Abstract
Background
L-carnitine (LC) is used as a supplement by recreationally-active, competitive and highly trained athletes. This systematic review aims to evaluate the effect of prolonged LC supplementation on metabolism and metabolic modifications.
Methods
A literature search was conducted in the MEDLINE (via PubMed) and Web of Science databases from the inception up February 2020. Eligibility criteria included studies on healthy human subjects, treated for at least 12 weeks with LC administered orally, with no drugs or any other multi-ingredient supplements co-ingestion.
Results
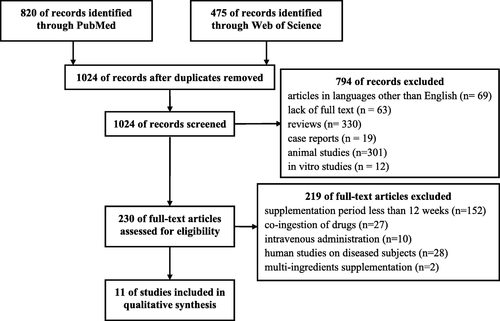
The initial search retrieved 1024 articles, and a total of 11 studies were finally included after applying inclusion and exclusion criteria. All the selected studies were conducted with healthy human subjects, with supplemented dose ranging from 1 g to 4 g per day for either 12 or 24 weeks. LC supplementation, in combination with carbohydrates (CHO) effectively elevated total carnitine content in skeletal muscle. Twenty-four-weeks of LC supplementation did not affect muscle strength in healthy aged women, but significantly increased muscle mass, improved physical effort tolerance and cognitive function in centenarians. LC supplementation was also noted to induce an increase of fasting plasma trimethylamine-N-oxide (TMAO) levels, which was not associated with modification of determined inflammatory nor oxidative stress markers.
Conclusion
Prolonged LC supplementation in specific conditions may affect physical performance. On the other hand, LC supplementation elevates fasting plasma TMAO, compound supposed to be pro-atherogenic. Therefore, additional studies focusing on long-term supplementation and its longitudinal effect on the cardiovascular system are needed.
Background
The main function of L-carnitine (LC) is the transport of long-chain fatty acids into the mitochondrial matrix for their conversion in energy, via β-oxidation process [Citation1]. Moreover, LC by the reaction with acetyl-CoA and maintaining the acetyl-CoA/CoA ratio in the cell regulates pyruvate dehydrogenase activity [Citation2]. LC also plays an important role in the regulation of metabolic pathways involved in skeletal muscle protein balance: proteolysis and protein synthesis [Citation3]. Furthermore, LC acts as anti-oxidant and anti-inflammatory compound [Citation3]; thus, it may attenuate the exercise-induced muscle damage.
The opinion that LC supplementation does not change metabolism is based mostly on short-term supplementation protocols [Citation4]. Recent studies demonstrate that prolonged supplementation, especially in combination with carbohydrates (CHO), may increase muscle total carnitine (TC) content in skeletal muscle [Citation5–Citation7]. Therefore, LC supplementation in specific conditions may affect physical performance. On the other hand, LC has been proposed as the red meat nutrient responsible for atherosclerosis promotion [Citation8]. As a potential link between red meat consumption and the increasing risk of cardiovascular disease, trimethylamine-N-oxide (TMAO) has been indicated [Citation8]. Since LC is still used by the athletes [Citation9, Citation10], the aim of this systematic review is to evaluate the effect of prolonged LC supplementation on metabolism/metabolic changes in healthy human subjects.
Methods
Eligibility criteria
The PICOS strategy was defined as follows: “P” (participants) human subjects, “I” (interventions) oral LC treatment, “C” (comparisons) between supplementation and placebo, supplementation and control, or pre- and post- supplementation, “O” (outcomes) muscle variables, and “S” (study design) randomized controlled trials, non-randomized controlled trials, non-randomized non-controlled trials.
Studies with the following criteria were excluded: described in languages other than English, articles without full-text availability, reviews and case reports. Subsequently, the following eligibility criteria were applied: a) healthy human subjects; b) supplementation at least for 12 weeks; c) oral LC administration; d) no drugs co-ingestion; e) no multi-ingredients supplementation.
Information sources and search
The literature was explored using the MEDLINE (via PubMed) and Web of Science databases, including all articles published from the inception up February 2020. The search was conducted using the terms: “carnitine supplementation” or “carnitine treatment” in combination with “exercise”, “training”, “athletic performance”, “muscle strength”, “muscle fatigue”, “muscle damage”, “muscle recovery”, “muscle synthesis” or “proteolysis”.
Study selection
Firstly, studies were assessed by title verification between databases (duplicates were removed). The second assessment performed by abstracts analysis, excluded studies in a language other than English, studies with lack of full text, reviews, case reports, animal studies and in-vitro studies. The last step was performed by analysis of full manuscripts based on the described above eligibility criteria.
Data collection process
The following information was compiled for each study: authors, year of publication, type of study, length of supplementation, a dose of supplementation and main effect. Lastly, the thematic analysis was carried out, to synthesize and interpret all the data that appeared from the included publications. The process of selecting papers, data collection as well as the quality assessment was performed independently by two authors (A.S., G.R.), and all disagreements were resolved by the discussion with the third author (R.O).
Results
Study selection
By the above-described search strategy, 1295 publications were identified. After the first selection, adjusted by duplicates, persisted 1024 articles. Of these, 794 were excluded after abstracts screening and identified articles in languages other than English, lack of full text or being review articles, case reports, animal or in-vitro studies. The full texts of 230 articles were screened by eligibility criteria. Finally, to the qualitative analysis were accepted 11 studies performed on healthy human subjects, treated for at least 12 weeks with LC administered orally, with no drugs or any other multi-ingredient supplements co-ingestion (Fig. ).
Description of the included studies
Table provides details and results of the 11 studies reviewed. Selected studies were published between 2002 and 2020. In the selected studies, participants were supplemented in a dose ranging from 1 g to 4,5 g per day for either 12 or 24 weeks, mostly by L-carnitine-L-tartrate (LCLT). In three studies, supplementations were combined with carbohydrates (CHO) [Citation5–Citation7], and in one with L-leucine [Citation18].
Table 1 Summary and results of the studies reviewed examining the LC supplementation
Muscle carnitine content was not affected following 12 weeks of LC supplementation alone [Citation11, Citation12]. On the other hand, LC supplementation in combination with CHO effectively elevated muscle TC after 12 [Citation6] and 24 weeks [Citation5]. Moreover, 12 weeks of supplementation alone [Citation13], or in combination with CHO [Citation6] promote the expression of the genes related to fatty acids and carnitine metabolism.
Twenty-four-weeks of LC supplementation alone did not affect muscle strength in healthy aged women [Citation15], but significantly increased muscle mass, improved physical effort tolerance and cognitive function in centenarians [Citation14].
In two studied groups of healthy aged woman, LC supplementation alone [Citation16, Citation17], or in combination with L-leucine [Citation18], induced an increase of fasting plasma TMAO levels. However, higher TMAO was not associated with determined inflammatory [Citation16] nor oxidative stress [Citation17] markers. Moreover, despite elevated TMAO, LC supplementation together with resistance training induced positive changes in mitochondrial DNA methylation of platelets [Citation18].
Discussion
The present findings have been debated in the six separate paragraphs, and for a better picture of LC supplementation, other studies were also disputed.
“Fat burner”
It has been assumed that LC supplementation, by increasing muscle carnitine content, optimizes fat oxidation and consequently reduces its availability for storage [Citation19]. Nevertheless, the belief that carnitine is a slimming agent has been negated in the middle of 90s [Citation20]. Direct measurements of carnitine in skeletal muscles failed to show any elevation in the muscle carnitine concentration following 14 days of 4 g/day [Citation21], or 6 g/day [Citation22] LC ingestion. These findings implied that LC supplementation was not able to increase fat oxidation and improve exercise performance by the proposed mechanism. Indeed, many original investigations, summarized in later review [Citation4], indicated that LC supplementation lasting up to 4 weeks, neither increase fat oxidation nor improve performance during prolonged exercises.
Since LC concentration in skeletal muscles is higher than that of blood plasma, active uptake of carnitine must take place [Citation23]. Stephens et al. [Citation24] noted that 5 h steady-state hypercarnitinemia (~ 10-fold elevation of plasma carnitine) induced by the intravenous LC infusion does not affect skeletal muscle TC content. On the other hand, similar intervention in combination with controlled hyperinsulinemia (~ 150mIU/L) elevates TC in skeletal muscle by ~ 15% [Citation24, Citation25]. Moreover, higher serum insulin maintained by the consumption of simple sugars resulted in augmented LC retention in healthy human subjects supplemented by LC for 2 weeks [Citation26]. Based on these results, Authors suggested that oral ingestion of LC, combined with CHO for activation carnitine transport into the muscles, should take ~ 100 days to increase muscle carnitine content by ~ 10% [Citation26]. This assumption has been confirmed in later studies [Citation5–Citation7]. These carefully conducted studies clearly showed that prolonged procedure (for ≥12 weeks) of a daily LC and CHO ingestion induced a raise of skeletal muscle TC levels [Citation5–Citation7], affecting exercise metabolism [Citation5], improving performance [Citation5] and energy expenditure [Citation6], without altering body composition [Citation6]. The lack of body fat stores loss may be explained by the 18% increase in body fat mass associated with CHO supplementation alone, noted in the control group [Citation6].
Nevertheless, 12 weeks of LC supplementation 2 g/day applied without CHO, elevated muscle TC only in vegetarian but not in omnivores [Citation12]. Neither exercise metabolism nor muscle metabolites were modified by augmented TC in vegetarian [Citation12].
Skeletal muscle protein balance regulation
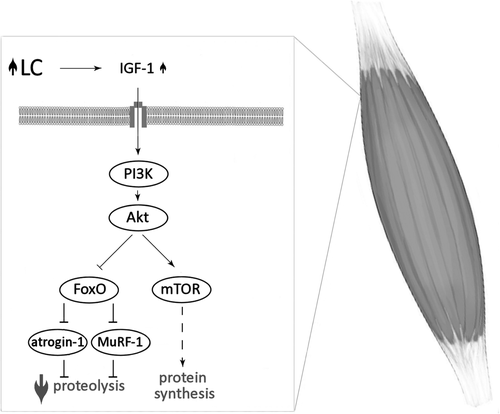
Skeletal muscle mass depends on the rates of protein synthesis and degradation. Elevated protein synthesis and attenuated proteolysis are observed during muscle hypertrophy. Both of these processes are mainly regulated by the signaling pathway: insulin-like growth factor-1 (IGF-1) – phosphoinositide-3-kinase (PI3K) – protein kinase B (Akt) – mammalian target of rapamycin (mTOR). The activation of mTOR leads to phosphorylation and activation of S6 kinases (S6Ks) and hyperphosphorylation of 4E-binding proteins (4E-BPs), resulting in the acceleration of protein synthesis. At the same time, Akt phosphorylates and inactivates forkhead box O (FoxO), thereby inhibit the responsible for proteolysis ubiquitin ligases: muscle-specific RING finger-1 (MuRF-1) and muscle atrophy F-box protein (atrogin-1), (for review see [Citation27–Citation29]).
The association between LC supplementation and the regulation of metabolic pathways involved in muscle protein balance have been shown in several animal studies (Fig. ) [Citation30–Citation35]. Four weeks of LC supplementation in rats increased plasma IGF-1 concentration [Citation33]. Elevated circulating IGF-1 led to an activation of the IGF-1–PI3K–Akt signalling pathway, causing augmented mTOR phosphorylation and higher phospho-FoxO/total FoxO ratio in skeletal muscle of LC supplemented rats [Citation33]. FoxO inactivation attenuated MURF-1 expression in quadriceps femoris muscle of supplemented rats (compared to control) [Citation33]. Moreover, LC administrated for 2 weeks suppresses atrogin-1 messenger RNA (mRNA) level in suspended rats’ hindlimb [Citation35], and only 7 days of LC administration downregulates MuRF-1 and atrogin-1 mRNAs reducing muscle wasting in a rat model of cancer cachexia [Citation32]. All these findings together might suggest that LC supplementation protect muscle from atrophy, especially in pathophysiological conditions.
Fig. 2 The association between LC supplementation and the regulation of metabolic pathways involved in muscle protein balance. L-carnitine (LC); insulin-like growth factor-1 (IGF-1); phosphoinositide-3-kinase (PI3K); protein kinase B (Akt); mammalian target of rapamycin (mTOR); forkhead box O (FoxO); muscle-specific RING finger-1 (MuRF-1); muscle atrophy F-box (atrogin-1); increase (![]()
In fact, administration of acetyl-L-carnitine 3 g/day for 5 months in HIV-seropositive patients induced ten-fold increase in serum IGF-1 concentration [Citation36]. Conversely, neither 3 weeks LC supplementation in healthy, recreationally weight-trained men [Citation37], nor 24 weeks LC supplementation in aged women [Citation15] did not affect circulating IGF-1 level concentration. Various effects might be due to different IGF-1 levels; significantly lower in the HIV-seropositive patients than in healthy subjects [Citation38]. Additionally, 8 weeks of LC supplementation in healthy older subjects, did not change total and phosphorylated mTOR, S6K and 4E-BP proteins level of vastus lateralis muscle [Citation39]. It must be highlighted that rat skeletal muscle TC increases ~ 50–70% following 4 weeks of LC supplementation [Citation33, Citation34], whereas comparable elevation has never been observed in human studies, even after 24 weeks of supplementation [Citation5, Citation7].
Body composition
These findings altogether suggest that prolonged LC supplementation might affect body composition in specific conditions.
Obesity
A recent meta-analysis, summarized studies focused on LC supplementation for a prolonged time (median 3 months) [Citation40]. Pooled results demonstrated a significant reduction in weight following LC supplementation, but the subgroups analysis revealed no significant effect of LC on body weight in subjects with body mass index (BMI) below 25 kg/m2. Therefore, authors suggested that LC supplementation may be effective in obese and overweight subjects. Surprisingly, intervention longer than 24 weeks showed no significant effect on BMI [Citation40].
Training
It has been assumed that a combination of LC supplementation with increased energy expenditure may positively affect body composition. However, either with aerobic [Citation41, Citation42] or resistance [Citation43] training, LC supplementation has not achieved successful endpoint. Six weeks of endurance training (five times per week, 40 min on a bicycle ergometer at 60% maximal oxygen uptake) together with LC supplementation (4 g/day) does not induce a positive effect on fat metabolism in healthy male subjects (% body fat 17.9 ± 2.3 at the beginning of the study) [Citation41]. Similarly, lack of LC effect has been reported in obese women [Citation42]. Eight weeks of supplementation (2 g/day) combined with aerobic training (3 sessions a week) had no significant effects on body weight, BMI and daily dietary intake in obese women [Citation42].
In the recent study, LC supplementation 2 g/day has been applied in combination with a resistance training program (4 days/week) to healthy men (age range 18–40 y.o.), for 9 weeks [Citation43]. Body composition, determined by dual energy X-ray absorptiometry, indicated no significant effect in fat mass and fat-free mass due to supplementation. Moreover, LC administration did not influence bench press results. The number of leg press repetitions and the leg press third set lifting volume increased in the LC group compared to the placebo group [Citation43]. Different LC effect in the limbs may be associated with the higher rates of glycogenolysis during arm exercise at the same relative intensity as leg exercise [Citation44].
Sarcopenia
Aged people have accelerated protein catabolism, which is associated with muscle wasting [Citation45]. LC could increase the amount of protein retention by inhibition of the proteolytic pathway. Six months of LC supplementation augmented fat free mass and reduced total body fat mass in centenarians [Citation14]. Such effect was not observed in elder women (age range 65–70 y.o.) after a similar period of supplementation [Citation15]. The effectiveness of LC supplementation may result from the age-wise distribution of sarcopenia. The prevalence of sarcopenia increased steeply with age, reaching 31.6% in women and 17.4% in men older than 80 years [Citation46]. In subjects below 70 years presarcopenia, but not sarcopenia symptoms were noted [Citation46].
Oxidative imbalance and muscle soreness
Muscle damage may occur during exercise, especially eccentric exercise. In the clearance of damaged tissues assist free radicals produced by neutrophils. Therefore, among other responses to exercise, neutrophils are released into the circulation. While neutrophil-derived reactive oxygen species (ROS) play an important role in breaking down damaged fragments of the muscle tissue, ROS produced in excess may also contribute to oxidative stress (for review see [Citation47, Citation48].
Based on the assumption that LC may provide cell membranes protection against oxidative stress [Citation49], it has been hypothesized that LC supplementation would mitigate exercise-induced muscle damage and improve post-exercise recovery. Since plasma LC elevates following 2 weeks of supplementation [Citation21, Citation22], short protocols of supplementation may be considered as effective in attenuating post-exercise muscle soreness. The findings indicated that 3 weeks of LC supplementation, in the amount 2-3 g/day, effectively alleviated pain [Citation50–Citation53]. It has been shown, through magnetic resonance imaging technique that muscle disruption after strenuous exercise was reduced by LC supplementation [Citation37, Citation51]. This effect was accompanied by a significant reduction in released cytosolic proteins such as myoglobin and creatine kinase [Citation50, Citation52, Citation53] as well as attenuation in plasma marker of oxidative stress - malondialdehyde [Citation51, Citation53, Citation54]. Furthermore, 9 weeks of LC supplementation in conjunction with resistance training revealed a significant increase of circulating total antioxidant capacity and glutathione peroxidase activity and decrease in malondialdehyde concentration [Citation43].
Risks of TMAO
In 1984 Rebouche et al. [Citation55], showed that rats, orally receiving radiolabeled LC, metabolized it to γ-butyrobetaine (up to 31% of the administered dose, present primary in feces) and TMAO (up to 23% of the administered dose, present primary in urine). On the contrary, these metabolites were not produced by the rats receiving the isotope intravenously and germ-free rats receiving the tracer orally, suggesting that orally ingested LC is in part degraded by the gut’s microorganisms [Citation55]. Similar observations were noted in later human studies [Citation56, Citation57], with the peak serum TMAO observed within hours following oral administration of the tracer [Citation56]. Prolonged LC treatment elevates fasting plasma TMAO [Citation16–Citation18, Citation58, Citation59]. Three months of oral LC supplementation in healthy aged women induced ten-fold increase of fasting plasma TMAO, and this level remained elevated for the further 3 months of supplementation [Citation16]. Four months after cessation of LC supplementation, plasma TMAO reached a pre-supplementation concentration, which was stable for the following 8 months [Citation60].
In 2011 Wang et al. [Citation61] suggested TMAO as a pro-atherogenic factor. Since diets high in red meat have been strongly related to heart disease and mortality [Citation62], LC has been proposed as the red meat nutrient responsible for atherosclerosis promotion [Citation8]. As a potential link between red meat consumption and the increasing risk of cardiovascular disease, TMAO has been indicated [Citation8]. Numerous later studies have shown the association between increased plasma TMAO levels with a higher risk of cardiovascular events [Citation63–Citation66]. The recent meta-analyses indicated that in patients with high TMAO plasma level, the incidence of major adverse cardiovascular events was significantly higher compared with patients with low TMAO levels [Citation67], and that all-cause mortality increased by 7.6% per each 10 μmol/L increment of TMAO [Citation68].
Since red meat is particularly rich in LC [Citation69], dietary intervention in healthy adults, indicated a significant increase in plasma and urine TMAO levels following 4 weeks of the red meat-enriched diet [Citation70]. The rise of plasma TMAO was on average three-fold compared with white meat and non-meat diets [Citation70]. Conversely, habitual consumption of red, processed or white meat did not affect plasma TMAO in German adult population [Citation71]. Similarly, a minor increase in plasma TMAO was observed following red meat and processed meat consumption in European multi-center study [Citation72].
In the previous century, the underlined function of TMAO was the stabilization of proteins against various environmental stress factors, including high hydrostatic pressure [Citation73]. TMAO was shown as widely distributed in sea animals [Citation74], with concentration in the tissue increasing proportionally to the depth of the fishes natural environment [Citation75]. Consequently, fish and seafood nutritional intake has a great impact on TMAO level in the human body [Citation76], significantly elevating also plasma TMAO concentration [Citation72]. Therefore, link between plasma TMAO and the risk of cardiovascular disease [Citation8] seems like a paradox, since more fish in the diet reduces this risk [Citation77].
Not only dietary modification may affect TMAO plasma levels. Due to TMAO excretion in urine [Citation56, Citation57], in chronic renal disease patients, TMAO elimination from the body fails, causing elevation of its plasma concentration [Citation78]. Therefore, higher plasma TMAO in humans was suggested as a marker of kidney damage [Citation79]. It is worthy to note that cardiovascular disease and kidney disease are closely interrelated [Citation80] and diminished renal function is strongly associated with morbidity and mortality in heart failure patients [Citation81]. Moreover, decreased TMAO urine excretion is associated with high salt dietary intake, increasing plasma TMAO concentration [Citation82].
The relation between TMAO and chronic disease can be ambiguous, involving kidney function [Citation79], disturbed gut-blood barrier [Citation83], or flavin-containing monooxygenase 3 genotype [Citation84]. Thus, whether TMAO is an atherogenic factor responsible for the development and progression of cardiovascular disease, or simply a marker of an underlined pathology, remains unclear [Citation85].
Adverse effects
Carnitine preparations administered orally can occasionally cause heart-burn or dyspepsia [Citation86]. No adverse events associated with LC administration were recorded at a dose 6 g/day for 12 months of supplementation in the patients with acute anterior myocardial infarction [Citation87], or at a dose 1.274 g/day (range 0.3–3 g/day) and duration 348 days (range 93–744 days) in patients with liver cirrhosis [Citation88]. Summarizing the risk associated with LC supplementation Hathcock and Shao [Citation89] indicated that intakes up to 2 g/day are safe for chronic supplementation.
Although the optimal dose of LC supplementation for myocardial infarction is 3 g/day in terms of all-cause mortality [Citation90], even lower LC intake elevates fasting plasma TMAO [Citation16–Citation18, Citation58, Citation59], which is ten-fold higher than control after 3 months of supplementation [Citation16, Citation17]. It is worthy to mention that Bakalov et al. [Citation91] analyzing European Medicine Agency database of suspected adverse drug reaction, noticed 143 cases regarding LC.
Strengths and limitations
The strength of this review is a focus on the period of LC treatment, very important aspect often missed in many articles dealing with this supplement. To date, only few studies have examined the effects of LC supplementation for at least 12 weeks, which is, on the other hand, the main limitation of the current review. This limitation is also magnified by the varied design of the studies available including different supplementation protocols and outcome measures. There is also a high degree of heterogeneity among participants of the analyzed studies. Therefore, the results should be taken with caution, and more research is required before definitive recommendations.
Conclusions
Lasting for several years opinion that LC supplementation does not change metabolism, especially exercise metabolism, is based mostly on short-term supplementation protocols. Nevertheless, LC is still used by elite [Citation9] and sub-elite [Citation10] athletes. Recent studies suggest that LC supplementation may elevate muscle TC content; therefore, modify muscle fuel metabolism and performance during the exercise. Due to insulin-mediated LC transport to the muscle, oral administration regimen should be combined with CHO. Because of LC poor bioavailability, it is likely that the supplementation protocol would take at least 3 months. Shorter period of supplementation may be effective in prevention of exercise-induced muscle damage, but not metabolic changes.
On the other hand, it is also clear that prolonged LC supplementation elevates fasting plasma TMAO [Citation16–Citation18, Citation58, Citation59], compound supposed to be pro-atherogenic [Citation61]. Therefore, additional studies focusing on long-term supplementation and its longitudinal effect on the TMAO metabolism and cardiovascular system are needed.
Authors’ contributions
Conceptualization: R.O.; Writing-original draft preparation: A.S., G.R. and R.O.; The authors declare that the content of this paper has not been published or submitted for publication elsewhere. All authors have read and agreed to the published version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
| LC | = | L-carnitine |
| TC | = | Total carnitine |
| TMAO | = | Trimethylamine-N-oxide |
| CHO | = | Carbohydrates |
| IGF-1 | = | Insulin-like growth factor-1 |
| PI3K | = | Phosphoinositide-3-kinase |
| Akt | = | Protein kinase B |
| mTOR | = | Mammalian target of rapamycin |
| S6K | = | S6 kinase |
| 4E-BP | = | 4E-binding protein |
| FoxO | = | Forkhead box O |
| MuRF-1 | = | Muscle-specific RING finger-1 |
| atrogin-1 | = | Muscle atrophy F-box |
| mRNA | = | Messenger RNA |
| BMI | = | Body mass index |
| ROS | = | Reactive oxygen species |
Acknowledgements
Not applicable.
Funding
This work was supported by National Science Centre in Poland, grant number 2014/15/B/NZ7/00893.
Availability of data and materials
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- BremerJCarnitine--metabolism and functionsPhysiol Rev198363 4 1420 1480 1:CAS:528:DyaL2cXmsFGgsQ%3D%3D https://doi.org/10.1152/physrev.1983.63.4.1420 6361812
- ArenasJHuertasRCamposYDiazAEVillalonJMVilasEEffects of L-carnitine on the pyruvate dehydrogenase complex and carnitine palmitoyl transferase activities in muscle of endurance athletesFEBS Lett1994341 1 91 93 1:CAS:528:DyaK2cXktVGqsbw%3D https://doi.org/10.1016/0014-5793(94)80246-7 8137928
- RingseisRKellerJEderKMechanisms underlying the anti-wasting effect of L-carnitine supplementation under pathologic conditions: evidence from experimental and clinical studiesEur J Nutr201352 5 1421 1442 1:CAS:528:DC%2BC3sXhtFGrsLbN https://doi.org/10.1007/s00394-013-0511-0 23508457
- BrassEPSupplemental carnitine and exerciseAm J Clin Nutr200072 2 Suppl 618S 623S 1:CAS:528:DC%2BD3cXlsFyhsbw%3D https://doi.org/10.1093/ajcn/72.2.618S 10919968
- WallBTStephensFBConstantin-TeodosiuDMarimuthuKMacdonaldIAGreenhaffPLChronic oral ingestion of L-carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humansJ Physiol2011589 Pt 4 963 973 1:CAS:528:DC%2BC3MXksFOmsLk%3D https://doi.org/10.1113/jphysiol.2010.201343 21224234 3060373
- StephensFBWallBTMarimuthuKShannonCEConstantin-TeodosiuDMacdonaldIAGreenhaffPLSkeletal muscle carnitine loading increases energy expenditure, modulates fuel metabolism gene networks and prevents body fat accumulation in humansJ Physiol2013591 18 4655 4666 1:CAS:528:DC%2BC3sXhsVCrurzK https://doi.org/10.1113/jphysiol.2013.255364 23818692 3784205
- ShannonCEGhasemiRGreenhaffPLStephensFBIncreasing skeletal muscle carnitine availability does not alter the adaptations to high-intensity interval trainingScand J Med Sci Sports201828 1 107 115 https://doi.org/10.1111/sms.12885 28345160
- KoethRAWangZLevisonBSBuffaJAOrgESheehyBTBrittEBFuXWuYLiL et al Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosisNat Med201319 5 576 585 1:CAS:528:DC%2BC3sXlsVCqu7w%3D https://doi.org/10.1038/nm.3145 23563705 3650111
- Baltazar-MartinsGBrito de SouzaDAguilar-NavarroMMunoz-GuerraJMDMPDel CosoJPrevalence and patterns of dietary supplement use in elite Spanish athletesJ Int Soc Sports Nutr201916 1 30 1:CAS:528:DC%2BC1MXhtl2qtL3O https://doi.org/10.1186/s12970-019-0296-5 31319850 6639916
- WardenaarFCCeelenIJVan DijkJWHangelbroekRWVan RoyLVan der PouwBDe VriesJHMensinkMWitkampRFNutritional supplement use by Dutch elite and sub-elite athletes: does receiving dietary counseling make a difference?Int J Sport Nutr Exerc Metab201727 1 32 42 1:CAS:528:DC%2BC1cXhsFKntLbP https://doi.org/10.1123/ijsnem.2016-0157 27615123
- WachterSVogtMKreisRBoeschCBiglerPHoppelerHKrahenbuhlSLong-term administration of L-carnitine to humans: effect on skeletal muscle carnitine content and physical performanceClin Chim Acta2002318 1–2 51 61 1:CAS:528:DC%2BD38XhslWjsb0%3D https://doi.org/10.1016/s0009-8981(01)00804-x 11880112
- NovakovaKKummerOBouitbirJStoffelSDHoerler-KoernerUBodmerMRobertsPUrwylerAEhrsamRKrahenbuhlSEffect of L-carnitine supplementation on the body carnitine pool, skeletal muscle energy metabolism and physical performance in male vegetariansEur J Nutr201655 1 207 217 1:CAS:528:DC%2BC2MXhsFWqur4%3D https://doi.org/10.1007/s00394-015-0838-9 25612929
- LohningerASendicALitzlbauerEHofbauerRStaniekHBleskyDSchwieglhoferCEderMBergmullerHMascherD et al Endurance exercise training and L-carnitine supplementation stimulates gene expression in the blood and muscle cells in young athletes and middle aged subjectsMonatshefte Fur Chemie2005136 8 1425 1442 1:CAS:528:DC%2BD2MXmsFKltbw%3D https://doi.org/10.1007/s00706-005-0335-6
- MalaguarneraMCammalleriLGarganteMPVacanteMColonnaVMottaML-Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: a randomized and controlled clinical trialAm J Clin Nutr200786 6 1738 1744 1:CAS:528:DC%2BD2sXhsVGhsL%2FI https://doi.org/10.1093/ajcn/86.5.1738 18065594
- Sawicka AK, Hartmane D, Lipinska P, Wojtowicz E, Lysiak-Szydlowska W, Olek RA. l-Carnitine Supplementation in Older Women. A Pilot Study on Aging Skeletal Muscle Mass and Function. Nutrients. 2018;10(2). https://doi.org/https://doi.org/10.3390/nu10020255.
- SamulakJJSawickaAKHartmaneDGrinbergaSPugovicsOLysiak-SzydlowskaWOlekRAL-Carnitine supplementation increases Trimethylamine-N-oxide but not markers of atherosclerosis in healthy aged womenAnn Nutr Metab201974 1 11 17 1:CAS:528:DC%2BC1MXisF2mt7w%3D https://doi.org/10.1159/000495037 30485835
- OlekRASamulakJJSawickaAKHartmaneDGrinbergaSPugovicsOLysiak-SzydlowskaWIncreased Trimethylamine N-oxide is not associated with oxidative stress markers in healthy aged womenOxidative Med Cell Longev20192019 6247169 1:CAS:528:DC%2BB3cXmsFansbg%3D https://doi.org/10.1155/2019/6247169
- BordoniLSawickaAKSzarmachAWinklewskiPJOlekRAGabbianelliRA pilot study on the effects of l-Carnitine and Trimethylamine-N-oxide on platelet mitochondrial DNA methylation and CVD biomarkers in aged womenInt J Mol Sci202021 3 1047 1:CAS:528:DC%2BB3cXhslGnu7nN https://doi.org/10.3390/ijms21031047
- GrunewaldKKBaileyRSCommercially marketed supplements for bodybuilding athletesSports Med199315 2 90 103 1:STN:280:DyaK3s7ovF2nuw%3D%3D https://doi.org/10.2165/00007256-199315020-00003 8446827
- HawleyJABrounsFJeukendrupAStrategies to enhance fat utilisation during exerciseSports Med199825 4 241 257 1:STN:280:DyaK1c3kvVOgtg%3D%3D https://doi.org/10.2165/00007256-199825040-00003 9587182
- BarnettCCostillDLVukovichMDColeKJGoodpasterBHTrappeSWFinkWJEffect of L-carnitine supplementation on muscle and blood carnitine content and lactate accumulation during high-intensity sprint cyclingInt J Sport Nutr19944 3 280 288 1:STN:280:DyaK2M%2FnvVyruw%3D%3D https://doi.org/10.1123/ijsn.4.3.280 7987362
- VukovichMDCostillDLFinkWJCarnitine supplementation: effect on muscle carnitine and glycogen content during exerciseMed Sci Sports Exerc199426 9 1122 1129 1:CAS:528:DyaK2MXpvVOksA%3D%3D https://doi.org/10.1249/00005768-199409000-00009
- ReboucheCJCarnitine movement across muscle cell membranes. Studies in isolated rat muscleBiochim Biophys Acta1977471 1 145 155 1:CAS:528:DyaE1cXhvFGqtQ%3D%3D https://doi.org/10.1016/0005-2736(77)90402-3 921970
- StephensFBConstantin-TeodosiuDLaithwaiteDSimpsonEJGreenhaffPLInsulin stimulates L-carnitine accumulation in human skeletal muscleFASEB J200620 2 377 379 1:CAS:528:DC%2BD28XhsFOksLY%3D https://doi.org/10.1096/fj.05-4985fje 16368715
- StephensFBConstantin-TeodosiuDLaithwaiteDSimpsonEJGreenhaffPLAn acute increase in skeletal muscle carnitine content alters fuel metabolism in resting human skeletal muscleJ Clin Endocrinol Metab200691 12 5013 5018 1:CAS:528:DC%2BD28XhtlGru7rL https://doi.org/10.1210/jc.2006-1584 16984983
- StephensFBEvansCEConstantin-TeodosiuDGreenhaffPLCarbohydrate ingestion augments L-carnitine retention in humansJ Appl Physiol (1985)2007102 3 1065 1070 1:CAS:528:DC%2BD2sXjvFOisLw%3D https://doi.org/10.1152/japplphysiol.01011.2006
- AttaixDVentadourSCodranABechetDTaillandierDCombaretLThe ubiquitin-proteasome system and skeletal muscle wastingEssays Biochem200541 173 186 1:CAS:528:DC%2BD2MXht1aitbzJ https://doi.org/10.1042/EB0410173 16250905
- SchiaffinoSDyarKACiciliotSBlaauwBSandriMMechanisms regulating skeletal muscle growth and atrophyFEBS J2013280 17 4294 4314 1:CAS:528:DC%2BC3sXht1ymu7nK https://doi.org/10.1111/febs.12253 23517348
- SanchezAMCandauRBBernardiHFoxO transcription factors: their roles in the maintenance of skeletal muscle homeostasisCell Mol Life Sci201471 9 1657 1671 1:CAS:528:DC%2BC3sXhslylsrfL https://doi.org/10.1007/s00018-013-1513-z 24232446
- KellerJRingseisRPriebeSGuthkeRKlugeHEderKDietary L-carnitine alters gene expression in skeletal muscle of pigletsMol Nutr Food Res201155 3 419 429 1:CAS:528:DC%2BC3MXisFyms7w%3D https://doi.org/10.1002/mnfr.201000293 20938991
- KellerJRingseisRKocALukasIKlugeHEderKSupplementation with l-carnitine downregulates genes of the ubiquitin proteasome system in the skeletal muscle and liver of pigletsAnimal20126 1 70 78 1:CAS:528:DC%2BC3MXhsF2ru7rM https://doi.org/10.1017/S1751731111001327 22436156
- BusquetsSSerpeRToledoMBetancourtAMarmontiEOrpiMPinFCapdevilaEMadedduCLopez-SorianoFJ et al L-Carnitine: an adequate supplement for a multi-targeted anti-wasting therapy in cancerClin Nutr201231 6 889 895 1:CAS:528:DC%2BC38Xnt1Krsbo%3D https://doi.org/10.1016/j.clnu.2012.03.005 22608917
- KellerJCouturierAHaferkampMMostEEderKSupplementation of carnitine leads to an activation of the IGF-1/PI3K/Akt signalling pathway and down regulates the E3 ligase MuRF1 in skeletal muscle of ratsNutr Metab (Lond)201310 1 28 1:CAS:528:DC%2BC3sXotFCmt7s%3D https://doi.org/10.1186/1743-7075-10-28
- KellerJRingseisREderKSupplemental carnitine affects the microRNA expression profile in skeletal muscle of obese Zucker ratsBMC Genomics201415 512 1:CAS:528:DC%2BC2cXhvVWrsbrI https://doi.org/10.1186/1471-2164-15-512 24952657 4078242
- JangJParkJChangHLimKL-Carnitine supplement reduces skeletal muscle atrophy induced by prolonged hindlimb suspension in ratsAppl Physiol Nutr Metab201641 12 1240 1247 1:CAS:528:DC%2BC28XhvVGgtrfF https://doi.org/10.1139/apnm-2016-0094 27841025
- Di MarzioLMorettiSD'AloSZazzeroniFMarcelliniSSmacchiaCAlesseECifoneMGDe SimoneCAcetyl-L-carnitine administration increases insulin-like growth factor 1 levels in asymptomatic HIV-1-infected subjects: correlation with its suppressive effect on lymphocyte apoptosis and ceramide generationClin Immunol199992 1 103 110 1:CAS:528:DyaK1MXkvV2rsbY%3D https://doi.org/10.1006/clim.1999.4727 10413658
- KraemerWJVolekJSFrenchDNRubinMRSharmanMJGomezALRatamessNANewtonRUJemioloBCraigBW et al The effects of L-carnitine L-tartrate supplementation on hormonal responses to resistance exercise and recoveryJ Strength Cond Res200317 3 455 462 https://doi.org/10.1519/1533-4287(2003)017<0455:teolls>2.0.co;2 12930169
- RondanelliMSolerteSBFioravantiMScevolaDLocatelliMMinoliLFerrariECircadian secretory pattern of growth hormone, insulin-like growth factor type I, cortisol, adrenocorticotropic hormone, thyroid-stimulating hormone, and prolactin during HIV infectionAIDS Res Hum Retrovir199713 14 1243 1249 1:CAS:528:DyaK2sXmsVeit70%3D https://doi.org/10.1089/aid.1997.13.1243 9310292
- EvansMGuthrieNPezzulloJSanliTFieldingRABellamineAEfficacy of a novel formulation of L-Carnitine, creatine, and leucine on lean body mass and functional muscle strength in healthy older adults: a randomized, double-blind placebo-controlled studyNutr Metab (Lond)201714 7 1:CAS:528:DC%2BC1cXjt1Cht74%3D https://doi.org/10.1186/s12986-016-0158-y
- AskarpourMHadiAMiraghajaniMSymondsMESheikhiAGhaediEBeneficial effects of l-carnitine supplementation for weight management in overweight and obese adults: an updated systematic review and dose-response meta-analysis of randomized controlled trialsPharmacol Res2020151 104554 1:CAS:528:DC%2BC1MXit1Oitr7E https://doi.org/10.1016/j.phrs.2019.104554 31743774
- LeeJKLeeJSParkHChaYSYoonCSKimCKEffect of L-carnitine supplementation and aerobic training on FABPc content and beta-HAD activity in human skeletal muscleEur J Appl Physiol200799 2 193 199 1:CAS:528:DC%2BD28XhtlCktrzO https://doi.org/10.1007/s00421-006-0333-3 17089153
- RafrafMKarimiMJafariAEffect of L-carnitine supplementation in comparison with moderate aerobic training on serum inflammatory parameters in healthy obese womenJ Sports Med Phys Fitness201555 11 1363 1370 1:STN:280:DC%2BC2MvnsF2ktA%3D%3D 25600905
- KoozehchianMSDaneshfarAFallahEAgha-AlinejadHSamadiMKavianiMKavehBMJungYPSabloueiMHMoradiN et al Effects of nine weeks L-Carnitine supplementation on exercise performance, anaerobic power, and exercise-induced oxidative stress in resistance-trained malesJ Exerc Nutrition Biochem201822 4 7 19 https://doi.org/10.20463/jenb.2018.0026 30661327 6343764
- AhlborgGJensen-UrstadMMetabolism in exercising arm vs. leg muscleClin Physiol199111 5 459 468 1:STN:280:DyaK38%2Fjs1Wntg%3D%3D https://doi.org/10.1111/j.1475-097x.1991.tb00818.x 1934942
- DohertyTJInvited review: Aging and sarcopeniaJ Appl Physiol (1985)200395 4 1717 1727 1:CAS:528:DC%2BD3sXotFyrtrc%3D https://doi.org/10.1152/japplphysiol.00347.2003
- VolpatoSBianchiLCherubiniALandiFMaggioMSavinoEBandinelliSCedaGPGuralnikJMZulianiG et al Prevalence and clinical correlates of sarcopenia in community-dwelling older people: application of the EWGSOP definition and diagnostic algorithmJ Gerontol A Biol Sci Med Sci201469 4 438 446 1:CAS:528:DC%2BC2cXjvVygtbc%3D https://doi.org/10.1093/gerona/glt149 24085400
- PeakeJSuzukiKNeutrophil activation, antioxidant supplements and exercise-induced oxidative stressExerc Immunol Rev200410 129 141 15633591
- PeakeJNosakaKSuzukiKCharacterization of inflammatory responses to eccentric exercise in humansExerc Immunol Rev200511 64 85 16385845
- FritzIBArrigoni-MartelliESites of action of carnitine and its derivatives on the cardiovascular system: interactions with membranesTrends Pharmacol Sci199314 10 355 360 1:CAS:528:DyaK2cXltler https://doi.org/10.1016/0165-6147(93)90093-y 8296391
- GiamberardinoMADraganiLValenteRDi LisaFSagginiRVecchietLEffects of prolonged L-carnitine administration on delayed muscle pain and CK release after eccentric effortInt J Sports Med199617 5 320 324 1:CAS:528:DyaK28Xls1Crur0%3D https://doi.org/10.1055/s-2007-972854 8858401
- VolekJSKraemerWJRubinMRGomezALRatamessNAGaynorPL-Carnitine L-tartrate supplementation favorably affects markers of recovery from exercise stressAm J Physiol Endocrinol Metab2002282 2 E474 E482 1:CAS:528:DC%2BD38XhtFGitbo%3D https://doi.org/10.1152/ajpendo.00277.2001 11788381
- SpieringBAKraemerWJVingrenJLHatfieldDLFragalaMSHoJYMareshCMAndersonJMVolekJSResponses of criterion variables to different supplemental doses of L-carnitine L-tartrateJ Strength Cond Res200721 1 259 264 https://doi.org/10.1519/00124278-200702000-00046 17313301
- HoJYKraemerWJVolekJSFragalaMSThomasGADunn-LewisCCodayMHakkinenKMareshCML-Carnitine l-tartrate supplementation favorably affects biochemical markers of recovery from physical exertion in middle-aged men and womenMetabolism201059 8 1190 1199 1:CAS:528:DC%2BC3cXptlalsr4%3D https://doi.org/10.1016/j.metabol.2009.11.012 20045157
- SpieringBAKraemerWJHatfieldDLVingrenJLFragalaMSHoJYThomasGAHakkinenKVolekJSEffects of L-carnitine L-tartrate supplementation on muscle oxygenation responses to resistance exerciseJ Strength Cond Res200822 4 1130 1135 https://doi.org/10.1519/JSC.0b013e31817d48d9 18545197
- ReboucheCJMackDLEdmonsonPFL-Carnitine dissimilation in the gastrointestinal tract of the ratBiochemistry198423 26 6422 6426 1:CAS:528:DyaL2MXjs1Kh https://doi.org/10.1021/bi00321a022
- ReboucheCJQuantitative estimation of absorption and degradation of a carnitine supplement by human adultsMetabolism199140 12 1305 1310 1:CAS:528:DyaK3MXmtlWjsL0%3D https://doi.org/10.1016/0026-0495(91)90033-S
- ReboucheCJChenardCAMetabolic fate of dietary carnitine in human adults: identification and quantification of urinary and fecal metabolitesJ Nutr1991121 4 539 546 1:CAS:528:DyaK3MXitVGrt7g%3D https://doi.org/10.1093/jn/121.4.539 2007906
- FukamiKYamagishiSSakaiKKaidaYYokoroMUedaSWadaYTakeuchiMShimizuMYamazakiH et al Oral L-carnitine supplementation increases trimethylamine-N-oxide but reduces markers of vascular injury in hemodialysis patientsJ Cardiovasc Pharmacol201565 3 289 295 1:CAS:528:DC%2BC2MXjvFSis7k%3D https://doi.org/10.1097/FJC.0000000000000197 25636076
- VallanceHDKoochinABranovJRosen-HeathABosdetTWangZHazenSLHorvathGMarked elevation in plasma trimethylamine-N-oxide (TMAO) in patients with mitochondrial disorders treated with oral l-carnitineMol Genet Metab Rep201815 130 133 1:CAS:528:DC%2BC1cXovVaisLk%3D https://doi.org/10.1016/j.ymgmr.2018.04.005 30023305 6047224
- Samulak JJ, Sawicka AK, Samborowska E, Olek RA. Plasma Trimethylamine-N-oxide following Cessation of L-carnitine Supplementation in Healthy Aged Women. Nutrients. 2019;11(6). https://doi.org/https://doi.org/10.3390/nu11061322.
- WangZKlipfellEBennettBJKoethRLevisonBSDugarBFeldsteinAEBrittEBFuXChungYM et al Gut flora metabolism of phosphatidylcholine promotes cardiovascular diseaseNature2011472 7341 57 63 1:CAS:528:DC%2BC3MXksVCrsrw%3D https://doi.org/10.1038/nature09922 21475195 3086762
- PanASunQBernsteinAMSchulzeMBMansonJEStampferMJWillettWCHuFBRed meat consumption and mortality: results from 2 prospective cohort studiesArch Intern Med2012172 7 555 563 https://doi.org/10.1001/archinternmed.2011.2287 22412075 3712342
- TangWHWangZLevisonBSKoethRABrittEBFuXWuYHazenSLIntestinal microbial metabolism of phosphatidylcholine and cardiovascular riskN Engl J Med2013368 17 1575 1584 1:CAS:528:DC%2BC3sXntVCmsrc%3D https://doi.org/10.1056/NEJMoa1109400 23614584 3701945
- TangWHWangZKennedyDJWuYBuffaJAAgatisa-BoyleBLiXSLevisonBSHazenSLGut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney diseaseCirc Res2015116 3 448 455 1:CAS:528:DC%2BC2MXhs12kt7o%3D https://doi.org/10.1161/CIRCRESAHA.116.305360 25599331
- SuzukiTHeaneyLMBhandariSSJonesDJNgLLTrimethylamine N-oxide and prognosis in acute heart failureHeart2016102 11 841 848 1:CAS:528:DC%2BC2sXmtFyntLg%3D https://doi.org/10.1136/heartjnl-2015-308826 26869641
- GruppenEGGarciaEConnellyMAJeyarajahEJOtvosJDBakkerSJLDullaartRPFTMAO is associated with mortality: impact of modestly impaired renal functionSci Rep20177 1 13781 1:CAS:528:DC%2BC1cXhsVyksbnL https://doi.org/10.1038/s41598-017-13739-9 29061990 5653802
- Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J Am Heart Assoc. 2017;6(7). https://doi.org/https://doi.org/10.1161/JAHA.116.004947.
- SchiattarellaGGSanninoAToscanoEGiuglianoGGargiuloGFranzoneATrimarcoBEspositoGPerrinoCGut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysisEur Heart J201738 39 2948 2956 1:CAS:528:DC%2BC1cXit1WgtrfN https://doi.org/10.1093/eurheartj/ehx342 29020409
- ReboucheCJEngelAGKinetic compartmental analysis of carnitine metabolism in the human carnitine deficiency syndromes. Evidence for alterations in tissue carnitine transportJ Clin Invest198473 3 857 867 1:CAS:528:DyaL2cXhtlKqurs%3D https://doi.org/10.1172/JCI111281 6707204 425090
- WangZBergeronNLevisonBSLiXSChiuSJiaXKoethRALiLWuYTangWHW et al Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and womenEur Heart J201940 7 583 594 1:CAS:528:DC%2BB3cXjsFeks74%3D https://doi.org/10.1093/eurheartj/ehy799 30535398
- RohrmannSLinseisenJAllenspachMvon EckardsteinAMullerDPlasma concentrations of Trimethylamine-N-oxide are directly associated with dairy food consumption and low-grade inflammation in a German adult populationJ Nutr2016146 2 283 289 1:CAS:528:DC%2BC28XhtVKksrbM https://doi.org/10.3945/jn.115.220103 26674761
- CheungWKeski-RahkonenPAssiNFerrariPFreislingHRinaldiSSlimaniNZamora-RosRRundleMFrostG et al A metabolomic study of biomarkers of meat and fish intakeAm J Clin Nutr2017105 3 600 608 1:CAS:528:DC%2BC2sXhtVOlu7zL https://doi.org/10.3945/ajcn.116.146639 28122782
- YanceyPHClarkMEHandSCBowlusRDSomeroGNLiving with water stress: evolution of osmolyte systemsScience1982217 4566 1214 1222 1:CAS:528:DyaL38XlsFyisbw%3D https://doi.org/10.1126/science.7112124 7112124
- GillettMBSukoJRSantosoFOYanceyPHElevated levels of trimethylamine oxide in muscles of deep-sea gadiform teleosts: a high-pressure adaptation?J Exp Zool1997279 4 386 391 1:CAS:528:DyaK2sXntlSmtrk%3D https://doi.org/10.1002/(sici)1097-010x(19971101)279:4<386::Aid-jez8>3.0.Co;2-k
- YanceyPHGerringerMEDrazenJCRowdenAAJamiesonAMarine fish may be biochemically constrained from inhabiting the deepest ocean depthsProc Natl Acad Sci U S A2014111 12 4461 4465 1:CAS:528:DC%2BC2cXjtlyhs78%3D https://doi.org/10.1073/pnas.1322003111 24591588 3970477
- ZhangAQMitchellSCSmithRLDietary precursors of trimethylamine in man: a pilot studyFood Chem Toxicol199937 5 515 520 1:CAS:528:DyaK1MXltVChu7c%3D https://doi.org/10.1016/S0278-6915(99)00028-9
- TongTYNApplebyPNBradburyKEPerez-CornagoATravisRCClarkeRKeyTJRisks of ischaemic heart disease and stroke in meat eaters, fish eaters, and vegetarians over 18 years of follow-up: results from the prospective EPIC-Oxford studyBMJ2019366 l4897 https://doi.org/10.1136/bmj.l4897 31484644 6724406
- BainMAFaullRFornasiniGMilneRWEvansAMAccumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysisNephrol Dial Transplant200621 5 1300 1304 1:CAS:528:DC%2BD28XjslKqsro%3D https://doi.org/10.1093/ndt/gfk056 16401621
- HauetTBaumertHGibelinHGodartCCarretierMEugeneMCitrate, acetate and renal medullary osmolyte excretion in urine as predictor of renal changes after cold ischaemia and transplantationClin Chem Lab Med200038 11 1093 1098 1:CAS:528:DC%2BD3MXns1CjtA%3D%3D https://doi.org/10.1515/CCLM.2000.162 11156334
- GansevoortRTCorrea-RotterRHemmelgarnBRJafarTHHeerspinkHJMannJFMatsushitaKWenCPChronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and preventionLancet2013382 9889 339 352 https://doi.org/10.1016/S0140-6736(13)60595-4 23727170
- DammanKValenteMAVoorsAAO'ConnorCMvan VeldhuisenDJHillegeHLRenal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysisEur Heart J201435 7 455 469 https://doi.org/10.1093/eurheartj/eht386 24164864
- BielinskaKRadkowskiMGrochowskaMPerlejewskiKHucTJaworskaKMotookaDNakamuraSUfnalMHigh salt intake increases plasma trimethylamine N-oxide (TMAO) concentration and produces gut dysbiosis in ratsNutrition201854 33 39 1:CAS:528:DC%2BC1cXos1Sms7o%3D https://doi.org/10.1016/j.nut.2018.03.004 29705499
- JaworskaKHucTSamborowskaEDobrowolskiLBielinskaKGawlakMUfnalMHypertension in rats is associated with an increased permeability of the colon to TMA, a gut bacteria metabolitePLoS One201712 12 1:CAS:528:DC%2BC1cXhsV2ru7%2FF https://doi.org/10.1371/journal.pone.0189310 29236735 5728578
- XuMBhattDKYeungCKClawKGChaudhryASGaedigkAPearceREBroeckelUGaedigkRNickersonDA et al Genetic and nongenetic factors associated with protein abundance of Flavin-containing Monooxygenase 3 in human liverJ Pharmacol Exp Ther2017363 2 265 274 1:CAS:528:DC%2BC1cXhtleltrbF https://doi.org/10.1124/jpet.117.243113 28819071 5697103
- UfnalMPhamKThe gut-blood barrier permeability - a new marker in cardiovascular and metabolic diseases?Med Hypotheses201798 35 37 1:CAS:528:DC%2BC28XhvFKnu7vI https://doi.org/10.1016/j.mehy.2016.11.012 28012600
- LangoRSmolenskiRTNarkiewiczMSuchorzewskaJLysiak-SzydlowskaWInfluence of L-carnitine and its derivatives on myocardial metabolism and function in ischemic heart disease and during cardiopulmonary bypassCardiovasc Res200151 1 21 29 1:CAS:528:DC%2BD3MXktVOisr0%3D https://doi.org/10.1016/s0008-6363(01)00313-3 11399244
- IlicetoSScrutinioDBruzziPD'AmbrosioGBoniLDi BiaseMBiascoGHugenholtzPGRizzonPEffects of L-carnitine administration on left ventricular remodeling after acute anterior myocardial infarction: the L-Carnitine Ecocardiografia Digitalizzata Infarto Miocardico (CEDIM) trialJ Am Coll Cardiol199526 2 380 387 1:CAS:528:DyaK2MXntlKht7Y%3D https://doi.org/10.1016/0735-1097(95)80010-E
- HiramatsuAAikataHUchikawaSOhyaKKodamaKNishidaYDaijoKOsawaMTeraokaYHondaF et al Levocarnitine use is associated with improvement in sarcopenia in patients with liver cirrhosisHepatol Commun20193 3 348 355 1:CAS:528:DC%2BC1MXjvFOjsbw%3D https://doi.org/10.1002/hep4.1309 30859147 6396356
- HathcockJNShaoARisk assessment for carnitineRegul Toxicol Pharmacol200646 1 23 28 1:CAS:528:DC%2BD28XptFCnsLw%3D https://doi.org/10.1016/j.yrtph.2006.06.007 16901595
- ShangRSunZLiHEffective dosing of L-carnitine in the secondary prevention of cardiovascular disease: a systematic review and meta-analysisBMC Cardiovasc Disord201414 88 1:CAS:528:DC%2BC2cXhvFKjs7jF https://doi.org/10.1186/1471-2261-14-88 25044037 4223629
- BakalovDSabitZTafradjiiska-HadjiolovaRRe: effect of l-carnitine supplementation on muscle cramps induced by stroke: a case reportNutrition202075-76 110771 https://doi.org/10.1016/j.nut.2020.110771 32268976