Abstract
Background
Previous studies have suggested that drug pricing could contribute to drug shortages; however, there is limited quantitative assessment of this potential causal association. This retrospective database analysis aimed to investigate the association between drug prices and drug shortage incidents in Saudi Arabia.
Methods
This was a retrospective database analysis study. Drugs with shortage notifications sent to the Saudi Food and Drug Authority (SFDA) between January 2017 and December 2020 were included. Each drug's foreign-to-Saudi price ratio (FTSPR) was calculated by dividing the mean international price by the Saudi price. Drugs were categorized into three groups based on their FTSPR: Group 1 (FTSPR > 1), Group 2 (FTSPR = 1), and Group 3 (FTSPR < 1). The primary outcome was the ratio of mean counts (mCR) between the three groups, with Group 3 serving as the control group. The analysis was adjusted for the measured confounders using a negative binomial regression model.
Results
A total of 900 drugs were included in the study, with 348 in Group 1, 345 in Group 2, and 209 in Group 3. The mean count in Group 1 was higher compared to Group 3 (mCR: 1.88; 95% confidence interval [CI] 1.24 to 2.83), while the mean counts between Group 2 and Group 3 were comparable (mCR: 1.39; 95% CI 0.92 to 2.09).
Conclusions
Our findings indicate an association between drug shortage incidents and higher prices of drugs outside Saudi Arabia. Further studies are needed to explore this causal relationship in different contexts.
Keywords:
Background
Drug shortage was defined by the United States [US] Federal Food, Drug, and Cosmetic Act as periods when the demand or projected demand for a drug exceeds the supply [Citation1]. It has emerged as a significant global challenge due to its multifaceted nature and the adverse clinical, social, and economic consequences it entails [Citation1–Citation21]. A recent study found that a large percentage of drug shortage incidents in the Netherlands were anticipated to have moderate to high negative clinical, humanistic or economic patient impacts [Citation22]. Various studies have identified clinical, economic, regulatory, and policy-related factors as contributors to drug shortages [Citation1–Citation21]. Among these factors, drug pricing has been identified as a potential risk; however, only a few studies have explored this causal association, and none was conducted in Saudi Arabia [Citation1–Citation21].
An analysis of a US database of commercial outpatient pharmacy claims, period 2008 to 2014, revealed that the duration of drug shortages was influenced by the gradual percentage increase in drug prices (i.e. higher percentage increases were associated with longer durations of drug shortages) [Citation11]. However, the study solely focused on generic drugs [Citation11]. A Canadian study examining a new generic pricing strategy implemented in 2014, known as the Tiered-Pricing Framework, did not find an association with the risk of disrupting the entry of generic drugs into Canada (although this study did not analyze actual shortage incidents) [Citation20]. Other studies indirectly addressed this causal association or considered it as a secondary outcome (e.g. a Canadian study suggested that markets with a single generic manufacturer and lower profit margins were more susceptible to shortages) [Citation21].
Limited research exists in Saudi Arabia regarding drug shortages in general and on the identification of risk factors related to such shortages in particular [Citation3, Citation14–Citation16]. Two studies have touched upon drug pricing as a potential risk factor for drug shortages, albeit without conducting quantitative causal analyses [Citation15, Citation16]. The pricing of both prescription and non-prescription drugs in Saudi Arabia is determined and regulated by the Saudi Food and Drug Authority (SFDA) in accordance with the SFDA pharmaceutical pricing guideline [Citation23]. Upon receiving initial marketing authorization approval from the SFDA, brand-name small molecules, biologics, and generic drugs intended for the public and private sectors in Saudi Arabia are subject to the SFDA pricing system [Citation23]. The initially assigned price undergoes a price-revaluation process throughout its regulatory life cycle, allowing for a maximum reduction of 30% under specific circumstances such as revising the prices of the entire therapeutic class, lowering the price in the manufacturer's country, renewing the marketing authorization, or modifying the status of the marketing authorization (i.e., variation) [Citation23]. This study aimed to evaluate the association between drug prices and the likelihood of drug shortage incidents using a Saudi drug shortage population.
Methods
Study design and data source
To identify drugs reported as experiencing shortages between January 2017 and December 2020, data were extracted from the database maintained by the Saudi Food and Drug Authority’s (SFDA) Drug Availability and Tracking Center. Various stakeholders, including marketing authorization holders, public and private health sectors, and patients, utilize different reporting systems such as the Track and Trace System to report incidents of drug shortages to the SFDA, usually referencing the SFDA’s drug registration number [Citation23]. However, in order to validate the occurrence of an actual drug shortage incident, confirmation from the SFDA (i.e., validation process) is required. In the study period, confirmation of the reported shortage incident relied on the marketing authorization holder. Presently, the confirmation of the reported shortage incident is accomplished by cross-referencing the SFDA Track and Trace System and/or verifying the existence of drug shortages with the marketing authorization holder.
All identified shortage incidents were unique for each SFDA drug registration number, meaning that no duplicate shortage incidents were found. The inclusion criteria did not consider the severity or anticipated negative clinical impact of the shortage. Once drugs with shortage incidents were identified, their local and international prices at the time of the shortage report were obtained from the SFDA database and the S&P Global Database [Citation24]. The latter is an information service provider that extracts the updated international prices from the authenticated and relevant sources within each country [Citation24]. Relevant data regarding potential confounding factors were extracted from both the internal and publicly available domains of the SFDA.
Study exposures, outcomes and confounders
For each drug, whether it was a brand-name small molecule, biologic or generic drug, the study calculated the foreign-to-Saudi price ratio (FTSPR) by dividing the mean price of the drug in the international market (numerator) by its price in Saudi Arabia (denominator) during the year of the shortage incident. Based on the FTSPR ratio, the drugs were classified into three groups: Group 1 included drugs with higher international prices compared to local prices (FTSPR ratio > 1), Group 2 included drugs with similar international and local prices (FTSPR ratio = 1), and Group 3 included drugs with higher local prices compared to international prices (FTSPR ratio < 1). Group 3 was chosen as the control arm for between-group comparisons, assuming that the likelihood of shortage due to pricing might be lower in this group given their high local prices.
The analysis of the study outcome was adjusted for several confounding variables, including the type of drug registration (brand-name small molecules, biologics, local, or international generics), route of administration (oral, injections, or other preparations), country of the manufacturer (local, regional, or international companies), cause of the shortage (as outlined in Table ), and whether the shortage incident was reported before or after the confirmation of human-to-human transmission of coronavirus disease 2019 (COVID-19) (pre-2020 vs. 2020). The selection of these confounding variables was based on direct acyclic graphs (DAGs) and previous literature indicating their potential as risk factors for drug shortages [Citation1–Citation21].
Table 1 Description of causes of drug shortage
Statistical analysis
Descriptive summaries were provided for the study groups, and Chi-square tests were used to explore differences in the distribution of the confounding variables among the groups. The main hypothesis of the study was that the number of shortage incidents in drugs that are more expensive in Saudi Arabia (Group 3) would be lower compared to the other groups. To test this hypothesis, mean count ratios (mCRs) were estimated between Group 1 vs. Group 3 and Group 2 vs. Group 3. A negative binomial regression model was employed to estimate the mCRs and their corresponding 95% confidence intervals while adjusting for the measured confounding variables. The use of a Poisson regression model was not feasible due to the presence of overdispersion in the data. All analyses were performed using RStudio Version 1.2.5033.
Results
During the study period, a total of 1082 drugs were reported to the SFDA as experiencing shortages, resulting in an average rate of 271 shortage incidents per year. The highest number of shortages occurred in 2019 (n = 544) and 2020 (n = 300). Pricing details necessary for calculating the FTSPR were available for 900 out of the 1082 drugs, representing 427 unique active pharmaceutical ingredients or fixed combinations. The median duration of shortage was 8.2 months, excluding drugs that were discontinued from the market and those reported in 2017 (the year during which the shortage notification system was established).
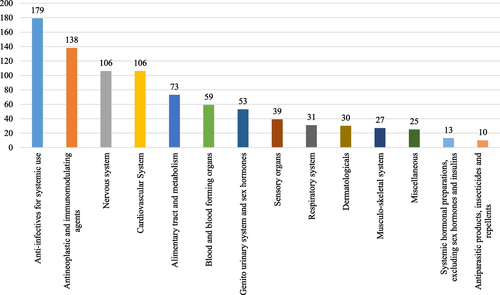
Among the 900 drugs with available pricing details, the majority were brand-name small molecules (47%) and international generics (38%) (Table ). In terms of preparations, 47% were oral and 40% were injectable. The most common reasons for shortage were voluntary discontinuation of registrations by the marketing authorization holders (47.5%) and marketing challenges (23.2%). Additionally, the majority of shortages occurred prior to the confirmation date of human-to-human transmission of COVID-19 (Table ). The shortages encompassed 13 main therapeutic classes based on the Anatomical-Therapeutic Chemical (ATC) classification system maintained by the World Health Organization, as depicted in Fig. . The most frequently reported drugs in shortage were anti-infectives for systemic use (19.9% of the 900 drugs) and antineoplastic and immunomodulating agents (15.3% of the 900 drugs). Notable examples of commonly reported drugs in shortage included temozolomide (n = 15), glucose/dextrose (n = 13), cephalexin (n = 13), methotrexate (n = 12), and warfarin (n = 11).
Table 2 Characteristics of the study groups
Most of the reported drugs with shortage during the study period belong to Group 1 (FTSPR > 1, n = 348), followed by drugs in Group 2 (FTSPR = 1, n = 343), and Group 3 (FTSPR < 1, n = 209). According to the negative binomial regression model, drug shortages were higher in Group 1 compared with Group 3 (mCR: 1.88; 95% confidence interval [CI] 1.24 to 2.83). However, the mean counts of between Group 2 and Group 3 were comparable, with an mCR of 1.39 (95% CI 0.92 to 2.09).
Discussion
Our study results demonstrated that drug shortages were mostly reported for drugs with higher prices in markets outside of Saudi Arabia. This group of drugs was also more likely to be associated with shortage incidents compared to drugs with higher prices in Saudi Arabia. The likelihood of shortage was found to be similar between drugs with comparable prices both inside and outside of Saudi Arabia, as well as drugs with higher prices in Saudi Arabia.
Our study revealed a potential association between drug pricing and the risk of shortage, which aligns with findings from a previous analysis of a US database of commercial outpatient pharmacy claims spanning from 2008 to 2014 [Citation11]. To the best of our knowledge, these two studies are the only available literature that quantitatively examine the relationship between drug pricing and the risk of shortage. It is important to note that the majority of shortage incidents included in our study occurred prior to the implementation of the updated pricing guideline in late 2020 (the guideline was officially published in early 2021) [Citation23]. One of the main objectives of updating the guideline was to mitigate the risk of drug shortage during pricing processes, such as by reviewing the availability of alternative treatment options and adjusting price-related requests in a manner that does not disrupt product supply [Citation23]. A future study should be conducted to evaluate the impact of this guideline update on the likelihood of drug shortages. Such studies may assist policy makers in incorporating drug availability considerations into the pricing mechanisms of pharmaceutical products.
In contrast to the United States and Europe, our study found that the most commonly reported therapeutic classes experiencing shortages in Saudi Arabia were anti-infectives for systemic use and antineoplastic and immunomodulating agents. In the US and Europe, the most commonly reported therapeutic classes with shortages were drugs for the central nervous system, followed by fluids/electrolytes in the US and drugs for the cardiovascular system in Europe [Citation11, Citation17, Citation19, Citation25]. The number of shortage incidents in 2020 in Saudi Arabia (n = 300) was lower compared to the US (n = 1106), Spain (n = 814), Norway (n = 800), Sweden (n = 890), and Finland (n = 1522) [Citation19]. The duration of shortages observed in our study was comparable to the reported shortage duration in the US (8.2 months vs. 8.4 months, respectively) [Citation11]. In our study, less than half of the drugs experiencing shortages were discontinued voluntarily by the marketing authorization holders, with reasons for discontinuation not provided in the system. The second most commonly reported reason for shortage was marketing challenges. On the other hand, supply/demand issues (where manufacturers are unwilling or unable to meet drug demand) and manufacturing issues (such as quality problems) were the most commonly reported reasons for shortage in the US [Citation17, Citation26]. A survey conducted by the European Association of Hospital Pharmacists in 2019 indicated that global shortages of active pharmaceutical ingredients and manufacturing problems were the most frequently cited possible reasons for drug shortages [Citation18]. Furthermore, similar to Europe, our study found that oral preparations were more likely to experience shortages, while in the US, injectable preparations were more prone to shortages [Citation11, Citation17, Citation19, Citation26].
Our study represents the first analysis to investigate the causal association between drug prices, or any other risk factor for drug shortage, and the likelihood of drug shortage in Saudi Arabia. It is also one of the few studies in the literature that quantitatively assessed this causal relationship. All shortage notification data in our study underwent validation by the SFDA upon receiving a notification; thereby enhancing the validity of the study data. However, our study has two limitations. Firstly, the reasons behind the voluntary discontinuation of marketing authorization for less than half of the studied drugs remain unknown. Secondly, due to the recent establishment of the Drug Availability and Tracking Center at that time, complete availability of the 2017 and 2018 drug shortage notifications was not achieved.
Conclusions
Our study found that drug pricing may be associated with the risk of shortage, with drugs that have higher prices outside Saudi Arabia being more likely to be in shortage. The study also highlights the need for further research on the impact of drug pricing on the risk of drug shortages and the need for measures to address the various reasons for drug shortages.
Disclaimer
The views expressed in this paper are those of the author(s) and not do not necessarily reflect those of the Saudi Food and Drug Authority or its stakeholders.
Author contributions
TAT and MAA wrote the manuscript. All authors designed and performed the research. MAA, OAA, MIA and TAT analyzed the data. TAT supervised the entire research.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Abbreviations
| US | = | United States |
| SFDA | = | Saudi Food and Drug Authority |
| FTSPR | = | Foreign-to-Saudi price ratio |
| COVID-19 | = | Coronavirus disease 2019 |
| DAGs | = | Direct acyclic graphs |
| mCR(s) | = | Mean count ratio(s) |
| ATC | = | Anatomical-Therapeutic Chemical |
| CI | = | Confidence interval |
Acknowledgements
None.
Funding
No funding was allocated for this study.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due confidentiality but are available from the corresponding author on reasonable request (some data are available from S&P Global Database but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Report | drug shortages: root causes and potential solutions. U.S. Food and Drug Administration. 2020. https://www.fda.gov/drugs/drug-shortages/report-drug-shortages-root-causes-and-potential-solutions. Accessed 01 Sept 2022.
- Chow CK, Nguyen TN, Marschner S, et al.Availability and affordability of medicines and cardiovascular outcomes in 21 high-income, middle-income and low-income countriesBMJ Glob Health20205 10.1136/bmjgh-2020-002640 33148540 7640501
- Alsheikh M, Seoane-Vazquez E, Rittenhouse B, et al.A comparison of drug shortages in the hospital setting in the United States and Saudi Arabia: an exploratory analysisHosp Pharm2016515370375 1:CAS:528:DC%2BC2sXns1Chsb4%3D 10.1310/hpj5105-370 27303090 4896345
- Lyengar S, Hedman L, Forte G, et al.Medicine shortages: a commentary on causes and mitigation strategiesBM Med201614124 10.1186/s12916-016-0674-7
- Pauwels K, Huys I, Casteels M, et al.Drug shortages in European countries: a trade-off between market attractiveness and cost containment?BMC Health Serv Res201414438 10.1186/1472-6963-14-438 25257912 4263120
- Kaakeh R, Sweet BV, Reilly C, et al.Impact of drug shortages on US health systemsAm J Health-Sys Pharm2011681918111819 10.2146/ajhp110210
- Fox ER, Tyler LSPotential association between drug shortages and high-cost medicationsPharmacotherapy20173713642 10.1002/phar.1861 27891635
- Tucker EL, Cao Y, Fox E, et al.The drug shortage era: a scoping review of the literature 2001–2019Clin Pharmacol Ther2020108611501155 10.1002/cpt.1934 32521038
- Alvizakos M, Detsis M, Grigoras CA, et al.The impact of shortages on medication prices: implications for shortage preventionDrugs20167615511558 10.1007/s40265-016-0651-7
- Phuong JM, Penm J, Chaar B, et al.The impacts of medication shortages on patient outcomes: a scoping reviewPLoS ONE2018145 10.1371/journal.pone.0215837
- Dave CV, Pawar A, Fox E, et al.Predictors of drug shortages and association with generic drug prices: a retrospective cohort studyValue Health2018211112861290 10.1016/j.jval.2018.04.1826 30442275
- Gupta DK, Huang S-MDrug shortages in the United States: a critical evaluation of root causes and the need for actionClin Pharmacol Ther2013932133135 1:STN:280:DC%2BC3szht1ymtQ%3D%3D 10.1038/clpt.2012.229 23337520
- Gatesman ML, Smith TJThe shortage of essential chemotherapy drugs in the United StatesNEJM20113651816531655 1:CAS:528:DC%2BC3MXhsVWqtbbP 10.1056/NEJMp1109772 22040130
- Alruthia YS, Alkofide H, Alajmi R, et al.Drug shortages in large hospitals in Riyadh: a cross-sectional studyAnn Saudi Med2017375375385 10.5144/0256-4947.2017.375 28988252 6074191
- Alruthia YS, Alwhaibi M, Alotaibi M, et al.Drug shortages in Saudi Arabia: root causes and recommendationsSaudi Pharm J2018267947951 10.1016/j.jsps.2018.05.002 30416350 6218331
- Alshehri S, Alshammari ADrug supply shortages in pharmacies: causes and solutions; a case study in King Khaled Eye Special HospitalInt Bus Manag2016101224532459
- Shukar S, Zahoor F, Hayat K, et al.Drug shortage: causes, impact, and mitigation strategiesFront Pharmacol202112 1:CAS:528:DC%2BB3MXhsl2gsr3M 10.3389/fphar.2021.693426 34305603 8299364
- 2019 EAHP medicine shortages report. European Association of Hospital Pharmacists. 2020. https://www.eahp.eu/sites/default/files/eahp_2019_medicines_shortages_report.pdf. Accessed 01 Sept 2022.
- Ravela R, Lyles A, Airaksinen MNational and transnational drug shortages: a quantitative descriptive study of public registers in Europe and the USABMC Health Serv Res2022221940 10.1186/s12913-022-08309-3 35869486 9306441
- Zhang W, Sun H, Guh DP, et al.The impact of tiered-pricing framework on generic entry in CanadaInt J Health Policy Manag2022116768776 33233033
- Zhang W, Guh DP, Sun H, et al.Factors associated with drug shortages in Canada: a retrospective cohort studyCMAJ Open202083E535E544 10.9778/cmajo.20200036 32873582 7641197
- Postma DJ, Notenboom K, De Smet PAGM, et al.Medicine shortages: impact behind numbersJ of Pharm Policy Pract202316144 10.1186/s40545-023-00548-x
- . Saudi Food and Drug Authority. 2021. https://www.sfda.gov.sa/ar/regulations/66357. Accessed 01 Sept 2022.
- RSD. Saudi Food and Drug Authority. 2018. https://rsd.sfda.gov.sa/registration-and-e-services-en.html. Accessed 01 Aug 2022.
- IHS Markit. https://ihsmarkit.com/index.html. Accessed 01 Sept 2022.
- Drug shortage statistics. American Society of Health-System Pharmacists. 2022. https://www.ashp.org/drug-shortages/shortage-resources/drug-shortages-statistics?loginreturnUrl=SSOCheckOnly. Accessed 01 Sept 2022.