Abstract
In fewer than 10 years, umbilical cord blood has transformed from a medical waste to a lifesaving treasure that parents in Taiwan are willing to store at great expense. As a blooming industry with yearly revenues of 600-700 million NT dollars, family banks annually store the cord blood of roughly 19,000 newborns, a number that constitutes 8-10% of newborns in Taiwan each year. Yet, given the predominant role market force plays in Taiwan's cord blood banking, how does it affect people's understanding of the technology and their imagination of their individuality and relationship with one another in the future? This paper reveals how private cord blood has successfully framed the practice of cord blood banking as a form of biological insurance, thereby downplayed the uncertainties inherent in the development of science, reinforced the private nature of the cord blood stored, while impoverishing parents' understanding and imagination of an alternative biosociality where cord blood can benefit more people. Moreover, by distinguishing between private cord blood banking and medical practice, this analogy of biological insurance also co-produced a more lenient governing framework based on parents' informed consent usually suitable for consumer transaction, but unwittingly placed physicians in a more vulnerable position to guard against conflicts of interest when providing information to prospective parents and collecting cord blood during the process of delivery. Hence, by exploring the technological, social, and regulatory contexts of the popularity of Taiwan's private cord blood banking, this paper seeks to make policy suggestions beyond informed consent hoping to empower people's role both as consumers as well as biological citizens to think more critically about the technology and to govern it more adequately.
Introduction and Analytical Framework
Cord blood stem cells are self-renewing primitive cells that have the ability to differentiate into specific tissue or body parts. Also known as “regenerative medicine,” transplantations of cord blood or other types of stem cells can be used to regenerate failed organs or tissue. Scientists are hoping that, should the technology mature, they can use it to treat Parkinson's disease, Alzheimer's disease, stroke, spinal cord injuries, arthritis, metabolism-related disease such as diabetes, and many other diseases.
With this potentiality, biomedical research based on cord blood or other human tissue have attracted venture-capital investments as well as funding from government and academia worldwide (CitationRajan 2006; CitationWaldby 2002). Under the name of “bioeconomy” (CitationRose 2007) or “tissue economies” (CitationWaldby and Mitchell 2006), the purpose of these investments is to capture the latent values in biological processes and renewable bioresources and eventually to improve people's health while facilitating sustainable developments in economies.
The prospect of stem cell medicine also opens up new possibilities for people to reflect upon their individuality and their relationship with others. Stem cell transplantation depends on the availability of tissues involving human leukocyte antigen (HLA)-type matches between donors and patients: by linking people's fates together on the basis of a shared genetic or somatic status, this type of transplantation suggests the possibility of forming a new bio-sociality, or communities whose members take care of each other, and this generalized practice could, in turn, affect one's individuality (CitationRainbow 1996; CitationRose 2007).
The problem is, given the strong market forces underlying the practice of cord blood banking worldwide, how would this factor shape the individuality and biosociality of people who share the same somatic or genetic status? Can stem cell transplantation truly benefit a great many people when it matures?
Using cord blood banking in Taiwan as an example, this paper responds to the aforementioned issues. Although still in a very primitive stage, stem cell science has been greeted by Taiwanese parents with enthusiasm. As a blooming industry with yearly revenues of 600-700 million NT dollars, Footnote1 family banks annually store the cord blood of roughly 19,000 newborns, a number that constitutes 8-10% of newborns in Taiwan each year. Footnote2 This popularity is extraordinary compared to the USA whose storage rate is only 2.6%, and Europe whose rate is 0.6%. Footnote3 One family bank even claimed that Taiwan's private cord blood banks' storage rate is the second highest in the world, with South Korea's storage rate (15-17%) taking the lead. Footnote4 Yet, the popularity of cord blood banking is limited to family banks, as Tzi-Chi, the largest nonprofit bank in Taiwan, stopped collecting cord blood at the end of 2008.
This paper argues that the market forces underlying cord blood banking (1) impoverish the public's understanding regarding the choices that cord blood banking may offer, (2) affect how the government regulated the practice, and (3) leave medical professionals more vulnerable to conflict of interests when providing information to their patients and when collecting cord blood during the process of delivery. Based on this observation, this paper proposes policy suggestions that seek to go beyond individuals' informed consent and bring public value and medical professionals' integrity back into the governing structure.
To conduct this analysis, this paper rests upon two theoretical concerns. The first concern is technological progressivism in the literature of the public's understanding of science. Looking into the biotechnology industry in the USA and India, Rajan (Citation2006) argues that, rather than attract capital investments with a marketable commodity, the industry attracts them with hopes, hype, and speculation of future returns. Likewise, in interpreting people's conception of science and technology, Daniel Lee Kleinman argues that scientism and technological progressivism are the two major features of our discursive landscape that hamper people's critical thinking about science and technology (CitationKleinman 2005). Scientism refers to a mistaken perception of seeing a clear division between fact and norm; facts are cognitively superior to norms; and facts should be left to trained scientists who follow a scientific method that is politically neutral and free of values. Complementing scientism is the discourse of technological progressivism, in which progress is mistakenly seen as a synonym for the good, and technology as the tool for all progressive projects (CitationKleinman 2005). These discourses permeate discussions about health, food safety, and environmental protection, and neglect how social and political factors also shape the technology and determine who benefits from it (CitationWinner 1986). Based on this theoretical concern, the current paper explores how family banks in Taiwan, by analogizing cord blood banking as a biological insurance for risk management, has downplayed the uncertainties characterizing the development of stem cell science and has limited people's access to information and people's choices relative to public donation.
A second analytical concern stems from the issue of “boundary-drawing” in the literature of governance of science. Originally developed by Gieryn (Citation1983), the analytical framework of “boundary work” is used to question the boundary drawn between science and non-science. For instance, by examining the debate over nuclear waste disposal in Yucca Mountain, Macfarlane argues that how issues are framed first influences what governance is needed and then serves to limit possibilities for democratic and open debate (2003). In the end, how the issue is framed and how the boundary of governance is drawn tends to co-produce each other (CitationMacfarlane 2003; CitationIrwin 2007). According to Irwin, this concept of co-production is significant for scientific governance (CitationIrwin 2007; Jasanoff Citation2004a, Citation2004b). First, it has shed light on how knowledge, expertise, technical practices, and material objects shape, sustain, subvert, or transform the relations of authority. Second, by addressing the co-production that takes place between society and nature, it avoids social and scientific determinism. Third, it allows us to examine the interplay among the cognitive, the material, the social, and the normative. Hence, how an issue is framed influences who governs the subject issue, according to what principle, and how. Vice versa, this boundary of governance further determines what kind of information is produced to the user of the technology and may shape how they perceive the product. Based on this theoretical concern, this paper will examine how family banks in Taiwan, by framing cord blood banking as a form of biological insurance, affect the assumption of the government's governing framework and co-produce a more lenient regulation designed for consumer transaction rather than medical practice, in turn leaving physicians' relationship with the family banks and their patients more vulnerable to conflicts of interest.
By using the term ‘governance’ rather than ‘regulation’ or ‘government’, this paper intends to broaden the discussion of regulation beyond government agencies' command and control. Indeed, Irwin(Citation2007) argues that ‘governance’ should not refer merely to coercive or non-coercive measures taken by the government; rather, it should include all activities conducted by actors including industry, scientific organizations, public and private pressure groups, and consumers, as well as the market. While all of these actors may seek to exercise certain control over a specific issue, each of them does so through different models of governance that presuppose different organizational mechanisms, operational assumptions, modes of thoughts, and consequential activities (CitationIrwin 2007).
The data of this analysis comes from the following sources. First, I examined the content on the websites of two of Taiwan's leading family banks from January to May, 2009. Second, using the keyword “cord blood,” Isearched the database of Taiwan's three major newspapers, Liberty Times, Chinatimes, and United Daily News, for the period encompassing the past ten years (i.e., between 1999 and 2009). Third, I collected and examined contracts between family banks and consumers from four companies, including the two leading family banks in the market, and another one that has the most cases of successful allogeneic transplantations. Fourth, I examine governmental regulation or regulatory decisions relevant to cord blood banking. And finally, to supplement the aforementioned data, I interviewed people familiar with the industry, including one parent who stored his/her child's cord blood in one of the leading family banks in the market, one obstetrician who has routine experience collecting cord blood for different family banks, and one scientist that worked in the industry and is familiar with the market.
I should mention one caveat regarding the terminology used in this paper. The literature on cord blood banking usually uses the term ‘private bank’ to refer to for-profit banks that store cord blood for autologous use, and the term ‘public bank’ to refer to nonprofit or government-funded banks that store cord blood for allogeneic uses (CitationIOM 2005). But increasingly, for-profit banks also have programs or inventories that provide cord blood for allogeneic uses. Hence, I will use the term ‘private cord blood banking’ to refer to the practices where parents store their newborns' cord blood for autologous use or for family members' needs—usually in a for-profit bank; I will use the term ‘family bank’ to refer to banks or programs that store this particular type of cord blood unit; and I will use ‘public bank’ or ‘public program’ to refer to those organizations that provide cord blood for allogeneic uses in the cases of needy strangers, regardless of whether the cord blood is stored in for-profit or not-for-profit banks.
Private Cord Blood Banking and the Framing of the Issues
Cord blood is the remainder of newborn infants' blood left in the placenta after delivery. It contains many hematopoietic stem cells (hereinafter HSC), which are primitive cells that can produce different types of blood cells and immune system cells. In addition to cord blood, HSC can also be found in bone marrow and peripheral blood.
Studies and professional societies unanimously recognized the advantage of using cord blood in stem cell transplantation in comparison with other sources of HSC (American Academy of Pediatrics, hereinafter CitationAAP, 2007; Royal College of Obstetricians and Gynaecologists, hereinafter CitationRCOG, 2006; the European Group on Ethics in Science and New Technologies, hereinafter CitationEU Group on Ethics 2004). First of all, because stem cells are collected from disposed umbilical cords and placenta, such collection is non-invasive and, hence, less risky than other procedures.
Second, since cord blood is put in storage, once it is needed, it is immediately available. Indeed, McGuckin and Forraz (Citation2008) estimate that, although there are 11 million bone marrow donors listed on a world registry, as many as 50% of patients awaiting bone marrow transplantation may never find a donor, not only because matching HLA and immunology is complicated, but also because locating donors is time consuming and difficult.
Third, because cord blood is a more primitive form of stem cell, only 4/6 or 5/6 of HLA types need to be matched with one another for a transplantation to succeed. Hence, cord blood is relatively more likely to produce rapid matches, which are often critical for patients in need of HSC transplantation. The lower threshold for matching also gives minority patients a better chance of finding matches, since there are typically fewer donors from minority groups. Finally, and most importantly, given its ability to create better compatibility between donor and host, cord blood transplantation is less likely than other sources of HSC to develop graft-versus-host disease.
Since the first successful case of an “umbilical cord blood” transplantation in 1988, the world has been anticipating more successes and wider applications of umbilical cord stem cell transplantations (CitationGluckman et al. 1989). Over the years, more than 6,000 patients have received cord blood transplantations donated chiefly from unrelated donors (CitationCopelan 2006).
Despite the rosy prospect of cord blood transplantation, whether it is wise to store the blood for future use is another issue. Yet, intriguingly, by framing this issue differently, medical societies and private cord blood banks have responded with opposite answers. Namely, by weighing the clinical benefits and risks of cord blood banking, medical societies have suggested that for newborns that do not come from high-risk families, storing their cord blood for autologous use is neither medically necessary nor safe and fair. Vice versa, by framing cord blood banking as a form of life insurance, family banks have argued that storing cord blood is a smart investment. The following section examines these assertions respectively.
Issue Framed by Medical Societies: Is Private Cord Blood Banking Clinically Cost Beneficial
The Merits of Storing Cord Blood for Autologous Use
Medical professional societies have repeatedly expressed their reservations about parents who, despite exhibiting no risk of related conditions, store their children's cord blood for the family's or the children's own needs (CitationAAP 2007; CitationRCOG 2006; CitationEU Group on Ethics 2004). For these children, the chance that they will need transplantation ranges from 1:217 to 1:20,000 (CitationAnnas 1999; CitationJohnson 1997; CitationNietfeld et al. 2008), and not until 2007 was the first case of autologous cord blood transplantation reported in a low-risk child (CitationHayani et al. 2007). Moreover, for a leukemia-stricken child who does need HSC transplantation, neither the child nor anyone else should use the child's cord blood, since it may carry leukemogenic mutations (CitationRCOG 2006; CitationEU Group on Ethics 2004).
Hence, the EU Group on Ethics pointed out that “the legitimacy of commercial cord blood banks for autologous use should be questioned as they sell a service which has presently no real use regarding therapeutic options” (CitationEU Group on Ethics 2004: 20). This assertion is echoed by other professional societies (CitationAAP 2007; CitationAmerican Medical Association 2007; CitationRCOG 2006; American College of Gynecology and Obstetrics, hereinafter CitationACOG 1997).
Efficiency and Equality of Cord Blood Units Stored for Autologous Use
Professional societies' second critique of private cord blood banking pertains to its effect on distributive justice. As of 2008, the National Marrow Donor Program in the USA reported that of all cord blood transplantations performed, cord blood units provided by public banks were 650 times greater in number than those provided by family banks. Footnote5 Ironically, family banks substantially store many more cord blood units than public banks. Footnote6
Both the larger number of cord blood units stored in family banks and lesser benefits achieved by this source of cord blood raise efficiency concerns and equality concerns for the practice of private cord blood banking. Hence, for instance, in its updated statement in 2007, the AAP recommends that physicians both discourage patients from private cord blood banking and encourage patients to donate to public registries (CitationAAP 2007). The French National Consultative Ethics Committee (i.e., “Comité Consultatif National d'Ethique) went as far as to say that family banks “contradict the principle of solidarity, without which no society can survive” and that if cord blood transplantation proves to be useful, “the principles of justice and equity should predominate and autologous storage should become routine and taken in charge by public authorities” (CitationEU Group on Ethics 2004). Italy even forbade private cord blood banking altogether (CitationEU Group on Ethics 2004).
Cord Blood Collections' Negative Effect on the Safety of Delivery
In addition to its low utility, cord blood banking also comes under fire for the potential risk it may bring to the process of delivery. A closer examination requires some explanation of the collection process. The collection of cord blood is generally simple. After sterile preparation, one can puncture the umbilical vein with a needle attached to a sterile and a closed-system collection bag that is placed lower than the placenta, so that the cord blood can flow into the bag with the help of gravity (CitationKurtzberg 2009). Through this process, an experienced collector can gather 100 ml of blood from a single placenta, which takes approximately 5-10 minutes (CitationKurtzberg 2009). The collector then labels the cord blood unit and ships it to a cord blood bank to be processed, tested, cryopreseved, and stored.
Most cord blood banks rely on obstetricians to collect the cord blood, who usually collect it in utero, namely, after the delivery of the newborn and before the delivery of the placenta. According to a survey by the Institute of Medicine (IOM) on cord blood banks in the United States, 19 out of the 21 cord blood banks that responded to the survey relied on obstetricians to collect the cord blood (CitationIOM 2005: 80). Only very few banks rely on their own trained personnel, who usually collect it ex utero, namely outside the delivery room after the whole process of delivery is over. Collecting cord blood ex utero is more costly since it requires additional personnel from the cord blood bank. But the process is less invasive, since it collects the cord blood only after the umbilical cord and placenta have been separated from the uterus. In contrast, studies indicate that collecting cord blood in utero usually yields larger amounts of cord blood (CitationSolves et al. 2003). No difference has been found in the CD34+ or cell counts in the two methods (CitationLasky et al. 2002).
So far, no case has been reported to suggest that in utero cord blood collection may adversely affect the mother's or newborns' safety during the process of delivery (CitationBallen 2005). But RCOG in the UK is concerned about whether the timing and the burden of in utero collection may divert physicians' attention away from the mother and the baby, and whether the procedure could be too dangerous for deliveries with complications, particularly in Cesarean sections, where timeliness is critical for minimizing blood loss in the surgery (2006). The RCOG argues that given the speculative value of cord blood banking, the aforementioned risk is ethically more problematic for most private cord blood banking, which usually does not have a more urgent need for the cord blood than do people who may need the cord blood to save their own or a relative's life (2006).
Issue Framed by Family Banks: Cord Blood banking as a Form of Biological Insurance
In contrast to medical societies' focus on clinical benefits and risks, family banks have framed the decision of cord blood banking as a biological insurance issue, through which risk-averse parents may manage their child's health risk by storing his or her cord blood in advance and may, thereby, reap the benefits of stem cell science in the future. Since it involves predicting the future, this framework is less rigorous regarding evidence and allows for considerably speculative calculations that render the decision similar to an investment decision. For instance, even though the odds of using one's cord blood might be currently low for a family, should the applications of cord blood transplantation continue to increase, in the long run, the odds that one might use the cord blood would increase.
In fact, despite their reservations in endorsing private cord blood banking, professional societies have been updating their statements as the technology develops. For instance, the AAP issued policy statements both in 1999, when the technology was primitive, and in 2007, when it was more developed (AAP Citation1999; Citation2007). Although its 1999 statement pointed out that “indications for autologous transplantation are limited, and the potential for future expansion is unlikely,” its 2007 statement, acknowledging the progresses both in clinical practices and in clinical trials, stated that “cord blood transplantation has been shown to be curative in patients with a variety of serious diseases” (CitationAAP 2007). Likewise, in 2006, the RCOG replaced its 2001 statement with a revised statement that approved routine use of cord blood transplantation for children with hemoglobinopathies, for children who have poor prognosis for acute myeloblastic leukemia and who lack a related donor, or for children with genetic diseases such as Hurler syndrome, where timely treatment is crucial (CitationRCOG 2006).
Preoccupied with the clinical risks and benefits, medical societies also have underestimated the difficulty of locating and acquiring compatible cord blood from a public registry, should one ever need it. Since one's HLA type is inherited from one's parents and ancestors, the chance of finding a matching donor depends tremendously on one's ethnicity. As of 2004, the ethnic composition of cord blood units stored in the National Marrow Donor Program, one of the two largest public HSC registries in the USA, is 52% white, 5% African American, 5% Asian, 13% Hispanic, 12% multiple race, 1% Native American, and 12% other or unknown (CitationBallen 2005). Moreover, Bone Marrow Donor Worldwide, the compiled list of HSC donors worldwide, consists of donors and cord blood units mostly of Northwest European origin (CitationOudshoorn et al. 2006). This would leave patients of Asian ethnicity less chance to find a match than those of European ethnicity.
Furthermore, compared to the cost of private cord blood banking, the fee for acquiring cord blood units from a public registry is often prohibitively high. For each unit of cord blood released, public banks collect a fee to cover the cost of screening, storing, and maintaining the registry and all its cord blood units, not just for the one that is being released. If the public banks do not have a large enough pool to match enough people to recoup their cost, they either charge a large amount, or simply stop operating. As a result, in 1996 nonprofit banks in the United States and Europe charged US$14,175-21,500 for each unit they released for transplantation, and the fee rose to US$32,256 in 2002. Footnote7 By 2008, McGuckin and Forraz (Citation2008) estimated that the fee range from US$20,000 to 50,000.
Because of these barriers, storing a newborn's cord blood for 20-25 years seems a relatively good bet although the odds of using the blood are low. Data from McGuckin and Forraz (Citation2008) indicate that the lump sum fee to store it for 20 years was approximately US$1,500-3,000 in 2008. This would be substantially lower than the cost of acquiring a compatible cord blood unit from a public registry, should one could ever find a matching donor.
Beyond The Competing Frames: Issues for Governance
While medical societies tend to frame cord blood banking as a clinical decision and question the proportionality between the related benefits and risks, family banks frame cord blood banking as a form of biological insurance and highlight the long-term gain from current risk management. Hence, as medical societies highlight the low odds that one may need one's cord blood, and focus on the unworthiness of the risk of collecting cord blood during delivery, the insurance analogy downplays the uncertainty surrounding one's possible need of one's cord blood and highlights the cost of finding a matched unit under the current landscape of cord blood depositories.
Yet, both of these approaches ignore the fact that science and technology, rather than develop in a social vacuum, are shaped in large part by how the society governs the research and development of science, who funds the research, for which direction and according to whose needs (Jasanoff Citation2004a, Citation2004b). Thus, while the insurance frame is mistaken by implying that the development of the science is only a matter of time, medical societies also neglect the fact that neither the development of science nor the odds that one may benefit from it are static.
Instead, both the development of stem cell science and the availability of cord blood for transplantation are issues that can be governed and, hence, should be subject to public debate. For instance, if the risk of collecting cord blood is a concern, a society can seek to regulate the practice to minimize the risk. Likewise, if the low availability of cord blood from a public registry is a concern, a society can mandate or subsidize public cord blood banks to make cord blood units more available to the neediest. It depends largely on what kind of society the public chooses collectively, and what kind of relationship each individual wants to form with others, all taking place in the tangled network of individual decisions and collective decisions.
In the end, although both discourse from the medical societies and family banks have some merits in them, without further exploring their social implication and corresponding governing structure, neither of them by itself provides a definite answer as to whether parents should or should not store their children's cord blood. In this regard, the following sections use cord blood banking in Taiwan to explore how family banks have successfully framed cord blood banking as a form of biological insurance, as well as how this frame has affected the public's understanding of the issue, has co-produced the governing framework, and has affected physicians' relationship with family banks and their patients.
Cord Blood Banking in Taiwan: The Co-production of an Insurance Analogy and a Market-oriented Governing Structure
As of 2009, Taiwan had eight family banks, Footnote8 organized as for-profit corporations, competing fiercely against each other in the island economy's private cord blood banking market. Depending on different programs in different banks, parents pay a lump sum fee of US$1,820-2,420 to store their newborn's cord blood in cryopreservation status for 20 years. Footnote9 Though quite a fortune, this price is not unaffordable for middle-class consumers in Taiwan. To begin with, the birth rate of Taiwan has been steadily decreasing to 1.10 children on average for each woman of fertile age, the lowest average in the world. Footnote10 Thus, parents have the ability and tendency to invest more in their only child. In 2007 regarding income, the annual per capita GDP of Taiwan was US$16,855, the annual average disposable income per household was US$27,996, and specifically regarding personal income distribution, the fourth 20% earned a disposable income of US$32,420 and the top 20% earned a disposable income of US$57,175. Footnote11 The minimum price that parents would have to pay to store their newborn's cord blood in 2007 was US$1,818, costing roughly the same as one year's worth of full insurance for a new mid-size automobile. Insofar as the latter investment insures only for 1 year, the price of storing a child's cord blood for 20 years is not extraordinary.
However, the popularity of cord blood banking is limited to family banks. As of 2008, there were four public banks that were collecting donated cord blood for unrelated patients. Footnote12 Among them, the Koo Foundation's Sun Yat-Sen Cancer Center and the Taiwan Blood Services Foundation have collected cord blood only from women that would give birth in an affiliated hospital. Though no statistics are available, these public programs' capacity for collection is thus highly limited. Another public bank, the Tzi-Chi Foundation, stopped collecting umbilical cord blood at the end of 2008, because with 12,549 units of cord blood samples stored since 2001, the bank's capacity was full.
Against this backdrop, the decision to engage in cord blood banking in Taiwan has become synonymous with storage for autologous use in family banks. Yet, how did family banks in Taiwan achieve such prosperity? More specifically, how did they succeed in framing cord blood banking as a form of biological insurance and occupy a dominant position in Taiwanese people's choice for cord blood banking?
How Did Family Banks Frame Cord Blood Banking as a Form of Biological Insurance
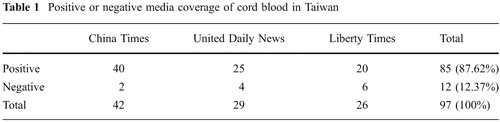
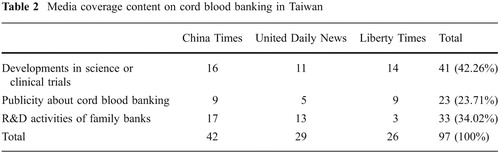
Taiwan's first family bank, Babybanks, was established in 2000, roughly a year ahead of the other major family bank, Healthbanks. Accompanying these economic activities, the media in Taiwan was quite favorable in its coverage of cord blood transplantation and of private cord blood banking. Using “cord blood” as the key words, a search of the three major newspapers' content from 1999 to 2009 yielded 97 news reports, among which 85 pieces, or 87.62%, are positive coverage (). Most of the positive coverage was about successful cord blood transplantation and successful development in cord blood applications (42.26%), along with research and development taking place in Taiwan's family banks (34.02%; ). The rest of the publicity about cord blood banking centered mainly on different celebrities' storage of their newborns' cord blood in different cord blood banks (34.02%).
Of all this media coverage, only 12 pieces (12.37%) were negative, six pieces (6.19%) were about two incidents where Taiwan's Fair Trade Commission punished the two leading family banks respectively for false representation in their commercial activities, three pieces (3.09%) involved individual physicians' reservations about the necessity of storing cord blood for autologous uses, and three pieces (3.09%) were about an actual incident where cord blood was contaminated before storage; also, two pieces (2.06%) covered physician warnings against possible contamination or inaccurate testing.
In addition to the media's favorable coverage of cord blood banking, family banks placed aggressive advertisements on television, on the radio, in magazines, in newspapers, on the internet, and in direct mailings to compete in this relatively new market. The businesses also set up service desks and posters in the waiting rooms of hospitals' obstetrics and gynecology departments. The combination of the media coverage and the commercial advertisements obviously made an impression on the general public. According to the Taiwan Genomic Survey conducted in 2004, 84%
of the participants had heard of “umbilical cord blood,” and well over one third (38%) could explain the term's meaning to others; only 14% had never heard of it. Footnote13
What is intriguing is, according to the aforementioned Taiwan Genomic Survey, of the randomly selected 1,632 participants, 63% would be willing to store their child's cord blood if they had a newborn. The willingness increased as their knowledge of cord blood issues increased. Footnote14 Yet, against the uncertain future of the science and the low odds of benefiting from the science, how did family banks succeed in persuading so many parents to actually store their children's cord blood?
My study shows that cord blood banks' major strategy has been to frame cord blood banking as a form of biological insurance. First, by framing cord blood banking as a form of biological insurance that is available to parents right after delivery of a child, the banks framed the procedure as a once-in-a-lifetime opportunity and downplayed the significant uncertainty surrounding these technological developments. Because the storage is like an insurance policy, the possibility that the cord blood may never be needed does not impair the storage's value. After all, what parents would want to run the risk that their children would have to undergo a stem cell transplantation?
In fact, this biological insurance framework implicitly allows for widely disseminated information that might not survive under stricter scientific scrutiny. For instance, when identifying the diseases that cord blood transplantations could help treat, one family bank listed not only Department of Health (DOH)-recognized conditions such as leukemia, severe aplastic anemia, and severe combined immunodeficiency, but also breast cancer, ovarian cancer, and brain tumors, which are not part of routine clinical practice. Footnote15 Another company's website has stated that “cord blood has been ‘widely used’ in diseases such as hematological malignancies, immunodeficiencies, inborn error metabolism, and ‘cancers’, and that research has proven' cord blood's usefulness in treating strokes, diabetes, liver disease, Parkinson's disease, heart injuries, muscle-stretching injuries, spinal injuries, and exercise-related joint injuries.” Footnote16 But the foregoing list of diseases actually blurs the boundaries among standard therapy, clinical trial therapy, and experimental therapy, of which only the first category is thought to be satisfactorily safe and effective for routine use. For instance, a pilot study on the use of autologous cord blood to treat type I diabetes acknowledges that “prolonged follow up and additional mechanistic effects are urgently needed to determine if umbilical cord blood derived stem cells can be used as part of safe and effective therapies for Type I diabetes” (CitationHaller et al. 2008, p. 710). Likewise, although there have been successful cases using autologous stem cells to treat infants' severe combined immunodeficiency (CitationCavazzana-Calvo et al. 2005; CitationChinen and Puck 2004), an AAP policy statement in 2007 recommended that, because some related patients had developed T-lymphocyte, this use requires more basic research before further attempts are made in clinical trials (CitationAAP 2007).
Second, this foregoing analogy to insurance further plays to parents' anxiety about “doing their best” for their children's well being. For instance, Hsu, Hsi-Di (徐熙娣), a popular celebrity, appears on TV spots urging parents “to do your best for your children.” Another celebrity, Tao, Jin-Yiing (陶晶瑩), appears on TV spots announcing her choice to store her newborn's cord blood in another family bank, whispering to her baby that it is “the first gift mommy is preparing for you. I hope you know how much I love you.” When she was interviewed about the experience of shooting the commercial, she confessed emotionally that, having had her first child somewhat later in life, she was unsure whether she would live to see her daughter married, but what she could be certain of would be that her daughter's cord blood would be in storage, strengthening the girl's chances of leading a healthy life. Footnote17
Unfortunately, as family banks promote their insurance analogy, they implicitly exclude parents' choice for donation. Indeed, according to the foregoing Taiwan Genomic Survey in 2004, assuming that it would cost parents US$300 a year to store their newborn's cord blood; of the 63% of participants who were willing to store their child's cord blood, 92% stated that they were still willing to donate their newborn's cord blood to save others, whereas only 6% stated that they were unwilling to do so (CitationTaiwan Genomic Survey 2005).
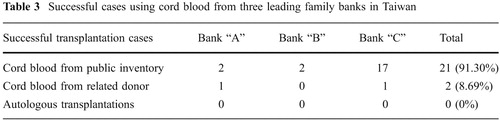
But driven by the market force of profit maximization, the publicity spots of Taiwan-based family banks have tended to downplay the existence and merit of public registry. For instance, of all successful transplantation cases publicized by the website of three leading family banks in Taiwan as of 2009, 91% have actually come from the family banks' inventory stored for public donation; only two cases, or 8%, are from related donors (). Although the family banks publicized these cases mainly to demonstrate that the quality of their cord blood in storage can meet the need for successful transplantation, the fact that these cord blood are from their inventory for public donation implicitly suggests that there are options other than private cord blood storage that family banks usually do not promote.
In fact, a comparison of the programs and contracts offered in the three leading family banks reveals a telling fact: except for a more expensive program in one family bank, and although many family banks allow parents to list a few family members as recipient, donations for needy strangers are not an option. To begin with, most programs are designed for autologous uses; hence, they do not test for HLA type before storage. HLA-type identification is designed as a supplement that incurs additional charges. Nor do most banks provide parents the option of listing
their cord blood in any public registry to preserve the possibility that needy strangers may benefit from the blood. Only a more expensive “mutual benefit program” in one bank has provided parents the option of listing their unit to help others. But since the program also allows parents to store the cord blood even if it is hepatitis positive, whether it meets the standard for allogeneic transplantation depends on each physician. Some physicians may refuse to use the blood because it may affect the success of transplantation; but other physicians may take into consideration the difficulty of finding another match and the urgency and necessity of performing the transplantation. Thus, even though theoretically, a person can end the storage contract and provide the unit to needy strangers, this step would be improbable because the person would, in all likelihood, not know about the existence of needy strangers; even if this knowledge were available, there still would be the problem of matching the cord blood's HLA type with the stranger unless either (1) HLA-type testing had taken place before storage for an extra charge or (2) the effort was made to thaw the cord blood for the purpose of testing the HLA type, which would then be a waste of blood if there were no match. Finally, even if there were a match, the cord blood might not meet the standard for allogeneic uses because the cord blood might be compromised (e.g., hepatitis positive) and unacceptable to the physician.Consequently, under the insurance analogy, the design of family banks' programs is not friendly to altruistic parents who are willing to store their newborns' cord blood for family members but who do not mind also providing it to needy strangers if the occasion were to arise. In this regard, a physician with knowledge about the matter estimated that the chance of finding a 6/6 match in Taiwan is 1:40,000, a 5/6 match is 1:6,000, and a 4/6 match is 1:4,000. Footnote18 Given the low probability of using one's cord blood and the lack of public banks in Taiwan, it would be more efficient and humane if privately stored cord blood units could find a place on a public registry, reserving to the parents the option of whether to provide the cord blood according to need. This option would also fulfill most parents' altruistic desire as reflected in the Taiwan Genomic Survey 2005.
The Governing Structure Co-produced: The Missing Chances for Public Donation
If cord blood stored in family banks is to be used for strangers, a governing structure must be able to meet general medical standards for transplantations. However, corresponding to family banks' insurance analogy, the DOH has also chosen to govern family banks more as an insurer catering to personal preference than as a medical practice subject to stricter regulation.
In fact, this attitude is more conducive to the government's policy for industrial development. Since 1980, the government has listed the biotech industry as one of the target industries to be developed using both public and private sectors. By 2005, the scale of investment in this industry reached US$760-910 million, with 60% from the government and 40% from the private sector (CitationWong 2007). Compared with other biotech industry, family bank has a low technological threshold and capital requirement for starting up, since all the bank does is test and store. Moreover, for its services, cord blood banking collects cash up front and can end the services if the client fails to pay for the following year's storage. Hence, compared with the biotech industry franchises that deal with a higher technological threshold, family banks have proven to be a rather safe investment.
For the 9 years before 2009, when the DOH finally passed the Regulation of Human Tissue Banks (hereinafter “the Regulation”), the only governmental regulation for family banks was the Standards for the Collection and Processing of Cord Blood (hereinafter “the Standards”) Footnote19 —a set of guidelines having no statutory authorization. Consequently, the DOH could neither punish nor ban nor impede family banks should they failed to meet the Standards. Thus, the nature of the family bank has not differed greatly from the natures of other for-profit companies, but has stood noticeably apart from medical institutions whose establishment is subject to approval and stricter regulation. Even the 2009 passage of the Regulation leaves it to be seen whether the DOH will shut down substandard family banks and how the DOH will protect the people who unfortunately stored their own or a loved one's cord blood in these banks.
At least before the end of 2009 and possibly also after 2009, parents thus relied mainly on contractual informed consent to protect their own interests. Jointly with the Commission of Consumer Protection of the Executive Yuan, the DOH promulgated at the end of 2007 a “Model Contract for Cord Blood Storage” (hereinafter Model Contract), Footnote20 hoping to remind parents of the uncertainty involved in cord blood transplantation. For instance, the second clause of the Model Contract requires that cord blood banks present the parents with the following information:
First, the clinical utility of umbilical cord blood is still in the stage of research and development: its applications and efficacy is still uncertain. Even if certain content in umbilical cord blood can be used for treatment of certain conditions, this does not mean that the cord blood stored by the parties of this contract has the same utility or efficacy. Second, the transplantation and potential application of umbilical cord blood are directly correlated with the number of stem cells in the umbilical cord blood, the survival rate of the thawed stem cells, the absence or presence of contamination therein, and the patient's weight.
The contract even requires a specific signature under the foregoing two clauses to ensure that consumers are fully aware of them. Footnote21
Under this governance structure, the focal point of regulation becomes whether the banks made false statements, a practice that falls within the jurisdiction of the Fair Trade Commission (FTC) rather than the DOH. In this capacity, the FTC punished the two leading family banks for making false statements in their advertisements, first in 2007 for falsely advertising that they were the leading company in the market, Footnote22 and again in 2008 for presenting false information when they compared their services with their competitors' services. Footnote23
Yet, this boundary-drawing between governance of consumer transactions and governance of medical practices has reinforced the private nature of cord blood banking and has minimized the chance that a stored unit could be donated for allogeneic uses serving unrelated patients in need. Unless the cord blood is stored for allogeneic uses from the start, the Standards do not require cord blood units to be free of such infections as hepatitis B or HIV, of bacterial contamination, or of blood-related genetic disorders. The rationale underlying this policy is that, while the infections or genetic disorders may harm the recipient of the transplantation, it will not harm the one who provided the cord blood, since he or she would already have the disease or genetic disorder, and antibiotics can eliminate the bacterial contamination. But the same infections or contaminations are usually unacceptable to public banks, and are banned for allogeneic transplantation by the Standards. But whether transplantation physicians would accept using contaminated cord blood in autologous transplantation is an open question. Article 7 of the Regulation passed in 2009 requires family banks to perform pre-storage tests for possible infections, but does not ban family banks from storing infected cord blood.
As a result of the different standard applied to cord blood stored for allogeneic uses and for autologous uses, Taiwan government's regulation reinforces cord blood's nature more as consumer goods governed by informed consent where consumer assumes their own risk than as a part of a medical procedure that must meet stricter standards. Regarding the family banks, one of the two leaders in this field allows parents to choose to store their child's cord blood if the cord blood is infected with hepatitis B+. Footnote24 The other leading family bank goes even further by allowing parents to store cord blood even if it has bacterial contamination, harbors infectious disease, or is less than 40 cc “as long as the law permits it.” Footnote25 In the end, although family banks are sure to profit from this storage, there is no guarantee that it will be usable should its owner ever need it. Hence, by placing private cord blood banking within the boundary of consumer transactions, and by leaving allogeneic transplantation within the boundary of medical practice, the Taiwan government has further hindered the possibility of using privately stored cord blood for altruistic purposes and has reinforced the perception that deposited cord blood is essentially private property.
The Medical Profession's Conflict of Interests in the Practice of Cord Blood Banking
The co-production between family banks' issue framing and the DOH's boundary drawing has profound effects beyond whether the quality of storage is in consumers' interests and whether the blood is for the public good. The co-production also affects Taiwan physicians' social relationship with their patients and with the family bank.
To begin with, Taiwan physicians' role in cord blood banking is more ambiguous than the roles played by the ACOG, the AAP, and the RCOG, which publicly discourage private cord blood banking. There have been individual boycotts and individual instances of advocacy, but as far as I can search from the literature or the media, no medial professional society has issued any formal statement on the issue. Indeed, my personal conversations with people from the industry indicate that there are obstetricians who personally refuse to collect cord blood for their patients during the process of delivery unless the blood is for public donation. There are also individual physicians who have expressed their disapproval of using blood unavailable for public donation. For instance, Dr. Yao-Chang Chen (陳耀昌), who is both the head of the Graduate Institute of Forensic Medicine of National Taiwan University and the co-founder of the Stem Cell Research Institute at the National Health Research Institute of Taiwan, does not oppose to people who can afford it store their newborns' cord blood for family uses, but extends two warnings to consumers: there is little likelihood that the blood will come in handy, and cord blood consumers should be wary of family banks' commercial tactics. Footnote26 A search in the three leading newspapers covering the 10 years between 1999 and 2009 reveals three instances where individual physicians publicly encouraged people to donate their child's cord blood rather than store it for private use. Footnote27 But these three instances constitute only 3.09% of all the sample period's cord blood coverage, which is predominately positive about current practices.
In contrast to the small levels of negative criticism targeting private cord blood banks, family banks often set up stands or posters in ob-gyn departments' patient waiting rooms and have sales representatives stationed there to promote their services. Such activities would be impossible without the departments' acquiescence. In fact, to increase their trustworthiness, the two leading family banks have websites listing dozens of physicians or other medical professionals as clients who store their child's cord blood in the family banks. The connection between family banks and esteemed clients is not limited to medical professionals. More than once, media outlets have reported that family banks provide free services to celebrities in exchange for the publicity. Footnote28
Physicians' involvement in promoting cord blood reached its peak when President Chen Shui-Bian's daughter and son-in-law, both of whom are medical professionals, chose to store their newborn's cord blood in one of the leading family banks. Footnote29 The child's father received a payment of US$560,000 for the publicity they created by eventually storing all three of their children's cord blood units in the bank. Footnote30
Such advertisement would be illegal for physicians if the Taiwan government treated cord blood banking as a medical practice. According to article 85 of Taiwan's Medical Practice Act, physicians cannot advertise information other than their name, licensure, location, business name, and business hours. Nor can physicians make use of news coverage to publicize medical research to achieve the same purpose. But according to the official in charge of biotechnology in the DOH, as long as family banks do not claim or imply that cord blood has any therapeutic effect, cord blood banking is not a medical practice subject to the regulation of the Medical Practice Act. Footnote31
Hence, as long as advertising does not involve false statements or misrepresentations, physicians who participate in these advertisements cannot be punished for this participation under any law. This limitation in the DOH's governing structure increases obstetricians' conflict of interest not only in their communication with their patient when it involves cord blood banking, but also in the process of delivery. My personal conversations with a scientist knowledgeable about the industry and with an obstetrician who routinely collect cord blood in deliveries show that family banks usually pay a fee to their clients' obstetricians for each cord blood unit collected during the process of delivery. The amount of the fee would range from US$45 to US$288, with an average between US$100 and 150, depending on the private negotiation between the sales representative and the physician or the ob-gyn department. The fee is paid after the cord blood arrives at the family bank and passes the routine tests for infection, contamination, and the like; furthermore, the cord blood must amount to a minimum of 40 ml.
As discussed in the earlier part of this paper, while it is still an open question as to whether the collection of cord blood actually distracts obstetricians from their obligation to maximize the safety of the mother and child during the process of delivery, the amount of the collection fee could create undue incentive for obstetricians to pay too much attention to the cord blood collection when they should be rigorously attending to the mother or the child. As of 2009, under a global budget system, Taiwan's National Health Insurance pays 8,902 points, or roughly less than US$269 dollars, to the provider for the delivery procedure, depending on whether the hospital is a medical center or belongs to other lower levels. Footnote32 Depending on the actual contract between the hospital and the obstetrician, the latter usually receives around 70% of the compensation, which amounts to US$188 for an obstetrician who works in a medical center. If the collection fee for umbilical cords substantially outshines the reward an obstetrician can gain from the National Health Insurance, unless the delivery is a critical case in which obstetricians would not dare to risk a malpractice suit, they might place great weight on ensuring that the cord blood is collected properly according to the given family bank's standards—and perhaps at the risk of compromising the primary duty to the safety of the mother and the child.
Yet, under the current governing framework that distinguishes cord blood banking from medical practice, even though the collection of cord blood is part of the process of delivery, which is no doubt a medical practice, the collection fee is not regulated. Nor does the fee lend itself to transparent regulation since the fee-related negotiations take place privately between physicians and the family banks.
Beyond Informed Consent: Bringing Public Values and Professionals' Integrity Back In
Given the underlying market forces that affect cord blood banking in Taiwan, I used the foregoing sections to examine how family banks have successfully framed cord blood banking as a form of biological insurance managing children's health risks, and I then analyzed how the DOH first distinguished cord blood banking from ordinary medical practices, thereby subjecting it to lenient standards and to informed consent. I further demonstrated how the DOH's standards have distinguished between cord blood stored for autologous uses and cord blood stored for allogeneic uses, a distinction that results in the storage of substandard units under conditions sometimes preventing members of the non-family population from accessing cord blood, despite their critical need for it.
Taiwan's experience in cord blood banking thus provides two lessons for governing a predominately private industry of cord blood banking. First, by allowing for and reinforcing the private nature of cord blood units, the governing structure has promoted the insurance analogy, has impoverished parents' understanding of the choices stem cell science has to offer, and has worked against altruistic parents that might consider public donation if pertinent information and options were available. As a result, without informed reflection or public debate, the market forces underlying the cord blood industry have shaped Taiwan into a society where cord blood is stored exclusively for autologous uses at the cost of a more efficient and altruistic model where people with the same somatic or genetic status can help each other.
Second, these same underlying forces not only erode the possibility of a more altruistic society, but also place medical professionals in a situation where they are more vulnerable to conflict of interest when providing information to patients or when collecting cord blood during the process of delivery. As the DOH does not regard cord blood banking as a form of medical practice, physicians are free to participate directly or indirectly in the advertisement or promotion of cord blood banking. This aspect of the scene has further limited the chance that parents can gain unbiased and substantially public-oriented information regarding cord blood banking. Moreover, the sometimes relatively lucrative rewards that physicians receive for collecting cord blood may unduly compromise their primary duty to ensure the safety of patients during delivery.
In addressing the threat to both the public good and the integrity of the medical profession, the government should take a more aggressive stance by imposing stricter regulations on the current practice of cord blood banking in Taiwan. Although setting up public registries or public cord blood banks may not be cost-efficient when the prospect of the science is still speculative, the government can bring the public good and the medical profession's integrity back into the current governing structure by making the following changes.
First, to maximize the public and private utility of stored cord blood units, the government should rearrange or simply eliminate the boundary between medical practice and cord blood banking, as well as the boundary between autologous storage and allogeneic storage, and should require stricter quality standards for the storage. These standards would include bans on banks' storage of infected and contaminated cord blood, and standardizing the practices of private and public banks according to clinically acceptable quality standards. This oversight would better ensure cord blood's use for transplantation and would further preserve the possibility that the neediest of patients—regardless of their family relation to the donor—would receive cord blood units.
Second, the government should encourage family banks to widen the availability of the public-donation choice to parents. For instance, of Taiwan's eight family banks, at least two store cord blood donated from people who cannot afford the fee for storage, but who are willing to donate the cord blood for others' use. Footnote33 Another for-profit cord blood bank in Taiwan offers a program where clients reserve the right to donate their cord blood to relatively needy people. Once a public registry discovers a possible recipient who needs the stored cord blood, the clients decide whether to donate the blood to the possible recipient; if the clients decide to make the donation, the family bank would reimburse the clients for storage payments made up to that point and would allow the donor to use the service of matching for free should the donor need the service in the future. Footnote34
But to expand and popularize the structure of the foregoing program, Taiwan must make related information more available. Hence, the government and professional societies should create guidelines to govern their own roles and other institutions' roles in providing information to the public about cord blood banking. For instance, the government and professional societies can bar physicians from providing a patient with any information about cord blood banking unless they explain two critical points: the difference between private and public cord blood banking, and a parent's option to donate a newborn's cord blood to public banks or public programs in private banks. These information not only will protect parents' right to informed consent and medical science's integrity, but also may increase cord blood donations and cord blood availability without burdening public expenditure.
A governing structure must also caution the potential risk that cord blood collection may bring to the process of delivery. Indeed, the RCOG recommends that, to prevent physicians from unnecessarily diverting their own attention away from the mother and baby, rules should stipulate that trained staff other than the delivery staff should collect cord blood after the delivery of the placenta, and that the collection should not alter the routine procedure of delivery (2006). Whether this is a cost-efficient recommendation requires further study. But at least service should not be made available when the physician believes that there may be a complication such as a nuchal cord or maternal hemorrhage (CitationACOG 2008; CitationRCOG 2006; CitationAAP 2007).
Finally, fees for cord blood collection should be standardized and kept within a reasonable range, so that undue incentive will not compromise the physician's primary duty to the safety of the mother and child. The fee should also be subject to disclosure according to other standards that exist to regulate physicians' possible conflicts of interest in relationships between physicians and pharmaceutical companies.
In the end, the consumer nature of Taiwan's cord blood banking highlights the challenges that arise when market forces and consumers' informed consent serve to govern a service permeated with hype for technical development. Each decision to store cord blood has social implications insofar as the decisions can strengthen or weaken both the public good and the perceived integrity of the medical profession. Consequently, a simple reliance upon individual informed consent is powerless not only against people's hyped-up enthusiasm for a science whose future is uncertain but also against the marketplace's systematic and pervasive bias for private interest over public good.
When the government or medical professionals fail to counterbalance the information and options provided by the private sector, citizens are deprived of alternate imaginations of their future individuality and their possible relationships with others; in short, citizens lose access to options that could serve their own interests and the public in better ways. Hence, to maximize the public good and to strengthen the medical profession's actual and perceived integrity, the government must strengthen and sharpen its interventions in the practice of cord blood banking.
Conclusion
Taiwan's experience highlights the downside of leaving the governance of a bioeconomy—permeated with people's hype for technical development—to a marketplace based squarely on people's informed consent. Although contracts generally warn parents that the development of science cannot be guaranteed, family banks' strategy of framing the issue of cord blood storage as an issue of biological insurance downplays the importance of this medical procedure's uncertain present and future. Likewise, by framing the issue as a commercial transaction rather than a medical practice, Taiwan's DOH has left consumers vulnerable to the industry's systematic bias against public donation and professionals' conflicts of interest in the practice of cord blood banking.
In the end, as citizens rather than mere consumers, parents lose the opportunity to reflect upon public values such as altruism and upon donors' relationship with people who, having the same genetic or somatic status as the donors, might need their cord blood for an immediate and critical transplantation. Moreover, because medical professionals' integrity has been compromised in the practice of cord blood banking, parents may lose both a potentially useful source of information serving to counter-balance cord blood banks' information and safeguards serving to protect the interests of parents and their children during the process of delivery. Hence, I argue that government must intervene in the cord blood industry to ensure that the blood meets medically acceptable standards for both autologous uses and allogeneic uses, while promoting the provision of information and options to the public regarding public donation. Moreover, given medical professionals' better access to the science on cord blood as well as their familiarity with the practices of the cord blood industry, I argue that the government or professional societies in Taiwan should establish guidelines for the professionals to regulate possible conflicts of interest when they provide information about cord blood banking to their patients and collect cord blood during the process of delivery.
Acknowledgment
Research of this paper is supported by the National Science Commission of Taiwan under the project “Law and Ethics of Biomedical Research in the Post-Genomic Era: Rethinking Research Subjects' Rights Toward Their Body and Tissue Samples (NSC 97-3112-H-010 -002).” I would like to thank Chia-Ling Wu for the inspiration and encouragement she generously provides all the time, Nicole S.Y. Huang, Hsiu-I Yang and the three anonymous reviewers for their invaluable comments and suggestions, the people who accepted my interviews, particularly Shawn Liu for patiently teaching me the science of cord blood banking and sharing with me his insights about the prospect of the science, and for H.Y. Tseng for her capable assistance. The errors and responsibility, however, are all mine.
Notes
1 No. 096179 Decision of Taiwan's Fair Trade Commission, 2007/12/18. The figure is based on the figure provided by the Ministry of Economic, which is equivalent to roughly 18-21 million US dollars in 2004 and matches the figures provided by the family banks themselves in the news coverage.
2 United Daily News, February 27, 2008, p. E2; Economic Daily News, January 9, 2008, p. D6.
3 Cryosave, umbilical cord blood stem cell storage should increase in Europe. Press release, September 11, 2007 available at https://www.cryo-save.com/company_news.html?id_news=76 (visited Jan 12, 2009).
4 Economic Daily News, January 9, 2008, p. D6.
5 The original data are unavailable from the National Marrow Donor Program. For more information, see Parent's Guide to Cord Blood Foundation, http://parentsguidecordblood.org/content/usa/medical/oddsofuse.shtml?navid=30 (visited May 17, 2009).
6 The actual amount is hard to be ascertained. The Association of Family Cord Blood Banks estimated that there are 750,000 units of cord blood stored in family banks in the United States. McGuckin and Forraz (Citation2008) estimate that there are approximately 700,000 units stored in private cord blood banks worldwide, with 500,000 in the USA and just over 135,000 in Europe. In contrast, according to statistics from the NMDP and the New York Blood Center, which are the two largest public inventories of cord blood units in the USA, there are only 110,000 units stored in public banks. Likewise, McGuckin & Forraz's study indicated that there were 276,000 cord blood units stored in approximately 34 public cord blood banks in about 20 countries (CitationMcGuckin and Forraz 2008).
7 In 1996, The New York Blood Center Placental Blood Program charged US$15,300, the US National Marrow Donor Program charged US$21,500, and the average of 15 European national marrow donor registries was US$14,175. The price rose substantially afterward. In 2002, the average price was US $32,256. See http://parentsguidecordblood.org/content/usa/medical/oddsofuse.shtml?navid=30 (visited May 27, 2009).
8 The eight companies are Baby Banks (訊聯), Health Banks (生寶), Stemcyte (美商永生), Sino Cell (再生緣), Alarvita (大展), Discovery (尖端), and two other banks—Chi-Fu (祈福) and Hong-Yeh (宏燁)—whose official English names are unavailable on the web.
9 The Epoch Times, March 25, 2006, available at http://www.epochtimes.com/b5/6/3/25/n1265949.htm (visited May 17, 2009).
10 Table 24, Birth Rate of Major Countries, International Statistics compiled by Ministry of Interior, available at http://sowf.moi.gov.tw/stat/national/j024.xls (visited Nov 25, 2009).
11 Directorate General of Budget, Accounting and Statistics, Executive Yuan of the Republic of China, Index of Income and Expenditure, Annual Report of Social Index Statistic 2008, available at http://eng.stat.gov.tw/public/data/dgbas03/bs2/socialindicator/2007/table_income.xls (visited Nov 25, 2009).
12 This includes the Buddist Tzi Chi Stem Cells Center (慈濟), the Taiwan Blood Services Foundation (台灣血液基金會), the cord blood bank set up by the Koo Foundation's Sun Yat-Sen Cancer Center (辜公亮和信治癌中心醫院), and the VIA Cord Blood Stem Foundation (信望愛基金會).
13 Taiwan Genomic Survey, No. 20, August 4, 2005 available at http://srda.sinica.edu.tw/webpages/gene/020.htm (3 of 3) [visited Dec 30, 2008]. This is regular survey that randomly selected samples people who are at least 18 in Taiwan. This particular survey on cord blood was conducted in April 2004 and had 1,632 participants.
14 Id. The fee for storage was set at 10,000 NT dollars per year.
15 http://www.babybanks.com.tw/SC/stemcell/cell_1_1_2.asp (2 of 4) [visited Dec 4, 2008].
16 http://www.healthbanks.com.tw/faq4-2.asp?no=50&Uno=42 [visited Jan 6, 2009].
17 Economic Daily, July 23, 2008, p. B4.
18 Hui-Ling Huang, Should I Store My Baby's Cord Blood? Common Health Magazine (康健雜誌), vol.114 (2008) available at http://www.commonhealth.com.tw/article/index.jsp?page=1&id=4449 (visited July 16, 2009).
19 Department of Health, No. 0910013376, January 18, 2002, Department of Health Gazette, vol. 31:11, pp. 26-35.
20 No. 0960051571, promulgated by the Department of Health, November 28, 2007.
21 Clause 2 of the Model Contract for Umbilical Cord Blood Storage, issued by the Department of Health, Yi Tzi Regulation No. 0960051571, November 28, 2007.
22 No. 096122, issued by the Fair Trade Commission, July 8, 2007; No. 096179, issued by the Fair Trade Commission, December 18, 2007.
23 No. 097112, issued by the Fair Trade Commission, August 21, 2009; No. 097111, issued by the Fair Trade Commission, August 21, 2008.
24 Contract on file with the author.
25 Contract on file with the author.
26 Epochtimes, 2006/6/7 available at http://www.epochtimes.com/b5/6/6/7/n1341918.htm (visited Nov 27, 2009).
27 Liberty Times 2008/6/11; Liberty Times 2006/3/6; United Daily News, Sep 18, 2007.
28 Liberty Times, 2005/6/20; Liberty Times, Dec 9, 2006.
29 China Times, May 27, 2006, p. A6.
30 Liberty Times, May 27, 2006.
31 Interview with Chin-Li Lin (林錦麗), Director of the Sixth Division, Department of Medical Affairs, covered in Hui-Ling Huang, Should I Store My Baby's Cord Blood? Common Health Magazine (康健雜誌), vol. 114 (2008), available at http://www.commonhealth.com.tw/article/index.jsp?page=1&id=4449 (visited July 16, 2009). See also interview with Hsueh-Jui Yuan (薛瑞元), Director of the Department of Medical Affairs, covered in Global Vision Magazine (遠見雜誌), August 2005, available at http://www.gvm.com.tw/Board/content.aspx?go=cover&ser=11187 (visited Nov 27, 2009).
32 For the procedure of delivery, Taiwan's National Health Insurance in 2009 began a case-payment system, which pays a lump sum of 36,335 points for the total cost of both vaginal birth and Caesarian section. The foregoing “8,902 points” refers to the compensation received by obstetricians for their work in these procedures. But hospitals withhold part of the compensation before doling out the remainder to the given obstetrician depending on the contract between the obstetrician and his or her hospital.
33 http://www.healthbanks.com.tw/faq2-3-2P.asp?no=192&Uno=1 (visited Jan 1, 2009); http://www.stemcyte.com.tw/news_detail.aspx?pid=P_00000027 (3 of 3) (visited Jan 16, 2009).
34 http://www.babybanks.com.tw/02_service/2_1a.html (2 of 2) (visited Jan 6, 2009).
References
- American Academy of Pediatrics (1999). Work Group on Cord Blood Banking. Cord blood banking for potential future transplantation: subject review. Pediatrics, 104(1), 116–118.
- American Academy of Pediatrics (2007). Policy statement on cord blood banking for potential future transplantation. Pediatrics, 119(1), 165–170.
- American College of Obstetricians and Gynecologists (1997). Committee opinion, routine storage of umbilical cord blood for potential future transplantation. International Journal of Gynecology & Obstetrics, 58, 257–259.
- American College of Obstetricians and Gynecologists (2008). Committee opinion: umbilical cord blood banking. Obstetrics & Gynecology, 111(2), 475–477.
- Annas G. (1999). Waste and longing—the legal status of placental blood banking. The New England Journal of Medicine, 340(19), 1521–1524.
- American Medical Association (2007). Umbilical Cord Blood Banking, Council on Ethical and Judicial Affairs Report 9-I-07, available at http://www.ama-assn.org/ama1/pub/upload/mm/469/ceja9i07doc.doc. Accessed 9 May 2010.
- Ballen K. K. (2005). New trends in umbilical cord blood transplantation. Blood, 105, 3786–3792.
- Cavazzana-Calvo M. Lagresle C. Hacein-Bey-Abina S. Fischer A. (2005). Gene therapy for severe combined immunodeficiency. Annual Review of Medicine, 56, 585–602.
- Chinen J. Puck J. M. (2004). Successes and risks of gene therapy in primary immunodeficiencies. The Journal of Allergy and Clinical Immunology, 113(4), 595–603.
- Copelan E. A. (2006). Hematopoietic stem cell transplantation. The New England Journal of Medicine, 354(17), 1813–1826.
- Gieryn T. F. (1983). Boundary-work and the demarcation of science from non-science: strains and interests in professional ideologies of scientists. American Sociological Review, 48, 781–795.
- Gluckman E. Broxmeyer H. A. Auerbach A. D. Friedman H. S. Douglas G. W. Devergie A. (1989). Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical cord blood from an HLA-identical sibling. The New England Journal of Medicine, 321(17), 1174–1178.
- Haller M. J. Viener H. L. Wasserfall C. Brusko T. Atkinson M. A. Schatz D. A. (2008). Autologous umbilical cord blood infusion for type 1 diabetes. Experimental Hematology, 36(6), 710–715.
- Hayani A. Lampeter B. Viswanatha D. Morgan D. Salvi S. N. (2007). First report of autologous cord blood transplantation in the treatment of a child with leukemia. Pediatrics, 119, e296–e300.
- Irwin A. (2007). STS perspective on scientific governance. In Hackett E. J. Amsterdamska O. Lynch M. Wajcman J. (Eds.), The handbook of science and technology studies (3rd ed., pp. 583–607). Cambridge, MA: MIT Press.
- Institute of Medicine (2005). Cord Blood: Establishing a National Hematopoietic Stem Cell Bank Program. Committee on Establishing a National Cord Blood Stem Cell Bank Program. Institute of Medicine, Washington, D.C.: National Academy of Academies Press. Available at http://www.nap.edu/openbook.php?878record_id=11269&page=79. Accessed 10 May 2009.
- Jasanoff S. (2004a). The idiom of co-production. In states of knowledge: the co-production of science and social order (pp. 1–12). London: Routledge.
- Jasanoff S. (2004b). Ordering knowledge, ordering society. In states of knowledge: the co-production of science and social order (pp. 13–45). London: Routledge.
- Johnson F. L. (1997). Placental blood transplantation and autologous banking—caveat emptor. Journal of Pediatric Hematology/Oncology, 19(3), 183–186.
- Kleinman D. L. (2005). Science and technology in society: from biotechnology to the internet. Malden, MA: Wiley-Blackwell.
- Kurtzberg J. (2009). Update on umbilical cord blood transplantation. Current Opinion in Pediatrics, 21, 22–29.
- Lasky L. C. Lane T. A. Miller J. P. (2002). In utero or ex utero cord blood collection: which is better? Transfusion, 42, 1261–1267.
- Macfarlane A. (2003). Underlying Yucca mountain: the interplay of geology and politics in nuclear waste disposal. Social Studies of Science, 33(5), 783–807.
- McGuckin C. P. Forraz N. (2008). Umbilical cord blood stem cells—an ethical source for regenerative medicine. Medicine and Law, 27, 147–165.
- Nietfeld J. J. Pasquini M. C. Logan B. R. Verter F. Horowitz M. M. (2008). Lifetime probabilities of hematopoietic stem cell transplantation in the US. Biology of Blood and Marrow Transplantation, 14(3), 316–322.
- Oudshoorn M. van Walraven S. M. Bakker J. N. A. Lie J. L. W. T. v.d. Zanden H. G. M. Heemskerk M. B. A. (2006). Hematopoietic stem cell donor selection: the europdonor experience. Human Immunology, 67, 405–412.
- Waldby C. (2002). Stem cells, tissue cultures and the production of biovalue. Health (London), 6, 305–323.
- Rainbow P. (1996). Artificiality and enlightenment: from sociobiology to biosociality. In essays on the anthropology of reason (pp. 91–111). Princeton, NJ: Princeton University Press.
- Rajan K. S. (2006). Biocapital: the constitution of postgenomic life. Durham & London: Duke University Press.
- Rose N. (2007). The politics of life itself: biomedicine, power and subjectivity in the twenty-first century. Princeton: Princeton University Press.
- Royal College of Obstetricians and Gynecologists 2006. Scientific Advisory Committee, Opinion Paper 2, Umbilical cord blood banking, London: Royal College of Obstetricians and Gynecologists. Available at http://www.rcog.org.uk/womens-health/clinical-guidance/umbilical-cord-blood-banking. Accessed 21 May 2009.
- Solves P. Mirabet V. Larrea L. Moraga R. Planelles D. Saucedo E. (2003). Comparison between two cord blood collection strategies. Acta Obstetricia et Gynecologica Scandinavica, 82, 439–442.
- Taiwan Genomic Survey 2005. No.20, August 4, Taiwan: Center for Survey Research, Academia Sinica. Available at http://srda.sinica.edu.tw/webpages/gene/020.htm (3 of 3). Accessed 30 Dec 2008.
- The European Group on Ethics in Science and New Technologies 2004. No.19, Opinion of The European Group on Ethics in Science and New Technologies to the European Commission: Ethical Aspects of Umbilical Cord Blood Banking, March 16.
- Winner L. (1986). Do artifacts have politics? In the whale and the reactor: a search for limits in an age of high technology (pp. 19–39). Chicago, IL: University of Chicago Press.
- Waldby C. Mitchell R. (2006). Tissue economies: blood, organs and cell lines in late capitalism. Durham & London: Duke University Press.
- Wong C.H. 2007. Biotech and Pharmaceutical Industry in Taiwan, report presented in the Monthly Meeting of the President's Office (). Available at https://www.cepd.gov.tw/att/files/. Accessed 28 Nov 2008.