Citation32, Citation198, Citation206, Citation207]; increase strength and/or muscle mass [Citation37, Citation41, Citation44, Citation45, Citation82, Citation208–Citation212]; minimize bone loss [Citation211, Citation212]; improve functional capacity in patients with knee osteoarthritis [Citation213] and fibromyalgia [Citation214]; positively influence cognitive function [Citation43, Citation83, Citation195]; and in some instances, serve as an anti-depressant [Citation215–Citation217].
For example, Gualano and associates supplemented patients with type II diabetes with a placebo or creatine (5 g/day) for 12 weeks during training. Creatine supplementation significantly decreased HbA1c and glycemic response to standardized meal as well as increased GLUT-4 translocation. These findings suggest that creatine supplementation combined with an exercise program improves glycemic control and glucose disposal in type 2 diabetic patients. Candow and others [Citation211] reported that low-dose creatine (0.1 g/kg/day) combined with protein supplementation (0.3 g/kg/day) increased lean tissue mass and upper body strength while decreasing markers of muscle protein degradation and bone resorption in older men (59–77 years). Similarly, Chilibeck et al. [Citation212] reported that 12 months of creatine supplementation (0.1 g/kg/day) during resistance training increased strength and preserved femoral neck bone mineral density and increased femoral shaft subperiosteal width in postmenopausal women. A recent meta-analysis [Citation80] of 357 elderly individuals (64 years) participating in an average of 12.6 weeks of resistance training found that participants supplementing their diet with creatine experienced greater gains in muscle mass, strength, and functional capacity. These findings were corroborated in a meta-analysis of 405 elderly participants (64 years) who experienced greater gains in muscle mass and upper body strength with creatine supplementation during resistance-training compared to training alone [Citation37]. These findings suggest that creatine supplementation can help prevent sarcopenia and bone loss in older individuals.
Finally, a number of studies have shown that creatine supplementation can increase brain creatine content generally by 5 – 15% [Citation218–Citation220]. Moreover, creatine supplementation can reduce mental fatigue [Citation221] and/or improve cognitive function [Citation83, Citation222–Citation225]. For example, Watanabe et al. [Citation221] reported that creatine supplementation (8 g/day for 5 days) reduced mental fatigue when subjects repeatedly performed a simple mathematical calculation as well as increased oxygen utilization in the brain. Rae and colleagues [Citation222] reported that creatine supplementation (5 g/day for 6 weeks) significantly improved working memory and intelligence tests requiring speed of processing. McMorris and coworkers [Citation224] found that creatine supplementation (20 g/day for 7 days) after sleep deprivation demonstrated significantly less decrement in performance in random movement generation, choice reaction time, balance and mood state suggesting that creatine improves cognitive function in response to sleep deprivation. This research group also examined the effects of creatine supplementation (20 g/day for 7 days) on cognitive function in elderly participants and found that creatine supplementation significantly improved performance on random number generation, forward spatial recall, and long-term memory tasks. Ling and associates [Citation225] reported that creatine supplementation (5 g/day for 15 days) improved cognition on some tasks. Since creatine uptake by the brain is slow and limited, current research is investigating whether dietary supplementation of creatine precursors like GAA may promote greater increases in brain creatine [Citation226, Citation227]. One recent study suggested that GAA supplementation (3 g/day) increased brain creatine content to a greater degree than creatine monohydrate [Citation227].
Pregnancy
Since creatine supplementation has been shown to improve brain and heart bioenergetics during ischemic conditions and possess neuroprotective properties, there has been recent interest in use of creatine during pregnancy to promote neural development and reduce complications resulting from birth asphyxia [Citation228–Citation237]. The rationale for creatine supplementation during pregnancy is that the fetus relies upon placental transfer of maternal creatine until late in pregnancy and significant changes in creatine synthesis and excretion occur as pregnancy progresses [Citation230, Citation232]. Consequently, there is an increased demand for and utilization of creatine during pregnancy. Maternal creatine supplementation has been reported to improve neonatal survival and organ function following birth asphyxia in animals [Citation228, Citation229, Citation231, Citation233–Citation235, Citation237]. Human studies show changes in the maternal urine and plasma creatine levels across pregnancy and association to maternal diet [Citation230, Citation232]. Consequently, it has been postulated that there may be benefit to creatine supplementation during pregnancy on fetal growth, development, and health [Citation230, Citation232]. This area of research may have broad implications for fetal and child development and health.
Safety
Since creatine monohydrate became a popular dietary supplement in the early 1990s, over 1,000 studies have been conducted and billions of servings of creatine have been ingested. The only consistently reported side effect from creatine supplementation that has been described in the literature has been weight gain [Citation5, Citation22, Citation46, Citation78, Citation91, Citation92, Citation112]. Available short and long-term studies in healthy and diseased populations, from infants to the elderly, at dosages ranging from 0.3 to 0.8 g/kg/day for up to 5 years have consistently shown that creatine supplementation poses no adverse health risks and may provide a number of health and performance benefits. Additionally, assessments of adverse event reports related to dietary supplementation, including in pediatric populations, have revealed that creatine was rarely mentioned and was not associated with any significant number or any consistent pattern of adverse events [Citation238–Citation240]. Unsubstantiated anecdotal claims described in the popular media as well as rare case reports described in the literature without rigorous, systematic causality assessments have been refuted in numerous well-controlled clinical studies showing that creatine supplementation does not increase the incidence of musculoskeletal injuries [Citation22, Citation111, Citation112, Citation241], dehydration [Citation111, Citation112, Citation117, Citation122, Citation127–Citation129, Citation242], muscle cramping [Citation76, Citation106, Citation111, Citation112, Citation117], or gastrointestinal upset [Citation22, Citation111, Citation112, Citation241]. Nor has the literature provided any support that creatine promotes renal dysfunction [Citation22, Citation51, Citation85, Citation114, Citation156, Citation172, Citation243–Citation248] or has long-term detrimental effects [Citation22, Citation23, Citation53, Citation155, Citation172]. Rather, as noted above, creatine monohydrate supplementation has been found to reduce the incidence of many of these anecdotally reported side effects.
With regard to the question of whether creatine has effect on renal function, a few case studies [Citation249–Citation252] reported that individuals purportedly taking creatine with or without other supplements presented with high creatinine levels and/or renal dysfunction [Citation249–Citation251]. Additionally, one study suggested that feeding rats with renal cystic disease 2 g/kg/d of creatine for 1 week (equivalent to 140 g/day for a 70 kg individual) and 0.4 g/kg/d (equivalent to 28 g/day for a 70 kg individual) for 4 weeks exacerbated disease progression. These reports prompted some concern that creatine supplementation may impair renal function [Citation253–Citation256] and prompted a number of researchers to examine the impact of creatine supplementation on renal function [Citation22, Citation51, Citation85, Citation114, Citation156, Citation172, Citation243–Citation248, Citation257–Citation259]. For example, Ferreira and associates [Citation260] reported that creatine feeding (2 g/kg/d for 10 weeks equivalent to 140 g/kg/d in a 70 kg person) had no effects on glomerular filtration rate and renal plasma flow in Wistar rats. Likewise, Baracho and colleagues [Citation261] reported that Wistar rats fed 0, 0.5, 1, or 2 g/kg/d of creatine did not result in renal and/or hepatic toxicity. Poortmans and coworkers reported that ingesting 20 g/day of creatine for 5 days [Citation243], and up to 10 g/day from 10 months to 5 years [Citation257] had no effect on creatine clearance, glomerular filtration rate, tubular resorption, or glomerular membrane permeability compared to controls. Kreider et al. [Citation22] reported that creatine supplementation (5–10 g/day for 21 months) had no significant effects on creatinine or creatinine clearance in American football players. Gualono and associates [Citation262] reported that 12 weeks of creatine supplementation had no effects on kidney function in type 2 diabetic patients. Finally, creatine supplement has been used as a means of reducing homocysteine levels and/or improving patient outcomes in patients with renal disease [Citation263–Citation265] as well as ameliorating birth asphyxia related renal dysfunction in mice [Citation228]. Moreover, long-term, high dose ingestion of creatine (up to 30 g/d for up to 5 years) in patient populations has not been associated with an increased incidence of renal dysfunction [Citation23, Citation155, Citation156, Citation172]. While some have suggested that individuals with pre-existing renal disease consult with their physician prior to creatine supplementation in an abundance of caution, these studies and others have led researchers to conclude that there is no compelling evidence that creatine supplementation negatively affects renal function in healthy or clinical populations [Citation5, Citation6, Citation22, Citation53, Citation259, Citation266, Citation267].
Performance-related studies in adolescents, younger individuals, and older populations have consistently reported ergogenic benefits with no clinically significant side effects [Citation5, Citation6, Citation22, Citation23, Citation53, Citation113, Citation129, Citation244, Citation245, Citation268]. The breadth and repetition of these findings provide compelling evidence that creatine monohydrate is well-tolerated and is safe to consume in healthy untrained and trained individuals regardless of age. Moreover, as noted above, the number of potential medical uses of creatine supplementation that can improve health and well-being as one ages and/or may provide therapeutic benefit in clinical populations ranging from infants to senior adults has continued to grow without identifying significant risks or adverse events even in these diseased or compromised special populations. It is no wonder that Wallimann and colleagues [Citation27] recommended that individuals should consume 3 g/day of creatine throughout the lifespan to promote general health.
Some critics of creatine supplementation have pointed to warnings listed on some product labels that individuals younger than 18 years of age should not take creatine as evidence that creatine supplementation is unsafe in younger populations. It’s important to understand that this is a legal precaution and that there is no scientific evidence that children and/or adolescents should not take creatine. As noted above, a number of short- and long-term studies using relatively high doses of creatine have been conducted in infants, toddlers and adolescents with some health and/or ergogenic benefit observed. These studies provide no evidence that use of creatine at recommended doses pose a health risk to individuals less than 18 years of age. Creatine supplementation may, however, improve training adaptations and/or reduce risk to injury, including in younger athletes. For this reason, it is our view that creatine supplementation is an acceptable nutritional strategy for younger athletes who: a.) are involved in serious/competitive supervised training; b.) are consuming a well-balanced and performance enhancing diet; c.) are knowledgeable about appropriate use of creatine; and d.) do not exceed recommended dosages.
Position of the international society of sports nutrition (ISSN)
After reviewing the scientific and medical literature in this area, the International Society of Sports Nutrition concludes the following in terms of creatine supplementation as the official Position of the Society:
Creatine monohydrate is the most effective ergogenic nutritional supplement currently available to athletes with the intent of increasing high-intensity exercise capacity and lean body mass during training.
Creatine monohydrate supplementation is not only safe, but has been reported to have a number of therapeutic benefits in healthy and diseased populations ranging from infants to the elderly. There is no compelling scientific evidence that the short- or long-term use of creatine monohydrate (up to 30 g/day for 5 years) has any detrimental effects on otherwise healthy individuals or among clinical populations who may benefit from creatine supplementation.
If proper precautions and supervision are provided, creatine monohydrate supplementation in children and adolescent athletes is acceptable and may provide a nutritional alternative with a favorable safety profile to potentially dangerous anabolic androgenic drugs. However, we recommend that creatine supplementation only be considered for use by younger athletes who: a.) are involved in serious/competitive supervised training; b.) are consuming a well-balanced and performance enhancing diet; c.) are knowledgeable about appropriate use of creatine; and d.) do not exceed recommended dosages.
Label advisories on creatine products that caution against usage by those under 18 years old, while perhaps intended to insulate their manufacturers from legal liability, are likely unnecessary given the science supporting creatine’s safety, including in children and adolescents.
At present, creatine monohydrate is the most extensively studied and clinically effective form of creatine for use in nutritional supplements in terms of muscle uptake and ability to increase high-intensity exercise capacity.
The addition of carbohydrate or carbohydrate and protein to a creatine supplement appears to increase muscular uptake of creatine, although the effect on performance measures may not be greater than using creatine monohydrate alone.
The quickest method of increasing muscle creatine stores may be to consume ~0.3 g/kg/day of creatine monohydrate for 5–7-days followed by 3–5 g/day thereafter to maintain elevated stores. Initially, ingesting smaller amounts of creatine monohydrate (e.g., 3–5 g/day) will increase muscle creatine stores over a 3–4 week period, however, the initial performance effects of this method of supplementation are less supported.
Clinical populations have been supplemented with high levels of creatine monohydrate (0.3 – 0.8 g/kg/day equivalent to 21–56 g/day for a 70 kg individual) for years with no clinically significant or serious adverse events.
Further research is warranted to examine the potential medical benefits of creatine monohydrate and precursors like guanidinoacetic acid on sport, health and medicine.
Conclusion
Creatine monohydrate remains one of the few nutritional supplements for which research has consistently shown has ergogenic benefits. Additionally, a number of potential health benefits have been reported from creatine supplementation. Comments and public policy related to creatine supplementation should be based on careful assessment of the scientific evidence from well-controlled clinical trials; not unsubstantiated anecdotal reports, misinformation published on the Internet, and/or poorly designed surveys that only perpetuate myths about creatine supplementation. Given all the known benefits and favorable safety profile of creatine supplementation reported in the scientific and medical literature, it is the view of ISSN that government legislatures and sport organizations who restrict and/or discourage use of creatine may be placing athletes at greater risk—particularly in contact sports that have risk of head trauma and/or neurological injury thereby opening themselves up to legal liability. This includes children and adolescent athletes engaged in sport events that place them at risk for head and/or spinal cord injury.
Acknowledgements
We would like to thank all of the participants and researchers who contributed to the research studies and reviews described in this position stand. Your dedication to conducing groundbreaking research has improved the health and well-being of countless athletes and patients.
Prepared as a Position Stand on behalf of the International Society of Sport Nutrition with approval of Editors-In-Chief, Founders, and Research Committee Members.
Funding
Support to prepare this manuscript was provided by the Council for Responsible Nutrition.
Availability of data and materials
Not applicable.
Authors’ contributions
RBK prepared the manuscript. Remaining coauthors reviewed, edited, and approved the final manuscript. The manuscript was then approved by the Research Committee and Editors-In Chief to represent the official position of the International Society of Sports Nutrition.
Competing interests
RBK is a co-founder of the International Society of Sports Nutrition (ISSN) and has received externally-funded grants from industry to conduct research on creatine, serves as a scientific and legal consultant, and is a university approved scientific advisor for Nutrabolt. He prepared this position stand update at the request of the Council for Responsible Nutrition and ISSN. DSK is a co-founder of the ISSN who works for a contract research organization (QPS). QPS has received research grants from companies who sell creatine. DSK sits in an advisory board (Post Holdings) to Dymatize that sells creatine. DSK declares no other conflicts of interest. JA is the CEO and co-founder of the ISSN; has consulted in the past for various sports nutrition brands. TNZ has received grants and contracts to conduct research on dietary supplements; has served as a paid consultant for industry; has received honoraria for speaking at conferences and writing lay articles about sports nutrition ingredients; receives royalties from the sale of several sports nutrition products; and has served as an expert witness on behalf of the plaintiff and defense in cases involving dietary supplements. TNZ is also co-inventor on multiple patent applications within the field of dietary supplements, applied nutrition and bioactive compounds. RW is the Chief Science Officer for Post Active Nutrition. ALA is CEO of Vitargo Global Sciences, Inc., a company that markets and sells a high insulinemic, starch-based carbohydrate. HLL has received research grants from companies who sell creatine and do business in the dietary supplement, natural products and medical foods industry. HLL is co-founder of Supplement Safety Solutions, LLC, serving as an independent consultant for regulatory compliance, safety surveillance and Nutravigilance to companies who sell creatine. Dr. Lopez is also co-inventor on multiple patent applications within the field of dietary supplements, applied nutrition and bioactive compounds. Remaining investigators have no competing interests to declare. The comments and positions taken in this paper do not constitute an endorsement by institution’s the authors are affiliated.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This paper was reviewed by the International Society of Sports Nutrition Research Committee and represents the official position of the Society.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- BertinM et al Origin of the genes for the isoforms of creatine kinaseGene2007392 1–2 273 282 1:CAS:528:DC%2BD2sXjs1Cgt70%3D 17329042 10.1016/j.gene.2007.01.007
- SuzukiT et al Evolution and divergence of the genes for cytoplasmic, mitochondrial, and flagellar creatine kinasesJ Mol Evol200459 2 218 226 1:CAS:528:DC%2BD2cXntlCkurY%3D 15486695 10.1007/s00239-004-2615-x
- SahlinKHarrisRCThe creatine kinase reaction: a simple reaction with functional complexityAmino Acids201140 5 1363 1367 1:CAS:528:DC%2BC3MXkvFWqt78%3D 21394603 10.1007/s00726-011-0856-8
- HarrisRCreatine in health, medicine and sport: an introduction to a meeting held at Downing College, University of Cambridge, July 2010Amino Acids201140 5 1267 1270 1:CAS:528:DC%2BC3MXkvFWqt7Y%3D 21509489 10.1007/s00726-011-0913-3
- BufordTW et al International Society of Sports Nutrition position stand: creatine supplementation and exerciseJ Int Soc Sports Nutr20074 6 17908288 2048496 10.1186/1550-2783-4-6
- KreiderRBJungYPCreatine supplementation in exercise, sport, and medicineJ Exerc Nutr Biochem201115 2 53 69 10.5717/jenb.2011.15.2.53
- HultmanE et al Muscle creatine loading in menJ Appl Physiol (1985)199681 1 232 237 1:CAS:528:DyaK28XkvFemtL0%3D
- GreenAL et al Carbohydrate ingestion augments skeletal muscle creatine accumulation during creatine supplementation in humansAm J Physiol1996271 5 Pt 1 E821 E826 1:CAS:528:DyaK28XntFyksr4%3D 8944667
- BalsomPDSoderlundKEkblomBCreatine in humans with special reference to creatine supplementationSports Med199418 4 268 280 1:STN:280:DyaK2M7hs1Ortg%3D%3D 7817065 10.2165/00007256-199418040-00005
- HarrisRCSoderlundKHultmanEElevation of creatine in resting and exercised muscle of normal subjects by creatine supplementationClin Sci (Lond)199283 3 367 374 1:CAS:528:DyaK3sXlvVKjtQ%3D%3D 10.1042/cs0830367
- BrosnanMEBrosnanJTThe role of dietary creatineAmino Acids201648 8 1785 1791 1:CAS:528:DC%2BC28XisFOgtbk%3D 26874700 10.1007/s00726-016-2188-1
- Paddon-JonesDBorsheimEWolfeRRPotential ergogenic effects of arginine and creatine supplementationJ Nutr2004134 10 Suppl 2888S 2894S 1:CAS:528:DC%2BD2cXosVOjsb4%3D 15465806 discussion 2895S
- BraissantO et al Creatine deficiency syndromes and the importance of creatine synthesis in the brainAmino Acids201140 5 1315 1324 1:CAS:528:DC%2BC3MXkvFWqtrc%3D 21390529 10.1007/s00726-011-0852-z
- WyssM et al Creatine and creatine kinase in health and disease--a bright future ahead?Subcell Biochem200746 309 334 18652084 10.1007/978-1-4020-6486-9_16
- BraissantO et al Dissociation of AGAT, GAMT and SLC6A8 in CNS: relevance to creatine deficiency syndromesNeurobiol Dis201037 2 423 433 1:CAS:528:DC%2BC3cXos1Gg 19879361 10.1016/j.nbd.2009.10.022
- BeardEBraissantOSynthesis and transport of creatine in the CNS: importance for cerebral functionsJ Neurochem2010115 2 297 313 1:CAS:528:DC%2BC3cXhtleqtr3E 20796169 10.1111/j.1471-4159.2010.06935.x
- Sykut-CegielskaJ et al Biochemical and clinical characteristics of creatine deficiency syndromesActa Biochim Pol200451 4 875 882 1:CAS:528:DC%2BD2MXisFSntbc%3D 15625559
- GanesanV et al Guanidinoacetate methyltransferase deficiency: new clinical featuresPediatr Neurol199717 2 155 157 1:STN:280:DyaK1c%2FjtlWhsg%3D%3D 9367297 10.1016/S0887-8994(97)00083-0
- Hanna-El-DaherLBraissantOCreatine synthesis and exchanges between brain cells: what can be learned from human creatine deficiencies and various experimental models?Amino Acids201648 8 1877 1895 1:CAS:528:DC%2BC28XisVakt70%3D 26861125 10.1007/s00726-016-2189-0
- BentonDDonohoeRThe influence of creatine supplementation on the cognitive functioning of vegetarians and omnivoresBr J Nutr2011105 7 1100 1105 1:CAS:528:DC%2BC3MXivFyntrk%3D 21118604 10.1017/S0007114510004733
- BurkeDG et al Effect of creatine and weight training on muscle creatine and performance in vegetariansMed Sci Sports Exerc200335 11 1946 1955 1:CAS:528:DC%2BD3sXoslWltbk%3D 14600563 10.1249/01.MSS.0000093614.17517.79
- KreiderRB et al Long-term creatine supplementation does not significantly affect clinical markers of health in athletesMol Cell Biochem2003244 1–2 95 104 1:CAS:528:DC%2BD3sXht12jsb8%3D 12701816 10.1023/A:1022469320296
- BenderAKlopstockTCreatine for neuroprotection in neurodegenerative disease: end of story?Amino Acids201648 8 1929 1940 1:CAS:528:DC%2BC28XmslKrug%3D%3D 26748651 10.1007/s00726-015-2165-0
- SchlattnerU et al Cellular compartmentation of energy metabolism: creatine kinase microcompartments and recruitment of B-type creatine kinase to specific subcellular sitesAmino Acids201648 8 1751 1774 1:CAS:528:DC%2BC28XhtVSktrzI 27318991 10.1007/s00726-016-2267-3
- YdforsM et al Modelling in vivo creatine/phosphocreatine in vitro reveals divergent adaptations in human muscle mitochondrial respiratory control by ADP after acute and chronic exerciseJ Physiol2016594 11 3127 3140 1:CAS:528:DC%2BC28XitVWju7s%3D 26631938 4887669 10.1113/JP271259
- WallimannTSchlosserTEppenbergerHMFunction of M-line-bound creatine kinase as intramyofibrillar ATP regenerator at the receiving end of the phosphorylcreatine shuttle in muscleJ Biol Chem1984259 8 5238 5246 1:CAS:528:DyaL2cXitVGisLs%3D 6143755
- WallimannTTokarska-SchlattnerMSchlattnerUThe creatine kinase system and pleiotropic effects of creatineAmino Acids201140 5 1271 1296 1:CAS:528:DC%2BC3MXkvFWqt7k%3D 21448658 3080659 10.1007/s00726-011-0877-3
- WallimannT et al Some new aspects of creatine kinase (CK): compartmentation, structure, function and regulation for cellular and mitochondrial bioenergetics and physiologyBiofactors19988 3–4 229 234 1:CAS:528:DyaK1MXltFWjtA%3D%3D 9914824 10.1002/biof.5520080310
- TarnopolskyMA et al Creatine transporter and mitochondrial creatine kinase protein content in myopathiesMuscle Nerve200124 5 682 688 1:CAS:528:DC%2BD3MXjvVCnsL8%3D 11317279 10.1002/mus.1055
- SantacruzLJacobsDOStructural correlates of the creatine transporter function regulation: the undiscovered countryAmino Acids201648 8 2049 2055 1:CAS:528:DC%2BC28XjvVGmtrs%3D 26951207 10.1007/s00726-016-2206-3
- BraissantOCreatine and guanidinoacetate transport at blood–brain and blood-cerebrospinal fluid barriersJ Inherit Metab Dis201235 4 655 664 1:CAS:528:DC%2BC38XpvVyrsrw%3D 22252611 10.1007/s10545-011-9433-2
- Campos-FerrazPL et al Exploratory studies of the potential anti-cancer effects of creatineAmino Acids201648 8 1993 2001 1:CAS:528:DC%2BC28XisFOnsb0%3D 26872655 10.1007/s00726-016-2180-9
- BalestrinoM et al Potential of creatine or phosphocreatine supplementation in cerebrovascular disease and in ischemic heart diseaseAmino Acids201648 8 1955 1967 1:CAS:528:DC%2BC28Xhtlaitbg%3D 26795537 10.1007/s00726-016-2173-8
- SaraivaAL et al Creatine reduces oxidative stress markers but does not protect against seizure susceptibility after severe traumatic brain injuryBrain Res Bull201287 2–3 180 186 1:CAS:528:DC%2BC38XitVequrc%3D 22051612 10.1016/j.brainresbull.2011.10.010
- RahimiRCreatine supplementation decreases oxidative DNA damage and lipid peroxidation induced by a single bout of resistance exerciseJ Strength Cond Res201125 12 3448 3455 22080314 10.1519/JSC.0b013e3182162f2b
- RiesbergLA et al Beyond muscles: the untapped potential of creatineInt Immunopharmacol201637 31 42 1:CAS:528:DC%2BC28XlvVOhtA%3D%3D 26778152 10.1016/j.intimp.2015.12.034
- CandowDGChilibeckPDForbesSCCreatine supplementation and aging musculoskeletal healthEndocrine201445 3 354 361 1:CAS:528:DC%2BC3sXhslWltbbN 24190049 10.1007/s12020-013-0070-4
- TarnopolskyMAClinical use of creatine in neuromuscular and neurometabolic disordersSubcell Biochem200746 183 204 18652078 10.1007/978-1-4020-6486-9_10
- KleyRATarnopolskyMAVorgerdMCreatine for treating muscle disordersCochrane Database Syst Rev20112 CD004760
- TarnopolskyMAPotential benefits of creatine monohydrate supplementation in the elderlyCurr Opin Clin Nutr Metab Care20003 6 497 502 1:STN:280:DC%2BD3M7ivFersA%3D%3D 11085837 10.1097/00075197-200011000-00013
- CandowDG et al Strategic creatine supplementation and resistance training in healthy older adultsAppl Physiol Nutr Metab201540 7 689 694 1:CAS:528:DC%2BC2MXoslOms7k%3D 25993883 10.1139/apnm-2014-0498
- MoonA et al Creatine supplementation: can it improve quality of life in the elderly without associated resistance training?Curr Aging Sci20136 3 251 257 1:CAS:528:DC%2BC2cXlt1Wjsg%3D%3D 24304199 10.2174/1874609806666131204153102
- RawsonESVeneziaACUse of creatine in the elderly and evidence for effects on cognitive function in young and oldAmino Acids201140 5 1349 1362 1:CAS:528:DC%2BC3MXkvFWqt74%3D 21394604 10.1007/s00726-011-0855-9
- CandowDGSarcopenia: current theories and the potential beneficial effect of creatine application strategiesBiogerontology201112 4 273 281 1:CAS:528:DC%2BC3MXptVykt7o%3D 21373890 10.1007/s10522-011-9327-6
- CandowDGChilibeckPDPotential of creatine supplementation for improving aging bone healthJ Nutr Health Aging201014 2 149 153 1:CAS:528:DC%2BC3cXlt1Sitrc%3D 20126964 10.1007/s12603-009-0224-5
- KreiderRBEffects of creatine supplementation on performance and training adaptationsMol Cell Biochem2003244 1–2 89 94 1:CAS:528:DC%2BD3sXht12jsb4%3D 12701815 10.1023/A:1022465203458
- CaseyA et al Creatine ingestion favorably affects performance and muscle metabolism during maximal exercise in humansAm J Physiol1996271 1 Pt 1 E31 E37 1:CAS:528:DyaK28XkslygtLs%3D 8760078
- GreenhaffPL et al Influence of oral creatine supplementation of muscle torque during repeated bouts of maximal voluntary exercise in manClin Sci (Lond)199384 5 565 571 1:CAS:528:DyaK3sXkvFCgtrk%3D 10.1042/cs0840565
- SteengeGRSimpsonEJGreenhaffPLProtein- and carbohydrate-induced augmentation of whole body creatine retention in humansJ Appl Physiol (1985)200089 3 1165 1171 1:CAS:528:DC%2BD3cXntV2jtLs%3D
- GreenwoodM et al Differences in creatine retention among three nutritional formulations of oral creatine supplementsJ Exerc Physiol Online20036 2 37 43
- VandenbergheK et al Long-term creatine intake is beneficial to muscle performance during resistance trainingJ Appl Physiol (1985)199783 6 2055 2063 1:CAS:528:DyaK1cXosVCr
- KimHJ et al Studies on the safety of creatine supplementationAmino Acids201140 5 1409 1418 1:CAS:528:DC%2BC3MXkvFWqt7w%3D 21399917 10.1007/s00726-011-0878-2
- JagerR et al Analysis of the efficacy, safety, and regulatory status of novel forms of creatineAmino Acids201140 5 1369 1383 21424716 3080578 10.1007/s00726-011-0874-6 1:CAS:528:DC%2BC3MXkvFWqt7s%3D
- Howard AN, Harris RC. Compositions containing creatine, U.S.P. Office, Editor. United States: United States Patent Office, United States Government; 1999.
- EdgarGShiverHEThe equilibrium between creatine and creatinine, in aqueous solution: the effect of hydrogen ionJ Am Chem Soc192547 1179 1188 1:CAS:528:DyaB2MXhtFOqsw%3D%3D 10.1021/ja01681a040
- DeldicqueL et al Kinetics of creatine ingested as a food ingredientEur J Appl Physiol2008102 2 133 143 1:CAS:528:DC%2BD2sXhtlSmu7vN 17851680 10.1007/s00421-007-0558-9
- PerskyAMBrazeauGAHochhausGPharmacokinetics of the dietary supplement creatineClin Pharmacokinet200342 6 557 574 1:CAS:528:DC%2BD3sXmtVGmurk%3D 12793840 10.2165/00003088-200342060-00005
- KreiderRB et al Effects of serum creatine supplementation on muscle creatine contentJ Exerc Physiologyonline20036 4 24 33
- SpillaneM et al The effects of creatine ethyl ester supplementation combined with heavy resistance training on body composition, muscle performance, and serum and muscle creatine levelsJ Int Soc Sports Nutr20096 6 19228401 2649889 10.1186/1550-2783-6-6 1:CAS:528:DC%2BC3cXksVKitb0%3D
- JagimAR et al A buffered form of creatine does not promote greater changes in muscle creatine content, body composition, or training adaptations than creatine monohydrateJ Int Soc Sports Nutr20129 1 43 1:CAS:528:DC%2BC38XhvVSiur%2FL 22971354 3479057 10.1186/1550-2783-9-43
- GalvanE et al Acute and chronic safety and efficacy of dose dependent creatine nitrate supplementation and exercise performanceJ Int Soc Sports Nutr201613 12 27034623 4815124 10.1186/s12970-016-0124-0 1:CAS:528:DC%2BC2sXis1ejt7s%3D
- CornishSMChilibeckPDBurkeDGThe effect of creatine monohydrate supplementation on sprint skating in ice-hockey playersJ Sports Med Phys Fitness200646 1 90 98 1:CAS:528:DC%2BD28XmtFelurk%3D 16596105
- DawsonBVladichTBlanksbyBAEffects of 4 weeks of creatine supplementation in junior swimmers on freestyle sprint and swim bench performanceJ Strength Cond Res200216 4 485 490 12423175
- GrindstaffPD et al Effects of creatine supplementation on repetitive sprint performance and body composition in competitive swimmersInt J Sport Nutr19977 4 330 346 1:CAS:528:DyaK1cXpsFGn 9407259 10.1123/ijsn.7.4.330
- JuhaszI et al Creatine supplementation improves the anaerobic performance of elite junior fin swimmersActa Physiol Hung200996 3 325 336 1:CAS:528:DC%2BC3cXjt1Wntbo%3D 19706374 10.1556/APhysiol.96.2009.3.6
- SilvaAJ et al Effect of creatine on swimming velocity, body composition and hydrodynamic variablesJ Sports Med Phys Fitness200747 1 58 64 1:CAS:528:DC%2BD2sXmtleksbs%3D 17369799
- KreiderRB et al Effects of creatine supplementation on body composition, strength, and sprint performanceMed Sci Sports Exerc199830 1 73 82 1:CAS:528:DyaK1cXhtVajsbg%3D 9475647 10.1097/00005768-199801000-00011
- StoneMH et al Effects of in-season (5 weeks) creatine and pyruvate supplementation on anaerobic performance and body composition in American football playersInt J Sport Nutr19999 2 146 165 1:CAS:528:DyaK1MXjvFKht7o%3D 10362452 10.1123/ijsn.9.2.146
- BembenMG et al Creatine supplementation during resistance training in college football athletesMed Sci Sports Exerc200133 10 1667 1673 1:CAS:528:DC%2BD3MXnvVCksL4%3D 11581550 10.1097/00005768-200110000-00009
- HoffmanJ et al Effect of creatine and beta-alanine supplementation on performance and endocrine responses in strength/power athletesInt J Sport Nutr Exerc Metab200616 4 430 446 1:CAS:528:DC%2BD2sXlvFylsbo%3D 17136944 10.1123/ijsnem.16.4.430
- ChilibeckPDMagnusCAndersonMEffect of in-season creatine supplementation on body composition and performance in rugby union football playersAppl Physiol Nutr Metab200732 6 1052 1057 18059577 10.1139/H07-072
- ClaudinoJG et al Creatine monohydrate supplementation on lower-limb muscle power in Brazilian elite soccer playersJ Int Soc Sports Nutr201411 32 24991195 4077550 10.1186/1550-2783-11-32
- KerksickCM et al Impact of differing protein sources and a creatine containing nutritional formula after 12 weeks of resistance trainingNutrition200723 9 647 656 1:CAS:528:DC%2BD2sXosF2it7Y%3D 17679046 10.1016/j.nut.2007.06.015
- KerksickCM et al The effects of creatine monohydrate supplementation with and without D-pinitol on resistance training adaptationsJ Strength Cond Res200923 9 2673 2682 19858753 10.1519/JSC.0b013e3181b3e0de
- VolekJS et al Creatine supplementation enhances muscular performance during high-intensity resistance exerciseJ Am Diet Assoc199797 7 765 770 1:CAS:528:DyaK2sXkslCqsbg%3D 9216554 10.1016/S0002-8223(97)00189-2
- VolekJS et al Physiological responses to short-term exercise in the heat after creatine loadingMed Sci Sports Exerc200133 7 1101 1108 1:CAS:528:DC%2BD3MXlsFSqsb0%3D 11445756 10.1097/00005768-200107000-00006
- VolekJS et al The effects of creatine supplementation on muscular performance and body composition responses to short-term resistance training overreachingEur J Appl Physiol200491 5–6 628 637 1:CAS:528:DC%2BD2cXjtFyis7Y%3D 14685870 10.1007/s00421-003-1031-z
- KreiderRB et al ISSN exercise & sport nutrition review: research & recommendationsJ Int Soc Sports Nutr20107 7 20181066 2853497 10.1186/1550-2783-7-7 1:CAS:528:DC%2BC3cXksFentr4%3D
- BranchJDEffect of creatine supplementation on body composition and performance: a meta-analysisInt J Sport Nutr Exerc Metab200313 2 198 226 1:CAS:528:DC%2BD3sXlsVemtrc%3D 12945830 10.1123/ijsnem.13.2.198
- DevriesMCPhillipsSMCreatine supplementation during resistance training in older adults-a meta-analysisMed Sci Sports Exerc201446 6 1194 1203 1:CAS:528:DC%2BC2cXotlKqt7c%3D 24576864 10.1249/MSS.0000000000000220
- LanhersC et al Creatine supplementation and lower limb strength performance: a systematic review and meta-analysesSports Med201545 9 1285 1294 25946994 10.1007/s40279-015-0337-4
- WirothJB et al Effects of oral creatine supplementation on maximal pedalling performance in older adultsEur J Appl Physiol200184 6 533 539 1:CAS:528:DC%2BD3MXktlaltbg%3D 11482548 10.1007/s004210000370
- McMorrisT et al Creatine supplementation and cognitive performance in elderly individualsNeuropsychol Dev Cogn B Aging Neuropsychol Cogn200714 5 517 528 17828627 10.1080/13825580600788100
- RawsonESClarksonPMAcute creatine supplementation in older menInt J Sports Med200021 1 71 75 1:CAS:528:DC%2BD3cXotlemsg%3D%3D 10683103 10.1055/s-2000-8859
- AguiarAF et al Long-term creatine supplementation improves muscular performance during resistance training in older womenEur J Appl Physiol2013113 4 987 996 1:CAS:528:DC%2BC3sXktlKhs70%3D 23053133 10.1007/s00421-012-2514-6
- TarnopolskyMAMacLennanDPCreatine monohydrate supplementation enhances high-intensity exercise performance in males and femalesInt J Sport Nutr Exerc Metab200010 4 452 463 1:CAS:528:DC%2BD3MXkvF2hsA%3D%3D 11099372 10.1123/ijsnem.10.4.452
- ZiegenfussTN et al Effect of creatine loading on anaerobic performance and skeletal muscle volume in NCAA division I athletesNutrition200218 5 397 402 1:CAS:528:DC%2BD38Xjt1Witr0%3D 11985944 10.1016/S0899-9007(01)00802-4
- AyoamaRHirumaESasakiHEffects of creatine loading on muscular strength and endurance of female softball playersJ Sports Med Phys Fitness200343 4 481 487 1:CAS:528:DC%2BD2cXivVemtrk%3D 14767409
- JohannsmeyerS et al Effect of creatine supplementation and drop-set resistance training in untrained aging adultsExp Gerontol201683 112 119 1:CAS:528:DC%2BC28XhtlKqs7rP 27523919 10.1016/j.exger.2016.08.005
- Ramirez-CampilloR et al Effects of plyometric training and creatine supplementation on maximal-intensity exercise and endurance in female soccer playersJ Sci Med Sport201619 8 682 687 26778661 10.1016/j.jsams.2015.10.005
- RodriguezNR et al Position of the American Dietetic Association, dietitians of Canada, and the American college of sports medicine: nutrition and athletic performanceJ Am Diet Assoc2009109 3 509 527 19278045 10.1016/j.jada.2009.01.005 1:CAS:528:DC%2BD1MXnvVWqsbk%3D
- ThomasDTErdmanKABurkeLMPosition of the academy of nutrition and dietetics, dietitians of Canada, and the American college of sports medicine: nutrition and athletic performanceJ Acad Nutr Diet2016116 3 501 528 26920240 10.1016/j.jand.2015.12.006
- FraczekB et al Prevalence of the use of effective ergogenic aids among professional athletesRocz Panstw Zakl Hig201667 3 271 278 27546324
- BrownDWyonMAn international study on dietary supplementation use in dancersMed Probl Perform Art201429 4 229 234 25433260
- McGuineTASullivanJCBernhardtDTCreatine supplementation in high school football playersClin J Sport Med200111 4 247 253 1:STN:280:DC%2BD38%2FgtVWltQ%3D%3D 11753062 10.1097/00042752-200110000-00007
- MasonMA et al Use of nutritional supplements by high school football and volleyball playersIowa Orthop J200121 43 48 1:STN:280:DC%2BD38%2FoslSjtQ%3D%3D 11813950 1888195
- LaBotzMSmithBWCreatine supplement use in an NCAA division I athletic programClin J Sport Med19999 3 167 169 1:STN:280:DyaK1MvktVyqug%3D%3D 10512346 10.1097/00042752-199907000-00009
- SheppardHL et al Use of creatine and other supplements by members of civilian and military health clubs: a cross-sectional surveyInt J Sport Nutr Exerc Metab200010 3 245 259 1:CAS:528:DC%2BD3cXntlyksro%3D 10997951 10.1123/ijsnem.10.3.245
- KnapikJJ et al Prevalence of dietary supplement use by athletes: systematic review and meta-analysisSports Med201646 1 103 123 26442916 10.1007/s40279-015-0387-7
- CaseyA et al Supplement use by UK-based British army soldiers in trainingBr J Nutr2014112 7 1175 1184 1:CAS:528:DC%2BC2cXhs12ku7fI 25119518 4189117 10.1017/S0007114514001597
- HuangSHJohnsonKPipeALThe use of dietary supplements and medications by Canadian athletes at the Atlanta and Sydney olympic gamesClin J Sport Med200616 1 27 33 16377972 10.1097/01.jsm.0000194766.35443.9c
- ScofieldDEUnruhSDietary supplement use among adolescent athletes in central Nebraska and their sources of informationJ Strength Cond Res200620 2 452 455 16686580
- NCAA National Study of Substance Use Habits of College Student-Athletes. 2014. [cited 2017 March 5, 2017]; Available from: http://www.ncaa.org/sites/default/files/Substance%20Use%20Final%20Report_FINAL.pdf. Accessed 22 Apr 2015.
- NelsonAG et al Muscle glycogen supercompensation is enhanced by prior creatine supplementationMed Sci Sports Exerc200133 7 1096 1100 1:CAS:528:DC%2BD3MXlsFSqsbw%3D 11445755 10.1097/00005768-200107000-00005
- CookeMB et al Creatine supplementation enhances muscle force recovery after eccentrically-induced muscle damage in healthy individualsJ Int Soc Sports Nutr20096 13 19490606 2697134 10.1186/1550-2783-6-13 1:CAS:528:DC%2BC3cXksVKiur8%3D
- SantosRV et al The effect of creatine supplementation upon inflammatory and muscle soreness markers after a 30 km raceLife Sci200475 16 1917 1924 1:CAS:528:DC%2BD2cXmsleqs7c%3D 15306159 10.1016/j.lfs.2003.11.036
- DeminiceR et al Effects of creatine supplementation on oxidative stress and inflammatory markers after repeated-sprint exercise in humansNutrition201329 9 1127 1132 1:CAS:528:DC%2BC3sXhtVSmu7nL 23800565 10.1016/j.nut.2013.03.003
- KreiderRB et al Effects of ingesting supplements designed to promote lean tissue accretion on body composition during resistance trainingInt J Sport Nutr19966 3 234 246 1:CAS:528:DyaK28XmtFWisLw%3D 8876343 10.1123/ijsn.6.3.234
- KreiderRB et al Effects of nutritional supplementation during off-season college football training on body composition and strengthJ Exerc Physiol Online19992 2 24 39
- EarnestCP et al The effect of creatine monohydrate ingestion on anaerobic power indices, muscular strength and body compositionActa Physiol Scand1995153 2 207 209 7778463 10.1111/j.1748-1716.1995.tb09854.x
- GreenwoodM et al Creatine supplementation during college football training does not increase the incidence of cramping or injuryMol Cell Biochem2003244 1–2 83 88 1:CAS:528:DC%2BD3sXht12jsLc%3D 12701814 10.1023/A:1022413202549
- GreenwoodM et al Cramping and injury incidence in collegiate football players Are reduced by creatine supplementationJ Athl Train200338 3 216 219 14608430 233174
- CancelaP et al Creatine supplementation does not affect clinical health markers in football playersBr J Sports Med200842 9 731 735 1:STN:280:DC%2BD1crptlyjtA%3D%3D 18780799 10.1136/bjsm.2007.030700
- SchroderHTerradosNTramullasARisk assessment of the potential side effects of long-term creatine supplementation in team sport athletesEur J Nutr200544 4 255 261 15309421 10.1007/s00394-004-0519-6 1:CAS:528:DC%2BD2MXmt1Gjsr8%3D
- RoseneJMWhitmanSAFogartyTDA comparison of thermoregulation with creatine supplementation between the sexes in a thermoneutral environmentJ Athl Train200439 1 50 55 15085212 385262
- Twycross-LewisR et al The effects of creatine supplementation on thermoregulation and physical (cognitive) performance: a review and future prospectsAmino Acids201648 8 1843 1855 1:CAS:528:DC%2BC28Xmt1Shtrg%3D 27085634 10.1007/s00726-016-2237-9
- WatsonG et al Creatine use and exercise heat tolerance in dehydrated menJ Athl Train200641 1 18 29 16619091 1421496
- WeissBAPowersMECreatine supplementation does not impair the thermoregulatory response during a bout of exercise in the heatJ Sports Med Phys Fitness200646 4 555 563 1:CAS:528:DC%2BD2sXit1yqs70%3D 17119520
- WrightGAGrandjeanPWPascoeDDThe effects of creatine loading on thermoregulation and intermittent sprint exercise performance in a hot humid environmentJ Strength Cond Res200721 3 655 660 17685723
- BeisLY et al The effects of creatine and glycerol hyperhydration on running economy in well trained endurance runnersJ Int Soc Sports Nutr20118 1 24 1:CAS:528:DC%2BC38XjvFaitrk%3D 22176668 3283512 10.1186/1550-2783-8-24
- EastonC et al The effects of a novel “fluid loading” strategy on cardiovascular and haematological responses to orthostatic stressEur J Appl Physiol2009105 6 899 908 19142656 10.1007/s00421-008-0976-3
- EastonCTurnerSPitsiladisYPCreatine and glycerol hyperhydration in trained subjects before exercise in the heatInt J Sport Nutr Exerc Metab200717 1 70 91 1:CAS:528:DC%2BD2sXjsFeks7c%3D 17460334 10.1123/ijsnem.17.1.70
- KilduffLP et al The effects of creatine supplementation on cardiovascular, metabolic, and thermoregulatory responses during exercise in the heat in endurance-trained humansInt J Sport Nutr Exerc Metab200414 4 443 460 1:CAS:528:DC%2BD2cXnvFajs70%3D 15467102 10.1123/ijsnem.14.4.443
- PolyviouTP et al Effects of glycerol and creatine hyperhydration on doping-relevant blood parametersNutrients20124 9 1171 1186 1:CAS:528:DC%2BC38XhtlOltL7J 23112907 3475229 10.3390/nu4091171
- PolyviouTP et al The effects of hyperhydrating supplements containing creatine and glucose on plasma lipids and insulin sensitivity in endurance-trained athletesJ Amino Acids20152015 352458 26167296 4488253 10.1155/2015/352458
- PolyviouTP et al Thermoregulatory and cardiovascular responses to creatine, glycerol and alpha lipoic acid in trained cyclistsJ Int Soc Sports Nutr20129 1 29 1:CAS:528:DC%2BC38XhvVSiurzL 22726625 3459729 10.1186/1550-2783-9-29
- LopezRM et al Does creatine supplementation hinder exercise heat tolerance or hydration status? a systematic review with meta-analysesJ Athl Train200944 2 215 223 19295968 2657025 10.4085/1062-6050-44.2.215
- RoseneJM et al The effects of creatine supplementation on thermoregulation and isokinetic muscular performance following acute (3-day) supplementationJ Sports Med Phys Fitness201555 12 1488 1496 1:STN:280:DC%2BC2MnksFygtA%3D%3D 25781214
- DalboVJ et al Putting to rest the myth of creatine supplementation leading to muscle cramps and dehydrationBr J Sports Med200842 7 567 573 1:STN:280:DC%2BD1cvjtVKksA%3D%3D 18184753 10.1136/bjsm.2007.042473
- HespelPDeraveWErgogenic effects of creatine in sports and rehabilitationSubcell Biochem200746 245 259 18652080 10.1021/bi061646s 1:CAS:528:DC%2BD28Xht12qtrvM
- HespelP et al Oral creatine supplementation facilitates the rehabilitation of disuse atrophy and alters the expression of muscle myogenic factors in humansJ Physiol2001536 Pt 2 625 633 1:CAS:528:DC%2BD3MXovVygsrg%3D 11600695 2278864 10.1111/j.1469-7793.2001.0625c.xd
- Op’t EijndeB et al Effect of oral creatine supplementation on human muscle GLUT4 protein content after immobilizationDiabetes200150 1 18 23 10.2337/diabetes.50.1.18
- JacobsPL et al Oral creatine supplementation enhances upper extremity work capacity in persons with cervical-level spinal cord injuryArch Phys Med Rehabil200283 1 19 23 11782827 10.1053/apmr.2002.26829
- TylerTF et al The effect of creatine supplementation on strength recovery after anterior cruciate ligament (ACL) reconstruction: a randomized, placebo-controlled, double-blind trialAm J Sports Med200432 2 383 388 14977662 10.1177/0363546503261731
- PerretCMuellerGKnechtHInfluence of creatine supplementation on 800 m wheelchair performance: a pilot studySpinal Cord200644 5 275 279 1:STN:280:DC%2BD283kslSkuw%3D%3D 16172624 10.1038/sj.sc.3101840
- KleyRAVorgerdMTarnopolskyMACreatine for treating muscle disordersCochrane Database Syst Rev20071 CD004760
- SullivanPG et al Dietary supplement creatine protects against traumatic brain injuryAnn Neurol200048 5 723 729 1:CAS:528:DC%2BD3cXosFSgt7w%3D 11079535 10.1002/1531-8249(200011)48:5<723::AID-ANA5>3.0.CO;2-W
- HausmannON et al Protective effects of oral creatine supplementation on spinal cord injury in ratsSpinal Cord200240 9 449 456 1:STN:280:DC%2BD38vivV2itg%3D%3D 12185606 10.1038/sj.sc.3101330
- PrassK et al Improved reperfusion and neuroprotection by creatine in a mouse model of strokeJ Cereb Blood Flow Metab200727 3 452 459 1:CAS:528:DC%2BD2sXjtlKitLo%3D 16773141 10.1038/sj.jcbfm.9600351
- AdcockKH et al Neuroprotection of creatine supplementation in neonatal rats with transient cerebral hypoxia-ischemiaDev Neurosci200224 5 382 388 1:CAS:528:DC%2BD3sXitFartLo%3D 12640176 10.1159/000069043
- ZhuS et al Prophylactic creatine administration mediates neuroprotection in cerebral ischemia in miceJ Neurosci200424 26 5909 5912 1:CAS:528:DC%2BD2cXlsleltLs%3D 15229238 10.1523/JNEUROSCI.1278-04.2004
- Allah YarRAkbarAIqbalFCreatine monohydrate supplementation for 10 weeks mediates neuroprotection and improves learning/memory following neonatal hypoxia ischemia encephalopathy in female albino miceBrain Res20151595 92 100 1:CAS:528:DC%2BC2cXhvF2mtL3J 25446460 10.1016/j.brainres.2014.11.017
- RabchevskyAG et al Creatine diet supplement for spinal cord injury: influences on functional recovery and tissue sparing in ratsJ Neurotrauma200320 7 659 669 12908927 10.1089/089771503322144572
- Freire RoyesLFCassolGThe effects of Creatine supplementation and physical exercise on traumatic brain injuryMini Rev Med Chem201616 1 29 39 1:CAS:528:DC%2BC2MXhvVGnsr%2FN 26202200 10.2174/1389557515666150722101926
- Stockler-IpsirogluSvan KarnebeekCDCerebral creatine deficiencies: a group of treatable intellectual developmental disordersSemin Neurol201434 3 350 356 25192512 10.1055/s-0034-1386772
- LongoN et al Disorders of creatine transport and metabolismAm J Med Genet C Semin Med Genet2011157C 1 72 78 21308988 10.1002/ajmg.c.30292 1:CAS:528:DC%2BC3MXjvFOgtrk%3D
- NasrallahFFekiMKaabachiNCreatine and creatine deficiency syndromes: biochemical and clinical aspectsPediatr Neurol201042 3 163 171 20159424 10.1016/j.pediatrneurol.2009.07.015
- Mercimek-MahmutogluS et al GAMT deficiency: features, treatment, and outcome in an inborn error of creatine synthesisNeurology200667 3 480 484 1:CAS:528:DC%2BD28Xntl2msbc%3D 16855203 10.1212/01.wnl.0000234852.43688.bf
- StrombergerCBodamerOAStockler-IpsirogluSClinical characteristics and diagnostic clues in inborn errors of creatine metabolismJ Inherit Metab Dis200326 2–3 299 308 1:CAS:528:DC%2BD3sXkvVCrsrs%3D 12889668 10.1023/A:1024453704800
- BattiniR et al Arginine:glycine amidinotransferase (AGAT) deficiency in a newborn: early treatment can prevent phenotypic expression of the diseaseJ Pediatr2006148 6 828 830 1:CAS:528:DC%2BD28XlsFGks74%3D 16769397 10.1016/j.jpeds.2006.01.043
- Stockler-IpsirogluS et al Guanidinoacetate methyltransferase (GAMT) deficiency: outcomes in 48 individuals and recommendations for diagnosis, treatment and monitoringMol Genet Metab2014111 1 16 25 1:CAS:528:DC%2BC3sXhvVKgt7rI 24268530 10.1016/j.ymgme.2013.10.018
- ValtonenM et al Central nervous system involvement in gyrate atrophy of the choroid and retina with hyperornithinaemiaJ Inherit Metab Dis199922 8 855 866 1:STN:280:DC%2BD3c%2FntFyjtw%3D%3D 10604138 10.1023/A:1005602405349
- Nanto-SalonenK et al Reduced brain creatine in gyrate atrophy of the choroid and retina with hyperornithinemiaNeurology199953 2 303 307 1:CAS:528:DyaK1MXltVKmtbo%3D 10430418 10.1212/WNL.53.2.303
- HeinanenK et al Creatine corrects muscle 31P spectrum in gyrate atrophy with hyperornithinaemiaEur J Clin Invest199929 12 1060 1065 1:CAS:528:DC%2BD3cXivFGnsA%3D%3D 10583455 10.1046/j.1365-2362.1999.00569.x
- Vannas-SulonenK et al Gyrate atrophy of the choroid and retina. A five-year follow-up of creatine supplementationOphthalmology198592 12 1719 1727 1:STN:280:DyaL287hvFOjsQ%3D%3D 4088625 10.1016/S0161-6420(85)34098-8
- SipilaI et al Supplementary creatine as a treatment for gyrate atrophy of the choroid and retinaN Engl J Med1981304 15 867 870 1:STN:280:DyaL3M7kvVKrug%3D%3D 7207523 10.1056/NEJM198104093041503
- EvangeliouA et al Clinical applications of creatine supplementation on paediatricsCurr Pharm Biotechnol200910 7 683 690 1:CAS:528:DC%2BD1MXhsVSmtL3E 19751179 10.2174/138920109789542075
- VerbruggenKT et al Global developmental delay in guanidionacetate methyltransferase deficiency: differences in formal testing and clinical observationEur J Pediatr2007166 9 921 925 1:CAS:528:DC%2BD2sXotVOmt7s%3D 17186272 10.1007/s00431-006-0340-8
- EnsenauerR et al Guanidinoacetate methyltransferase deficiency: differences of creatine uptake in human brain and muscleMol Genet Metab200482 3 208 213 1:CAS:528:DC%2BD2cXlsVSgu78%3D 15234333 10.1016/j.ymgme.2004.04.005
- OgbornDI et al Effects of creatine and exercise on skeletal muscle of FRG1-transgenic miceCan J Neurol Sci201239 2 225 231 22343158 10.1017/S0317167100013275
- LouisM et al Beneficial effects of creatine supplementation in dystrophic patientsMuscle Nerve200327 5 604 610 1:CAS:528:DC%2BD3sXktVSqtbs%3D 12707981 10.1002/mus.10355
- BanerjeeB et al Effect of creatine monohydrate in improving cellular energetics and muscle strength in ambulatory Duchenne muscular dystrophy patients: a randomized, placebo-controlled 31P MRS studyMagn Reson Imaging201028 5 698 707 1:CAS:528:DC%2BC3cXmsVejsL8%3D 20395096 10.1016/j.mri.2010.03.008
- FelberS et al Oral creatine supplementation in Duchenne muscular dystrophy: a clinical and 31P magnetic resonance spectroscopy studyNeurol Res200022 2 145 150 1:STN:280:DC%2BD3c3is1Oisg%3D%3D 10763500 10.1080/01616412.2000.11741051
- RadleyHG et al Duchenne muscular dystrophy: focus on pharmaceutical and nutritional interventionsInt J Biochem Cell Biol200739 3 469 477 1:CAS:528:DC%2BD2sXntlygsg%3D%3D 17137828 10.1016/j.biocel.2006.09.009
- TarnopolskyMA et al Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophyNeurology200462 10 1771 1777 1:CAS:528:DC%2BD2cXjs1yltbw%3D 15159476 10.1212/01.WNL.0000125178.18862.9D
- AdhihettyPJBealMFCreatine and its potential therapeutic value for targeting cellular energy impairment in neurodegenerative diseasesNeuromolecular Med200810 4 275 290 1:CAS:528:DC%2BD1MXhtlKktA%3D%3D 19005780 2886719 10.1007/s12017-008-8053-y
- VerbessemP et al Creatine supplementation in Huntington’s disease: a placebo-controlled pilot trialNeurology200361 7 925 930 1:CAS:528:DC%2BD3sXns1Chsb8%3D 14557561 10.1212/01.WNL.0000090629.40891.4B
- DedeogluA et al Creatine therapy provides neuroprotection after onset of clinical symptoms in Huntington’s disease transgenic miceJ Neurochem200385 6 1359 1367 1:CAS:528:DC%2BD3sXkslOkt70%3D 12787055 2866522 10.1046/j.1471-4159.2003.01706.x
- AndreassenOA et al Creatine increase survival and delays motor symptoms in a transgenic animal model of Huntington’s diseaseNeurobiol Dis20018 3 479 491 1:STN:280:DC%2BD3Mzps1GgsA%3D%3D 11447996 10.1006/nbdi.2001.0406
- FerranteRJ et al Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s diseaseJ Neurosci200020 12 4389 4397 1:CAS:528:DC%2BD3cXktVCkt7Y%3D 10844007
- MatthewsRT et al Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington’s diseaseJ Neurosci199818 1 156 163 1:CAS:528:DyaK1cXjtVKqtg%3D%3D 9412496
- BenderA et al Long-term creatine supplementation is safe in aged patients with Parkinson diseaseNutr Res200828 3 172 178 1:CAS:528:DC%2BD1cXksVahtL0%3D 19083405 10.1016/j.nutres.2008.01.001
- HassCJCollinsMAJuncosJLResistance training with creatine monohydrate improves upper-body strength in patients with Parkinson disease: a randomized trialNeurorehabil Neural Repair200721 2 107 115 17312085 10.1177/1545968306293449
- BenderA et al Creatine supplementation in Parkinson disease: a placebo-controlled randomized pilot trialNeurology200667 7 1262 1264 1:CAS:528:DC%2BD28XhtFyqtLfF 17030762 10.1212/01.wnl.0000238518.34389.12
- KomuraK et al Effectiveness of creatine monohydrate in mitochondrial encephalomyopathiesPediatr Neurol200328 1 53 58 12657421 10.1016/S0887-8994(02)00469-1
- TarnopolskyMAPariseGDirect measurement of high-energy phosphate compounds in patients with neuromuscular diseaseMuscle Nerve199922 9 1228 1233 1:CAS:528:DyaK1MXmtV2itbY%3D 10454718 10.1002/(SICI)1097-4598(199909)22:9<1228::AID-MUS9>3.0.CO;2-6
- TarnopolskyMARoyBDMacDonaldJRA randomized, controlled trial of creatine monohydrate in patients with mitochondrial cytopathiesMuscle Nerve199720 12 1502 1509 1:STN:280:DyaK1c%2FltFShug%3D%3D 9390662 10.1002/(SICI)1097-4598(199712)20:12<1502::AID-MUS4>3.0.CO;2-C
- AndreassenOA et al Increases in cortical glutamate concentrations in transgenic amyotrophic lateral sclerosis mice are attenuated by creatine supplementationJ Neurochem200177 2 383 390 1:CAS:528:DC%2BD3MXjtVOlur0%3D 11299300 10.1046/j.1471-4159.2001.00188.x
- ChoiJK et al Magnetic resonance spectroscopy of regional brain metabolite markers in FALS mice and the effects of dietary creatine supplementationEur J Neurosci200930 11 2143 2150 19930399 2846694 10.1111/j.1460-9568.2009.07015.x
- DeraveW et al Skeletal muscle properties in a transgenic mouse model for amyotrophic lateral sclerosis: effects of creatine treatmentNeurobiol Dis200313 3 264 272 1:CAS:528:DC%2BD3sXlvFOkt7Y%3D 12901841 10.1016/S0969-9961(03)00041-X
- DroryVEGrossDNo effect of creatine on respiratory distress in amyotrophic lateral sclerosisAmyotroph Lateral Scler Other Motor Neuron Disord20023 1 43 46 1:CAS:528:DC%2BD38XmtFWgt7s%3D 12061948 10.1080/146608202317576534
- EllisACRosenfeldJThe role of creatine in the management of amyotrophic lateral sclerosis and other neurodegenerative disordersCNS Drugs200418 14 967 980 1:CAS:528:DC%2BD2MXit1CjsA%3D%3D 15584767 10.2165/00023210-200418140-00002
- MazziniL et al Effects of creatine supplementation on exercise performance and muscular strength in amyotrophic lateral sclerosis: preliminary resultsJ Neurol Sci2001191 1–2 139 144 1:CAS:528:DC%2BD3MXnslCitbc%3D 11677005 10.1016/S0022-510X(01)00611-6
- VielhaberS et al Effect of creatine supplementation on metabolite levels in ALS motor corticesExp Neurol2001172 2 377 382 1:CAS:528:DC%2BD3MXosFGhsbg%3D 11716561 10.1006/exnr.2001.7797
- HultmanJ et al Myocardial energy restoration of ischemic damage by administration of phosphoenolpyruvate during reperfusion. A study in a paracorporeal rat heart modelEur Surg Res198315 4 200 207 1:CAS:528:DyaL3sXkvF2jsrs%3D 6873118 10.1159/000128354
- ThelinS et al Metabolic and functional effects of creatine phosphate in cardioplegic solution. Studies on rat hearts during and after normothermic ischemiaScand J Thorac Cardiovasc Surg198721 1 39 45 1:STN:280:DyaL2s3jsVWkuw%3D%3D 3589594 10.3109/14017438709116917
- OsbakkenM et al Creatine and cyclocreatine effects on ischemic myocardium: 31P nuclear magnetic resonance evaluation of intact heartCardiology199280 3–4 184 195 1:CAS:528:DyaK3sXhvVejtrw%3D 1511465 10.1159/000175002
- ThoreliusJ et al Biochemical and functional effects of creatine phosphate in cardioplegic solution during aortic valve surgery—a clinical studyThorac Cardiovasc Surg199240 1 10 13 1:STN:280:DyaK38zjs1amtQ%3D%3D 1631861 10.1055/s-2007-1020103
- BoudinaS et al Alteration of mitochondrial function in a model of chronic ischemia in vivo in rat heartAm J Physiol Heart Circ Physiol2002282 3 H821 H831 1:CAS:528:DC%2BD38Xit1GhtLc%3D 11834475 10.1152/ajpheart.00471.2001
- LaclauMN et al Cardioprotection by ischemic preconditioning preserves mitochondrial function and functional coupling between adenine nucleotide translocase and creatine kinaseJ Mol Cell Cardiol200133 5 947 956 1:CAS:528:DC%2BD3MXjtlSlurg%3D 11343417 10.1006/jmcc.2001.1357
- ConorevEASharovVGSaksVAImprovement in contractile recovery of isolated rat heart after cardioplegic ischaemic arrest with endogenous phosphocreatine: involvement of antiperoxidative effect?Cardiovasc Res199125 2 164 171 1:STN:280:DyaK38%2Fns1Cgtw%3D%3D 1742767 10.1093/cvr/25.2.164
- SharovVG et al Protection of ischemic myocardium by exogenous phosphocreatine. I. Morphologic and phosphorus 31-nuclear magnetic resonance studiesJ Thorac Cardiovasc Surg198794 5 749 761 1:STN:280:DyaL1c%2Fjslantw%3D%3D 3669703
- AnyukhovskyEP et al Effect of phosphocreatine and related compounds on the phospholipid metabolism of ischemic heartBiochem Med Metab Biol198635 3 327 334 1:STN:280:DyaL283kt1yrsg%3D%3D 3718764 10.1016/0885-4505(86)90090-3
- SharovVG et al Protection of ischemic myocardium by exogenous phosphocreatine (neoton): pharmacokinetics of phosphocreatine, reduction of infarct size, stabilization of sarcolemma of ischemic cardiomyocytes, and antithrombotic actionBiochem Med Metab Biol198635 1 101 114 1:CAS:528:DyaL28Xhs1Wktb8%3D 3778674 10.1016/0885-4505(86)90064-2
- GualanoB et al Creatine supplementation in the aging population: effects on skeletal muscle, bone and brainAmino Acids201648 8 1793 1805 1:CAS:528:DC%2BC28Xms1Kqu7Y%3D 27108136 10.1007/s00726-016-2239-7
- EarnestCPAlmadaALMitchellTLHigh-performance capillary electrophoresis-pure creatine monohydrate reduces blood lipids in men and womenClin Sci (Lond)199691 1 113 118 1:CAS:528:DyaK28XltVaisbs%3D 10.1042/cs0910113
- DeminiceR et al Creatine supplementation prevents fatty liver in rats fed choline-deficient diet: a burden of one-carbon and fatty acid metabolismJ Nutr Biochem201526 4 391 397 1:CAS:528:DC%2BC2MXitVCkurs%3D 25649792 10.1016/j.jnutbio.2014.11.014
- DeminiceR et al Creatine supplementation prevents hyperhomocysteinemia, oxidative stress and cancer-induced cachexia progression in Walker-256 tumor-bearing ratsAmino Acids201648 8 2015 2024 1:CAS:528:DC%2BC28XhtVent70%3D 26781304 10.1007/s00726-016-2172-9
- LawlerJM et al Direct antioxidant properties of creatineBiochem Biophys Res Commun2002290 1 47 52 1:CAS:528:DC%2BD38XhsFCisg%3D%3D 11779131 10.1006/bbrc.2001.6164
- RakpongsiriKSawangkoonSProtective effect of creatine supplementation and estrogen replacement on cardiac reserve function and antioxidant reservation against oxidative stress in exercise-trained ovariectomized hamstersInt Heart J200849 3 343 354 1:CAS:528:DC%2BD1cXpt1Wqsrg%3D 18612191 10.1536/ihj.49.343
- RahimiR et al Effects of creatine monohydrate supplementation on exercise-induced apoptosis in athletes: a randomized, double-blind, and placebo-controlled studyJ Res Med Sci201520 8 733 738 1:CAS:528:DC%2BC28XitFeltrzO 26664419 4652305 10.4103/1735-1995.168320
- Deminice R, Jordao AA. Creatine supplementation decreases plasma lipid peroxidation markers and enhances anaerobic performance in rats. Redox Rep. 2015;21(1):31–36.
- GualanoB et al Creatine in type 2 diabetes: a randomized, double-blind, placebo-controlled trialMed Sci Sports Exerc201143 5 770 778 1:CAS:528:DC%2BC3MXkvVGrtb8%3D 20881878 10.1249/MSS.0b013e3181fcee7d
- Op’t EijndeB et al Creatine supplementation increases soleus muscle creatine content and lowers the insulinogenic index in an animal model of inherited type 2 diabetesInt J Mol Med200617 6 1077 1084 16685419
- AlvesCR et al Creatine-induced glucose uptake in type 2 diabetes: a role for AMPK-alpha?Amino Acids201243 4 1803 1807 1:CAS:528:DC%2BC38XhtlOlsrbL 22349765 10.1007/s00726-012-1246-6
- SmithRNAgharkarASGonzalesEBA review of creatine supplementation in age-related diseases: more than a supplement for athletesF1000Res20143 222 25664170 4304302
- PatraS et al A short review on creatine-creatine kinase system in relation to cancer and some experimental results on creatine as adjuvant in cancer therapyAmino Acids201242 6 2319 2330 1:CAS:528:DC%2BC38XmvFGks7w%3D 21769499 10.1007/s00726-011-0974-3
- CaneteS et al Does creatine supplementation improve functional capacity in elderly women?J Strength Cond Res200620 1 22 28 16503684
- CandowDGChilibeckPDEffect of creatine supplementation during resistance training on muscle accretion in the elderlyJ Nutr Health Aging200711 2 185 188 1:CAS:528:DC%2BD2sXmtFensLk%3D 17435961
- CandowDG et al Comparison of creatine supplementation before versus after supervised resistance training in healthy older adultsRes Sports Med201422 1 61 74 24392772 10.1080/15438627.2013.852088
- CandowDG et al Low-dose creatine combined with protein during resistance training in older menMed Sci Sports Exerc200840 9 1645 1652 1:CAS:528:DC%2BD1cXhtVeis73F 18685526 10.1249/MSS.0b013e318176b310
- ChilibeckPD et al Effects of creatine and resistance training on bone health in postmenopausal womenMed Sci Sports Exerc201547 8 1587 1595 1:CAS:528:DC%2BC2MXht1WntbrJ 25386713 10.1249/MSS.0000000000000571
- NevesMJr et al Beneficial effect of creatine supplementation in knee osteoarthritisMed Sci Sports Exerc201143 8 1538 1543 1:CAS:528:DC%2BC3MXovF2jsLc%3D 21311365 10.1249/MSS.0b013e3182118592
- AlvesCR et al Creatine supplementation in fibromyalgia: a randomized, double-blind, placebo-controlled trialArthritis Care Res (Hoboken)201365 9 1449 1459 1:CAS:528:DC%2BC3sXhtlKku7bL 10.1002/acr.22020
- RoitmanS et al Creatine monohydrate in resistant depression: a preliminary studyBipolar Disord20079 7 754 758 1:CAS:528:DC%2BD1cXisVKq 17988366 10.1111/j.1399-5618.2007.00532.x
- D’AnciKEAllenPJKanarekRBA potential role for creatine in drug abuse?Mol Neurobiol201144 2 136 141 21399936 10.1007/s12035-011-8176-2 1:CAS:528:DC%2BC3MXht1amtr7P
- Toniolo RA, et al. Cognitive effects of creatine monohydrate adjunctive therapy in patients with bipolar depression: Results from a randomized, double-blind, placebo-controlled trial. J Affect Disord. 2016.
- DechentP et al Increase of total creatine in human brain after oral supplementation of creatine-monohydrateAm J Physiol1999277 3 Pt 2 R698 R704 1:CAS:528:DyaK1MXmt1Gju7w%3D 10484486
- LyooIK et al Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine-monohydratePsychiatry Res2003123 2 87 100 1:CAS:528:DC%2BD3sXmsFOgurs%3D 12850248 10.1016/S0925-4927(03)00046-5
- PanJWTakahashiKCerebral energetic effects of creatine supplementation in humansAm J Physiol Regul Integr Comp Physiol2007292 4 R1745 R1750 1:CAS:528:DC%2BD2sXks1Kitb0%3D 17185404 10.1152/ajpregu.00717.2006
- WatanabeAKatoNKatoTEffects of creatine on mental fatigue and cerebral hemoglobin oxygenationNeurosci Res200242 4 279 285 1:CAS:528:DC%2BD38Xjt1Srt7s%3D 11985880 10.1016/S0168-0102(02)00007-X
- RaeC et al Oral creatine monohydrate supplementation improves brain performance: a double-blind, placebo-controlled, cross-over trialProc Biol Sci2003270 1529 2147 2150 1:CAS:528:DC%2BD2cXht1eqsQ%3D%3D 14561278 1691485 10.1098/rspb.2003.2492
- McMorrisT et al Creatine supplementation, sleep deprivation, cortisol, melatonin and behaviorPhysiol Behav200790 1 21 28 1:CAS:528:DC%2BD2sXhvVSmtA%3D%3D 17046034 10.1016/j.physbeh.2006.08.024
- McMorrisT et al Effect of creatine supplementation and sleep deprivation, with mild exercise, on cognitive and psychomotor performance, mood state, and plasma concentrations of catecholamines and cortisolPsychopharmacology (Berl)2006185 1 93 103 1:CAS:528:DC%2BD28XhslWnsro%3D 10.1007/s00213-005-0269-z
- LingJKritikosMTipladyBCognitive effects of creatine ethyl ester supplementationBehav Pharmacol200920 8 673 679 1:CAS:528:DC%2BD1MXhsVersLzI 19773644 10.1097/FBP.0b013e3283323c2a
- OstojicSMGuanidinoacetic acid as a performance-enhancing agentAmino Acids201648 8 1867 1875 1:CAS:528:DC%2BC2MXhs1eiu7vM 26445773 10.1007/s00726-015-2106-y
- OstojicSM et al Guanidinoacetic acid versus creatine for improved brain and muscle creatine levels: a superiority pilot trial in healthy menAppl Physiol Nutr Metab201641 9 1005 1007 1:CAS:528:DC%2BC28XhsVWjt73P 27560540 10.1139/apnm-2016-0178
- Ellery SJ, et al. Renal dysfunction in early adulthood following birth asphyxia in male spiny mice, and its amelioration by maternal creatine supplementation during pregnancy. Pediatr Res. 2017.
- LaRosaDA et al Maternal creatine supplementation during pregnancy prevents acute and long-term deficits in skeletal muscle after birth asphyxia: a study of structure and function of hind limb muscle in the spiny mousePediatr Res201680 6 852 860 1:CAS:528:DC%2BC2sXht1Oqs7Y%3D 27466898 10.1038/pr.2016.153
- EllerySJWalkerDWDickinsonHCreatine for women: a review of the relationship between creatine and the reproductive cycle and female-specific benefits of creatine therapyAmino Acids201648 8 1807 1817 1:CAS:528:DC%2BC28XjtFChsrY%3D 26898548 10.1007/s00726-016-2199-y
- EllerySJ et al Dietary creatine supplementation during pregnancy: a study on the effects of creatine supplementation on creatine homeostasis and renal excretory function in spiny miceAmino Acids201648 8 1819 1830 1:CAS:528:DC%2BC2MXitVOjsbjJ 26695944 10.1007/s00726-015-2150-7
- DickinsonH et al Creatine supplementation during pregnancy: summary of experimental studies suggesting a treatment to improve fetal and neonatal morbidity and reduce mortality in high-risk human pregnancyBMC Pregnancy Childbirth201414 150 24766646 4007139 10.1186/1471-2393-14-150
- BortoluzziVT et al Co-administration of creatine plus pyruvate prevents the effects of phenylalanine administration to female rats during pregnancy and lactation on enzymes activity of energy metabolism in cerebral cortex and hippocampus of the offspringNeurochem Res201439 8 1594 1602 1:CAS:528:DC%2BC2cXpvVSlsrc%3D 24916961 10.1007/s11064-014-1353-8
- ValletJLMilesJRRempelLAEffect of creatine supplementation during the last week of gestation on birth intervals, stillbirth, and preweaning mortality in pigsJ Anim Sci201391 5 2122 2132 1:CAS:528:DC%2BC3sXotl2gtr8%3D 23463559 10.2527/jas.2012-5610
- EllerySJ et al Creatine pretreatment prevents birth asphyxia-induced injury of the newborn spiny mouse kidneyPediatr Res201373 2 201 208 1:CAS:528:DC%2BC3sXit1GgsLY%3D 23174701 10.1038/pr.2012.174
- DickinsonH et al Maternal dietary creatine supplementation does not alter the capacity for creatine synthesis in the newborn spiny mouseReprod Sci201320 9 1096 1102 1:CAS:528:DC%2BC2cXhs1OmtLrP 23427185 3745711 10.1177/1933719113477478
- IrelandZ et al A maternal diet supplemented with creatine from mid-pregnancy protects the newborn spiny mouse brain from birth hypoxiaNeuroscience2011194 372 379 1:CAS:528:DC%2BC3MXht1eitrbJ 21640166 10.1016/j.neuroscience.2011.05.012
- GellerAI et al Emergency department visits for adverse events related to dietary supplementsN Engl J Med2015373 16 1531 1540 1:CAS:528:DC%2BC28Xot1ajs7g%3D 26465986 10.1056/NEJMsa1504267
- ZorzelaL et al Serious adverse events associated with pediatric complementary and alternative medicineEur J Integr Med20146 467 47 10.1016/j.eujim.2014.05.001
- FDA. CFSAN Adverse Event Reporting System (CAERS). 2017. [cited 2017 March 27, 2017]; Available from: https://www.fda.gov/Food/ComplianceEnforcement/ucm494015.htm. Accessed 18 Apr 2017.
- GreenwoodM et al Creatine supplementation patterns and perceived effects in select division I collegiate athletesClin J Sport Med200010 3 191 194 1:STN:280:DC%2BD3M%2Fmt1eitw%3D%3D 10959929 10.1097/00042752-200007000-00007
- HileAM et al Creatine supplementation and anterior compartment pressure during exercise in the heat in dehydrated menJ Athl Train200641 1 30 35 16619092 1421498
- PoortmansJR et al Effect of short-term creatine supplementation on renal responses in menEur J Appl Physiol Occup Physiol199776 6 566 567 1:CAS:528:DyaK2sXnslSms70%3D 9404870 10.1007/s004210050291
- RobinsonTM et al Dietary creatine supplementation does not affect some haematological indices, or indices of muscle damage and hepatic and renal functionBr J Sports Med200034 4 284 288 1:STN:280:DC%2BD3M%2FktlSksQ%3D%3D 10953902 1724224 10.1136/bjsm.34.4.284
- GroeneveldGJ et al Few adverse effects of long-term creatine supplementation in a placebo-controlled trialInt J Sports Med200526 4 307 313 1:CAS:528:DC%2BD2MXltlShsb0%3D 15795816 10.1055/s-2004-817917
- GualanoB et al Effects of creatine supplementation on renal function: a randomized, double-blind, placebo-controlled clinical trialEur J Appl Physiol2008103 1 33 40 1:CAS:528:DC%2BD1cXhvF2qt7k%3D 18188581 10.1007/s00421-007-0669-3
- LugaresiR et al Does long-term creatine supplementation impair kidney function in resistance-trained individuals consuming a high-protein diet?J Int Soc Sports Nutr201310 1 26 1:CAS:528:DC%2BC3sXptFanu7s%3D 23680457 3661339 10.1186/1550-2783-10-26
- FarquharWBZambraskiEJEffects of creatine use on the athlete’s kidneyCurr Sports Med Rep20021 2 103 106 12831718 10.1249/00149619-200204000-00007
- ThorsteinsdottirBGrandeJPGarovicVDAcute renal failure in a young weight lifter taking multiple food supplements, including creatine monohydrateJ Ren Nutr200616 4 341 345 17046619 10.1053/j.jrn.2006.04.025
- Kuehl K, Goldberg L, Elliot D, Renal insufficiency after creatine supplementation in a college football athlete (Abstract). Med Sci Sports Exerc. 1998;30:S235.
- PritchardNRKalraPARenal dysfunction accompanying oral creatine supplementsLancet1998351 9111 1252 1253 1:STN:280:DyaK1czgsF2jtw%3D%3D 9643752 10.1016/S0140-6736(05)79319-3
- BarisicN et al Effects of oral creatine supplementation in a patient with MELAS phenotype and associated nephropathyNeuropediatrics200233 3 157 161 1:STN:280:DC%2BD38vkt12lsg%3D%3D 12200746 10.1055/s-2002-33679
- JuhnMSTarnopolskyMPotential side effects of oral creatine supplementation: a critical reviewClin J Sport Med19988 4 298 304 1:STN:280:DyaK1M7gtVGgsA%3D%3D 9884794 10.1097/00042752-199810000-00007
- JuhnMSOral creatine supplementation: separating fact from hypePhys Sportsmed199927 5 47 89 1:STN:280:DC%2BC3c%2FksVOhsw%3D%3D 20086718 10.3810/psm.1999.05.839
- BenziGIs there a rationale for the use of creatine either as nutritional supplementation or drug administration in humans participating in a sport?Pharmacol Res200041 3 255 264 1:CAS:528:DC%2BD3cXhtFWjsL0%3D 10675277 10.1006/phrs.1999.0618
- BenziGCeciACreatine as nutritional supplementation and medicinal productJ Sports Med Phys Fitness200141 1 1 10 1:CAS:528:DC%2BD3MXjvValu7o%3D 11317142
- PoortmansJRFrancauxMLong-term oral creatine supplementation does not impair renal function in healthy athletesMed Sci Sports Exerc199931 8 1108 1110 1:CAS:528:DyaK1MXlsVCqsr4%3D 10449011 10.1097/00005768-199908000-00005
- FrancauxM et al Effect of exogenous creatine supplementation on muscle PCr metabolismInt J Sports Med200021 2 139 145 1:CAS:528:DC%2BD3cXit12gt7s%3D 10727076 10.1055/s-2000-11065
- PoortmansJRFrancauxMAdverse effects of creatine supplementation: fact or fiction?Sports Med200030 3 155 170 1:STN:280:DC%2BD3cvks1ynuw%3D%3D 10999421 10.2165/00007256-200030030-00002
- FerreiraLG et al Effects of creatine supplementation on body composition and renal function in ratsMed Sci Sports Exerc200537 9 1525 1529 1:CAS:528:DC%2BD2MXhtVajs7jF 16177604 10.1249/01.mss.0000177555.94271.44
- BarachoNC et al Study of renal and hepatic toxicity in rats supplemented with creatineActa Cir Bras201530 5 313 318 26016930 10.1590/S0102-865020150050000002
- GualanoB et al Creatine supplementation does not impair kidney function in type 2 diabetic patients: a randomized, double-blind, placebo-controlled, clinical trialEur J Appl Physiol2011111 5 749 756 1:CAS:528:DC%2BC3MXnvFKkt7k%3D 20976468 10.1007/s00421-010-1676-3
- TaesYE et al Creatine supplementation does not decrease total plasma homocysteine in chronic hemodialysis patientsKidney Int200466 6 2422 2428 1:CAS:528:DC%2BD2MXisVyitg%3D%3D 15569335 10.1111/j.1523-1755.2004.66019.x
- ShelmadineBD et al The effects of supplementation of creatine on total homocysteineJ Ren Nurs20124 6 278 283 10.12968/jorn.2012.4.6.278
- ShelmadineBD et al Effects of thirty days of creatine supplementation on total homocysteine in a pilot study of end-stage renal disease patientsJ Ren Nurs20124 4 6 11
- PlineKASmithCLThe effect of creatine intake on renal functionAnn Pharmacother200539 6 1093 1096 1:CAS:528:DC%2BD2MXltlOns7s%3D 15886291 10.1345/aph.1E628
- PerskyAMRawsonESSafety of creatine supplementationSubcell Biochem200746 275 289 18652082 10.1007/978-1-4020-6486-9_14
- GualanoB et al In sickness and in health: the widespread application of creatine supplementationAmino Acids201243 2 519 529 1:CAS:528:DC%2BC38XhtVemurbF 22101980 10.1007/s00726-011-1132-7
- WilliamsMHFacts and fallacies of purported ergogenic amino acid supplementsClin Sports Med199918 3 633 649 1:STN:280:DyaK1MzjvFSgtw%3D%3D 10410846 10.1016/S0278-5919(05)70173-3
