Abstract
As is often the case with cutting-edge biomedical technology, the introduction of gene therapy in Japan provoked discussions of ethics and safety while offering little hope for dramatic recovery. Nevertheless, only 5 years after the first authorized clinical trial in the USA, the first Japanese trial was conducted in 1995. This trial event garnered extensive media coverage but yielded little opposition. To understand the public reaction, a range of sources were examined, including news media and popular scientific magazines. In the case of Japan, public acceptance did not mean enlightened consent; rather, it meant that interactions among researchers, the media, and the populace brought about a new interpretation of the technology.
Scientific knowledge is first presented in the form of articles in peer-reviewed journals, then by publication in newspapers, magazines, and popular science books. However, documents regarding research planning and funding precede the research itself. Articles highlighting anticipation or expectation of research results appear in the media, and as the research continues and progresses, these sources may report it repeatedly. The reaction of readers is fed back to the press and, hence, the researchers. This is the case in research such as gene therapy, which is comparatively well known, as well as in controversial types of human genetic research. As a result, various discourses, from professional to amateur, surround the research.
The present study looks at a range of Japanese discourses on gene therapy. The main source is Japan's leading newspaper, Asahi shimbun. Newspapers are usually seen as the most reliable source of scientific and technological news in Japan (CitationNational Institute of Science and Technology Policy 2001). In 1985, the Japanese phrase meaning “gene therapy” (遺伝子治療) appeared for the first time in Asahi shimbun; since then, it has appeared in about 1,000 articles. Another source is popular books on gene therapy. Of the 38 Japanese books with “gene therapy” in their titles, 14 were aimed at professionals, to judge by their contents, publishers, and price. This leaves 24 popular books, some of which are translations. The translations represent an important part of a broader Japanese discourse, because they were regarded as having value by Japanese publishers. In addition, I look at several popular journals that carried articles on the subject are investigated. The discourses constructed in these books and magazines are also examined in the present study, so as to clarify the social image and interpretation of gene therapy in the media.
After reviewing the background history of gene therapy, we will examine the Japanese discourse on gene therapy at three specific moments: before the first Japanese trial, around the time of the Japanese first trial, and around the time when the Human Genome Project released its first important findings. Over time, concerns about ethics and danger were replaced by clinical and industrial perspectives. In addition, the suggestion that gene therapy amounted to “playing God” has lost its force, and it has come to be seen instead as a concrete application of the findings of the Human Genome Project. Japan did not experience the rapid backlash against gene therapy that occurred in the USA in the late 1990s (CitationStockdale 1999a).
Among these discourses, this paper places a particular emphasis on those associating gene therapy with the Human Genome Project. This type of association is neither necessary nor inevitable. The first target diseases of gene therapy have been single-gene disorders such as adenosine deaminase (ADA) deficiency and X-linked combined immunodeficiency disease. The genes responsible for such serious diseases had been identified by pioneering researchers before the advent of the Human Genome Project in 1990. The latter aimed to determine the human base sequence, not genes themselves. As was already known, most human diseases are not genetically determined, even though they are affected and controlled by many genes. In Japan, however, there was a perception that the Human Genome Project was a basic research initiative with important applications for gene therapy. Some may view this interpretation, which is based on the linear model (CitationBush 1945), as inadequate, but statements associating gene therapy with the Human Genome Project repeatedly appeared in the media and in popular books during the period in question. In cases where the effects of the Human Genome Project are discussed, gene therapy is not always mentioned. But there are times when the connection is taken for granted or emphasized. This association is often related to the public understanding of gene therapy, and one of my principal concerns is to identify the factors that naturalized the association natural as well as the results of the association. Once we grasp those connections, we can see why the practice of human gene therapy was readily accepted in Japan.
Specifically citing gene therapy and the genetic diagnosis of a fertilized egg, Japanese sociologist Jiro Nudeshima (Citation1995) pointed out that “there is a great difference between the actual conditions of advanced medicine and its public image”. In a book about gene therapy of cancer, Teruaki Fukami (Citation1996) pointed out the use of clever rhetoric to remove any negative associations from gene therapy. However, neither of these authors addressed the topic in any detail. Such is the goal of the present paper.
In terms of biomedical technology, Japanese society is generally more conservative than most European and East Asian countries. This is the case, for example, with organ transplants (CitationHayashi 2002; CitationLock 2002). In October 1997, Japan's Organ Transplantation Law came into effect, legalizing the removal of organs from brain-dead donors for transplant purposes. However, as of March 2010, only about 80 such organ transplants had been performed. There are many opponents to organ transplants from such donors for the reasons which include religious, philosophical, sociological, and medical misgivings. As a result, Japanese patients sometimes choose to travel to other countries to await opportunities for transplant. Conversely, Eurotransplant, for example, reports that there were more than 1,500 postmortem liver donors within its catchment area in 2008 (CitationEurotransplant International Foundation 2009).
In Japan, more than 10,000 children are born annually through in vitro fertilization. Hundreds of gynecological clinics provide infertile clients with such services, but the Japan Society of Obstetrics and Gynecology prohibits egg donations and any kind of surrogate conception (CitationTsuge 1999). As a result, few Japanese obstetricians exploit such reproductive technologies, and infertile couples tend to go abroad to be parents. Furthermore, many Japanese obstetricians do not have a positive attitude toward prenatal testing, so the technique is not generally used with pregnant women in the country. In addition to such hesitation in accepting biomedical technology, the use of alternative medicine is very popular, and there is a widespread distrust of medicine and doctors in Japan. Still, gene therapy has been conducted with little opposition. Why? Popular attitudes toward science and technology tend to be investigated only when resistance is significant, such as in the cases of nuclear power and genetically modified organisms. But it remains important to ask why some technologies are readily accepted while others are not, especially in a case when the social history of Japanese medical technology would have predicted protests.
I shall devote some attention to the use of the term “gene therapy”. Its definition is not completely clear, and it has changed over time. At the beginning, it referred to the treatment of diseases through the administration of genes or of cells into which genes were inserted. Gene therapy in this sense was first planned for hereditary diseases thought to be caused by a single gene. However, soon after the first approved trial, many kinds of cancers, HIV infections, and other common diseases were targeted for gene therapy. Today, some experiments with applications for therapeutic development can be included under the name of gene therapy. New categories, such as cell therapy, have also appeared.
Historically speaking, popular response to biological and medical innovations has regularly prompted politicians, officials, and academics to modify their approach in Japan. One thinks of the period around 1980, when citizens challenged recombinant DNA experiments; of the 1960s, when patients and their families claimed a role in formulating standard care; and, during the same period, the efforts of disabled people to intervene in national medical and welfare policy making. A former physicist named Jinzaburo Takagi established an organization called the Citizens' Nuclear Information Center in 1975, modeled on Frank von Hippel's idea of citizen scientists. Citizens' Nuclear Information Center has addressed many problems regarding occupational exposure to radioactive rays.
In addition, the concept of deliberative democracy encouraged Japanese officials and STS researchers to introduce a participatory approach to policy making in the areas of science and technology around 2000. Since then, there have been a number of participatory Technology Assessment (pTA) projects and many other related activities. Needless to say, Japan has tended to follow corresponding movements in other developed countries.
Following or contemporaneous with these civic movements, the concepts of biological citizenship (e.g., CitationRose and Novas 2004), genetic citizenship (e.g., CitationHeath et al. 2004), and scientific citizenship (e.g., CitationIrwin 2001) appeared around 2000. Though these concepts are not identical, they all share the idea of civic engagement and all are based on the inclusion of public opinion in biological and biomedical research and policy making. However, there are many opinions on the role citizens should play and on what constitutes public opinion.
Irwin and Wynne (Citation1996) emphasized the importance of lay experts' knowledge in social/scientific issues, while Collins and Evans (Citation2002) called for a reevaluation of the roles of professional experts. Irwin (Citation2001) suggested that the meaning of civic engagement was ambiguous.
The idea of genetic citizenship surfaced after the advent of the Human Genome Project, and many patient groups embraced the idea (CitationStockdale 1999b). Though the autonomy of those involved in making decisions about genetic treatments is often assumed, from the viewpoint of subjectification theory—stemming from the ideas of Michel Foucault—this style of involvement or engagement might impose on patients the “responsibility for the self to manage its present in the light of a knowledge of its own future” (CitationRose and Novas 2004: 441–442, CitationMatsubara 2007; CitationKayukawa 2010).
The concept of public involvement implies not only interested parties but also a wide range of people. Public engagement can be understood as a legitimization of the process of policy making, no matter what the role of the public might be. Still, the question of what the public is and must be remains open. In the present paper, I assume both that mass media discourses are jointly constructed by the press and its readers and that public opinion can be investigated by looking at those discourses. This is not a normative but a descriptive study.
The notion that popular opposition to technology occurs as a result of misunderstanding or of inaccurate reporting has been criticized many times (e.g., CitationIrwin and Wynne 1996). If the public's impression is more than a mere misunderstanding, then it is important to investigate the construction of that impression. Still, discourse analysis applied to the mass media has such limitations that it is nearly impossible to go beyond the appearance of such discourses, just as a questionnaire cannot go beyond the data gathered. In the present paper, “the public” refers to what can be recognized in various discourses rather than representing specific people or groups.
1 Gene Therapy Emerging Abroad
Most sources agree that the first gene therapy trials were conducted in 1980 by an American doctor on two patients suffering from beta thalassemia, one in Israel and the other in Italy. Dr. Martin Cline of the University of California, Los Angeles, led the project. This was about a decade before the Human Genome Project began. The trials gave rise to huge ethical problems because Cline failed to disclose their details through review board, and a broad debate ensued (CitationYonemoto 1985).
In 1982, an official US government report entitled Splicing Life was published by the President's Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. It provided the key to human gene therapy by underscoring the difference between somatic cell therapy and germ line cell therapy (CitationNukaga 2008). It said: “Gene therapy offers the greatest promise for those single-gene defects in which an identifiable product is expressed in a discrete subpopulation of cells” (CitationPresident's Commission 1982).
In 1984, the Recombinant DNA Advisory Committee of the National Institute of Health issued guidelines on gene therapy under the title The Points to Consider in the Design and Submission of Protocols for the Transfer of Recombinant DNA Molecules into the Genome of One or More Human Subjects. A report by the Office of Technology Assessment titled Human Gene Therapy was published in the same year.
As a result of these reports, gene therapy came to be seen by the end of the 1980s as a significant treatment method for severe genetic diseases. As long as it was applied to human somatic cells, there seemed to be no specific ethical difficulty. The problem lay in the balance of efficiency and risk (CitationMurray 1990). Even in an article published in a bioethical journal, gene therapy was discussed rather optimistically: “Researchers hope that one day gene therapy will cure inherited disorders such as thalassemia and sickle cell anemia. […] There is a growing consensus that gene therapy addressed to somatic or general body cells (and not affecting sperm, eggs, or germ line cells able to give rise to them) can and should be attempted, so long as clinical trials meet rigorous stipulations” (CitationGrobstein and Flower 1984: 13).
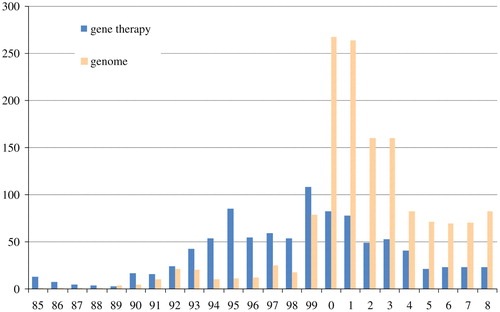
In Japan, the Asahi shimbun first reported on gene therapy in 1985 (), presenting it as an advanced biomedical technology that was nearing reality in the USA. Related newspaper articles published that year often included commentary explaining what gene therapy was. By 1990, the term was associated with treatment for intractable diseases, discussion of bioethical matters, or animal experiments. The ethical issue was not yet sharply focused; one article stated that gene therapy was akin to stepping into “the domain of the gods” (Asahi shimbun, August 26, 1985: 22). Articles on the subject were usually on the science or culture pages rather than in the general news section.
Few popular books on gene therapy appeared in the 1980s. The one important exception was the translation of Theodore Friedmann's Gene Therapy: Fact and Fiction in Biology's New Approaches to Disease, published in 1986. This was comprehensive treatment of the topic to appear in Japan. On the other hand, gene therapy was treated in several articles in popular science magazines during that decade. Even business magazines introduced the subject (CitationYamaguchi 1989). Most such articles were very short, with little information and no particular stance pro or con. Friedmann's book (CitationFriedmann 1986), by contrast, commented extensively on ethical issues, as did Isono (Citation1981), written by a biologist and popularizer of science. But ethicists and sociologists had yet to discover this subject.
Fig. 1 The number of articles in the Asahi shimbun containing the term “gene therapy” (遺伝子治療) and “genome” (ゲノム) (Source: Asahi shimbun article database, “Kikuzo II”)
One popular journal, Gijutsu to ningen (Technology and Human Beings), long skeptical of science and technology, regarded gene therapy as an important issue. First seen as a matter analogous to recombinant DNA technology, gene therapy seemed like eugenics reborn (CitationYonemoto 1982). However, such ethical criticism gradually softened, only to be replaced by concerns about the health risks of gene therapy (Fukumoto Citation1987, Citation1988) after discussions of the prohibition of germ line cell therapy were launched.
In spite of increasingly frequent discussions in the press, many people had little heard about gene therapy. According to a public opinion survey on the life sciences conducted in 1985 by the General Administrative Agency of the Cabinet (Citation1986), relatively high rate of respondents were unfamiliar with the terms “gene therapy” (19.4%) and “gene recombination” (23.5%). Still, the debate over this technology's ethical repercussions has begun.
2 Gene Therapy and Ethical Issues
In 1990, Japanese television and print news reported that William French Anderson and his colleagues had initiated a gene therapy trial at the United States' National Institutes of Health. The Council for International Organizations of Medical Sciences held a conference on the therapy in July 1990, while the trial was being reviewed. The term “gene therapy” appeared in large type on the front page of a Japanese newspaper for the first time that year. From that time on, the term occurred with greater frequency (). No such trials were planned in Japan that year. In fact, there was not even a movement to establish guidelines for gene therapy. By and large, the topic cropped up in discussions of human genetics and ethics.
During that period, two ethical issues specific to gene therapy were often mentioned. Some felt that manipulating genes could be seen as “recreating” human beings, and they deemed this impermissible. Others heard echoes of that discredited pseudoscience, eugenics. They feared that human gene therapy might be extended beyond the scope of therapy to the population at large.
Newspapers and popular books soon took up these concerns. In the Asahi shimbun, for example, a discourse emerged linking human gene therapy to a refashioning of human beings: eight relevant articles appeared between 1985 and 1995 (Asahi shimbun, May 22, 1985: 1; May 31, 1985: 4; June 1, 1987: 4; August 4, 1990: 11; September 16, 1990: 3; April 6, 1993: 3; March 11, 1995: 4; and October 15, 1995 [Osaka ed.]: 8), but never after 1995. In addition, discourses also took up the matter of patients' rights (CitationDNA Mondai Kenkyukai 1994).
At the beginning, gene therapy was associated with recombinant DNA technology rather than with basic human genome research. Over time, this changed. To understand this relationship, the story of human genome research must first be investigated.
3 The Human Genome Project and Changes in the Ethical Framework of Gene Therapy
An international consortium—the Human Genome Organization—collaborated on a vast enterprise—the Human Genome Project—to determine the primary structure of the nuclear DNA shared by all human beings. This was the first international organization created to investigate a single area of biological research. It was founded in 1989 and research was started in 1990 by the Department of Energy and the National Institutes of Health (NIH) in the USA. Several countries joined, and extensive research work was carried out. The end result was the release of a working draft of the human genome sequence in 2000, followed by a complete description in 2003. As is well known, a private firm called Celera Genomics, founded by former NIH researcher Craig Venter, played an important role in the last stages of sequencing.
The implications of this project in the history of science have been discussed by many scientists, historians, and sociologists (e.g., CitationConrad and Gabe 1999). The resulting database has given rise to new kinds of biological and biomedical research, and novel methods for diagnosis and therapy have been planned and tried out. Many ethical, legal, and social problems have also arisen, and there has been controversy over related patents. It is therefore clear that the project has taken on various meanings that change according to one's viewpoint; there cannot be only one true meaning, as the following sections show.
At the outset, the project was expected to continue for several decades (CitationWatson 1990) and was seen as a long-term basic research initiative requiring extensive funding and national support. The Japanese government and several other countries provided about ten million dollars of funding annually. In the late 1980s, researchers appealed to the Japanese government to support human genome research directly. In August 1990, the Japanese Council for Science and Technology implemented a national policy to promote human genome research and seek out partners for international cooperation. As a result, it is said that the Japanese contribution to human genome analysis made up about 6% of the total (CitationSakaki 2001). From 1991 to 2009, most of the funding for genomic research has been provided by the Ministry of Education, Culture, Sports, Science, and Technology. The third budgetary revision, which applied to the period from 2000 to 2004, was among the Millennium Projects aimed at promoting technological innovation.
When the Council for International Organizations of Medical Sciences convened in Tokyo and Inuyama City in 1990 for its 24th conference, the subject was “Genetics, Ethics and Human Values: Human Genome Mapping, Genetic Screening and Therapy”. Gene therapy was consistently presented as an issue of human genetics. The council adopted a declaration known as the “Inuyama Declaration,” stating: “Alterations in somatic cells, which will affect only the DNA of the treated individual, should be evaluated like other innovative therapies. Particular attention by independent ethical review committees is necessary, especially when gene therapy involves children, as it will for many of the disorders in question” (CitationCIOMS 1990).
The language suggests that gene therapy did not appear to present any specific ethical problems. In the early 1990s, researchers hoped to avoid potential controversies by restricting the therapy's application to severe diseases and germ line cells not influenced by gene recombination. Such limitations were specified in two guidelines announced jointly by Japan's Ministry of Health and Welfare and the Ministry of Education in 1994.
During the 1990s, the US government devoted about 5% of the budget for the Human Genome Project to studying associated ethical, legal, and social issues. Subsequently, government-sponsored genomic research in Japan also devoted funds to the study of ELSI, which is often referred to as “studies on points of contact with society”. The first report on these studies was published in 1996 (CitationKato 1996). A single chapter was devoted to ethical discussions of human gene therapy. Yasuyuki Sakaki (Citation1995), the leader of the genome research program funded by the Ministry of Education from 1996 to 2000, proposed an ELSI project and explained genetic diagnosis and gene therapy, including their ethical problems.
With the advent of the Human Genome Project, the ethical issues of gene therapy came to be interpreted as an aspect of human genetics. Fears related to the “recreation” of human beings and eugenics were displaced by the problems of risk and patients' rights.
4 Gene Therapy Discussed: Approaching Japanese Trial
How did the Human Genome Project affect attitudes toward gene therapy? The main focus of the present paper is public interpretations of gene therapy, but a brief attention to the top genome scientists must come first.
Any fundamental research project faces the problem of raison d'être; without having clarified this, its utility will remain unclear. In the case of the Human Genome Project, the value of scientific discovery was emphasized early on. James Watson (Citation1990) wrote: “When finally interpreted, the genetic messages encoded within our DNA molecules will provide the ultimate answers to the chemical underpinnings of human existence. They will not only help us understand how we function as healthy human beings, but will also explain, at the chemical level, the role of genetic factors in a multitude of diseases, such as cancer, Alzheimer's disease, and schizophrenia, that diminish the individual lives of so many millions of people” (44).
In this quotation, the project's importance is stated from a biological and a biomedical viewpoint. Indeed, there are resemblances between genome sequencing research and the enumerative study of natural history. Some bacterial genome projects were completed in the 1990s, the first of which was the sequencing of Haemophilus influenzae in 1995. In animals, the sequencing of the Caenorhabditis elegans (a type of nematode) genome was completed in 1998. The genome sequencing of Arabidopsis thaliana, the first plant to have its genome sequenced, was completed in 2000. Homo sapiens is, of course, one of the target species of genome sequencing research, and comparative investigation will tell us the place of human beings in nature. In fact, this is an important scientific theme. At the same time, though, human genome research naturally leads to the discovery of pathological mechanisms. However, finding pathological processes (mainly in terms of genetics) is one thing, but treatment is another. For Watson, the first leader of the Human Genome Project, the application of the decoded human DNA sequence was a matter of secondary importance.
Still, innumerable claims proliferated about the medical applications of this grand project. The diagnosis of hereditary diseases was a natural area of interest. However, the development of gene therapy was not always seen as the project's principal goal. For example, the report issued by the Office of Technology Assessment in 1984, which investigated gene therapy from a range of viewpoints, was cool in its evaluation of the contribution of the genome project: “Gene mapping will not improve gene therapy directly, and for most diseases the ability to make a diagnosis will precede the availability of an effective treatment” (CitationOffice of Technology Assessment 1988: 64).
This seemed to be the standard view on the relationship between the Human Genome Project and gene therapy among medical researchers. On the other hand, a more positive discourse appears, without contradicting the above view, among those championing the project. The director of the Human Genome Center at Lawrence Berkeley National Laboratory wrote: “The cost of finding individual disease genes, one at a time, is often staggering. The ultimate benefits of finding even one major disease gene that might not have been observed by methods less systematic than the genome project could recoup the entire cost of that project” (CitationCantor 1990: 51).
The Inuyama Declaration (Citation1990) stipulates: “The genome project will produce knowledge of relevance to human gene therapy, which will very soon be clinically applicable to a few rare but very burdensome recessive disorders.” When they suggested connections between the Human Genome Project and gene therapy, ethicists seemed to presuppose another association, as indicated by the following passage: “That increased understanding [i.e., the results of the project] will eventually provide insights into the prevention and treatment of the 3,000 known inherited disorders, and of conditions caused by our (gene-governed) physiological reactions to pathogens, toxins, and mutagens of external origin” (CitationJuengst 1991: 71).
The scientists and policy-makers who pushed for the Human Genome Project did not open with rosy predictions about its practical applications; the association with gene therapy cropped up far more frequently in studies produced by those working on ELSI. The Japanese bioethicist Rihito Kimura outlined his opinions on the ethical issues in a newspaper article, pressing for the regulation of the nascent “gene therapy industry” (Asahi shimbun, December 4, 1993: 5). The piece presupposed not only that the genome project and gene therapy were ethically commensurable, but also that the project would ineluctably lead to gene therapy in the near future. The Japanese ELSI research project on human genetics made an issue of gene therapy (CitationKato 1996).
On the other hand, associations that do not relate to ethics can be seen in the media. In the early 1990s “gene therapy” became an increasingly familiar term, but “genome” made fewer appearances in newspapers (). Early on, the Asahi shimbun reported the following in an article about the Human Genome Project: “The identification of genes that cause diseases has been developing rapidly, and the idea of gene modification for radical treatment will inevitably be promoted. […] Knowing the gene that accounts for the disease makes gene therapy the radical solution” (Asahi shimbun, August 7, 1990: 4). An article on Japan's relatively slow progress toward initiating gene therapy trials stated: “New genes are being rapidly discovered as the Human Genome Project progresses” (Asahi shimbun, April 7, 1993 [evening ed.]: 3).
While genetic researchers and practitioners did not always see eye to eye, they generally shared the assumption that genomic research had an essentially practical goal. Compared with fields such as astronomy or particle physics, research on human biology rarely treads on the territory of pure science. For taxpayers, it is natural to think that such research—especially when it uses large amounts of public money—must provide some benefit to people's lives. But such motivations do not appear to have driven genome researchers at the start of the Human Genome Project. The two early leaders of Japan's human genome research, Ken'ichi Matsubara and Yasuyuki Sakaki, were molecular biologists rather than physicians, and they held PhDs rather than MDs. Somewhat paradoxically, the applications of genome sequencing attracted far more publicity than its purely scientific accomplishments, in spite of the researchers' professional inclinations. The tendency was to see the Human Genome Project as the basic research and human gene therapy as its application.
As we saw earlier, phrases such as “radical resolution” and “radical treatment” were used to describe gene therapy in the late 1980s and the 1990s. Here is another example: “[Gene therapy is] a cutting-edge medical technique used to treat gene-deficiency disorders by supplying normal genes from an external source. It can be thought of as a kind of transplant, and it is expected to offer radical treatment for more than two thousand types of disease, both hereditary and nonhereditary” (Asahi shimbun, May 31, 1985: 4).
In addition, phrases such as “ultimate treatment” (Asahi shimbun, April 12, 1995 [evening ed.]: 12, November 27, 1995, [Gunma ed.]: no page number) and “ultimate medicine” (Asahi shimbun, August 1, 1995 [evening ed.]: 1) also appeared. In April 1993, a feature on gene therapy was titled “Ultimate Treatment Begins” (Asahi shimbun, April 7, 1993: 3). It is clear that these expressions reflected the public interpretation of gene therapy at the time.
This suggests that when the genome project and gene therapy were associated, genetic determinism slipped in. If a particular gene causes a disease, identifying that gene might lead to a treatment. In fact, the metaphor of a “blueprint” was often used in this context (CitationHayashi 2004), as it was in the USA (CitationNelkin and Lindee 1995). As an example, one newspaper article said that gene therapy was “the treatment of diseases by manipulating genes as a ‘blueprint’ of the body” (Asahi shimbun, September 18, 1990: 4).
This deterministic interpretation and the association of gene therapy with the Human Genome Project were widespread in popular literature in the early 1990s. An English-language book on human genetics by Robin McKie, The Genetic Jigsaw, was translated into Japanese in 1992. The following passage appears before a discussion of gene therapy: “We must envision the future when new genetics has developed, that is, the ability of scientists who intend not only to investigate inherited abnormality but also to cure it. It is, in a manner, the ultimate goal of molecular biology to manipulate human beings on a cellular level” (CitationMcKie 1992: 184).
The Japanese title of this book was, literally, “The Front Line of Gene Therapy(遺 伝子治療最前線),” as if the ultimate goal of human genetic research was gene therapy. The title also reminds us that the term “genome” was less well known than “gene therapy” at the time.
Robert Shapiro's The Human Blueprint: The Race to Unlock the Secrets of Our Genetic Script was translated in 1993. It was one of the earliest books published in Japan to include the word “genome” its title. Shapiro wrote: “The ultimate resolution is to find a way of rewriting the genetic information on DNA. Such a resolution is applicable not only to sickle cell anemia but also to other genetic diseases. This method of treating genetic conditions by artificially changing disease-causing parts of DNA is called gene therapy” (CitationShapiro 1993: 118).
A Japanese science journalist Shun'ichi Takebe wrote the following: “To what extent should man interfere with the path of evolution? The answer lies in human gene therapy” (Asahi shimbun, August 26, 1985: 22). Gene therapy was viewed as the ultimate type of treatment.
In a press interview, pediatrician Yukio Sakiyama, who conducted the first Japanese trial on gene therapy, reflected on the birth of the medicine: “Gene therapy promised to be an effective treatment, but unlike an antibiotic used to cure pneumonia, it is far from effective” (CitationAnon 1997: 57).
Earlier presentations had insisted on the ethical issues. One might say that such concerns related and the strong expectations surrounding the use of the genomic insights are two sides of the same coin: both originate from a deterministic view of genetics. The association of gene therapy with the Human Genome Project supports this view.
In the USA the first applications of gene therapy seemed to go well (CitationAnderson 1992), and in the 1990s, hundreds of clinical investigations were conducted. European countries and Japan followed. In 1993, a newspaper article declared: “Gene therapy may be applied not only to genetic diseases but also to cancers, and it is also known to be effective against AIDS” (Asahi shimbun, April 16, 1993: 1). Although scientists had no evidence to shore up such claims, newspapers routinely using the word “success” about the application of gene therapy (Asahi Shimbun, April 2, 1994 [evening ed.]: 14, April 13, 1994 [evening ed.]: 10).
5 The First Japanese Trial: Gene Therapy Coming to be Recognized
In August 1995, the first Japanese trial on human gene therapy was conducted at Hokkaido University (CitationOnodera et al. 1998). A 4-year-old girl suffering from ADA deficiency received an injection of her own cells, which had been manipulated using recombinant DNA technology. The injections were repeated 11 times; the trial ended about one and a half years after the first administration. In August 1997, a posteriori assessment by the university's institutional review board concluded that the trial had been safe and effective (CitationHayashi 2003).
After that trial, an attempt to treat an HIV infection was planned and approved by Kumamoto University in 1995 but was never implemented. After 1997, patients with several kinds of cancer and with peripheral arterial disease became subjects for human gene therapy. All treatment took place at university hospitals.
As the Human Genome Project proceeded, expectations for its influence on human pathology seemed to grow. The English pathologist John Savill wrote: “The completion of the human genome project should make the search for ‘disease’ genes much quicker and will increase still further the importance of these gene based approaches toward diseases” (CitationSavill 1997: 126). In this quotation, scientific results are underscored, and no direct influence on therapy is mentioned. The biological researchers know well that clinical trials are very distant from “gene based approaches toward diseases”. One newspaper reported the first trial as the top story of the day in its August 1 and August 8 evening editions. The headline read: “Man Handles Blueprint of Life” (Asahi shimbun, August 1, 1995: 1). After approval from the institutional review board, a series of reports covered the preparations, the therapeutic process, and the patient's discharge from the hospital in April 1997. Thanks to this coverage, which included regular descriptions of the patient's physical condition, millions of readers witnessed the “success” of the country's first gene therapy trial, which culminated with the former patient's enrolment in elementary school. The story arguably played an important role in the public acceptance of human gene therapy.
A series of further clinical trials was reported in the late 1990s. In addition to good results from animal-based experiments, the effectiveness and ostensible success of human trials were reported several times. In 1999, a large number of articles contained the term “gene therapy” (). Many newspaper reports on the subject that year detailed the approval and implementation of gene therapy trials at Japanese university hospitals. Some medical mishaps in foreign trials were reported, but bioethical problems received scant coverage in the press. Most newspaper articles on gene therapy in the late 1990s were favorable, and ethical problems took a backseat. Attention to and expectations for this new biotechnology-based therapy naturally increased in the late 1990s.
In 1999, the Ministry of Health, Labor, and Welfare decided to simplify and expedite the approval process for gene therapy, and there was little opposition. Even when the case of Jesse Gelsinger, an 18-year-old American who died during a gene therapy trial, was reported in Japanese newspapers, this was simply viewed as a rare failure among thousands of American trials (e.g., Asahi Shimbun, December 3, 1999 [evening ed.]).
In the 1990s, several books on gene therapy appeared, some in translation (CitationNichols 1992; CitationThompson 1995; CitationLyon and Gorner 1998; CitationCohen 1999) and others by Japanese authors. French biologist Daniel Cohen wrote in his book: “Owing to the acceleration of the Human Genome Project, gene identification for most diseases will be complete within ten or twenty years” (CitationCohen 1999: 110).
Keiya Ozawa, a gene therapy researcher, wrote a popular science fiction novel on the subject in 1994. One of the characters says: “Thanks to the development of human genome decoding, the range of applications for gene therapy has been expanded” (CitationOzawa 1994: 191).
On the other hand, in his book on the subject, Yasuyuki Sakaki stated that gene therapy was “not directly related to the Human Genome Project” (CitationSakaki 1995: 105).
It is clear that the government and many biomedical researchers promoted gene therapy in the 1990s; this promotion appears to have led to an increasingly close association with the Human Genome Project. This trend was partially a result of Japan's policy on science and technology. After the bubble economy burst in 1991, the government began preparations for the promotion of advanced technology, aiming for a transformation from a traditional industrial economy to a knowledge-based society. This led to increased emphasis on cutting-edge medical technologies. In 1993, Japan's Ministry of Health, Labor, and Welfare allocated 150 million yen to gene therapy research.
In November 1995, the Science and Technology Basic Law was enacted. It stipulated that the government would formulate a Science and Technology Basic Plan every 5 years, and that the national promotion of science and technology would be implemented in line with these plans. In April 2000, the Industrial Technology Enhancement Act (sometimes referred to as Japan's Bayh-Dole Act) came into effect.
The health ministry set up the Health Science Council in 1997 to rationalize those institutions promoting innovation in the health sciences. The council established the Assessment Subcommittee for Advanced Medical Care to investigate problems related to advanced biomedical technology, and gene therapy trials became one of the subcommittee's main concerns. After the first subcommittee met in 1997, the ministry's assessment of gene therapy trials got under way: the process of reviewing applications for clinical trials would subsequently be in constant motion. Japanese gene therapy, even at the basic level, started with a great push from the government. The first trial at Hokkaido University had been discussed by a preliminary advisory panel set up especially by the council.
6 Gene Therapy after the Completion of the Human Genome Decoding
The release of the draft version of the human genome sequence in June 2000 received extensive media exposure. The following passage is representative: “Now, the decoding of the human genome—the entire set of genetic information—is almost complete. […] There is great potential for making genomic drugs and gene therapy based on human genomic information the ultimate personalized medicine” (Asahi shimbun, January 4, 2001: 6).
In 2000, an opinion piece entitled “Gene Therapy: The Patient as Master” was publishedinthe Asahi shimbun. The author was a high school student, and the piece reflects popular attitudes. It begins: “The human genome has been decoded, and the development of gene therapy is now expected” (Asahi shimbun, July 22, 2000: 12).
During this period, everyone imagined a close relationship between the Human Genome Project and human gene therapy. Indeed, some cast gene therapy in industrial terms in the 1990s. Fumimaro Takaku, a famous physician and medical researcher, wrote: “In the near future, gene therapy will undoubtedly be conducted in clinical practice. […] Some say that the twenty-first century will be the century of gene therapy” (CitationTakaku 1994: 2).
In the 1980s and 1990s, articles on gene therapy were rarely written from an economic point of view. After 2000, however, the term “gene therapy” began to appear frequently in the economics sections of newspapers. For example, one article predicted: “Gene therapy's market size is expected to grow to about 10 trillion yen within a few years” (Asahi shimbun, July 5, 2002: 12). The establishment of a gene therapy joint venture between a private company and a university was also reported, and the article opened with the following remark: “The human genome has been decoded, and global competition is now turning up the heat” (Asahi shimbun, October 30, 2000: 31). These articles provide definitive evidence of the tremendous financial expectations for gene therapy that arose as the Human Genome Project entered its final phase.
During this period, many popular science books on the theme of human genetics were published in Japan. Many of these books assured readers of a close connection between the Human Genome Project and medical practice. Nobuyoshi Shimizu, a molecular biologist and world-leading researcher on human genetics, wrote: “It is obvious that in the near future the results of genetic analysis may be applied in the field of medicine for diagnosis and treatment. The method of therapy will be divided into two parts, one with medicine, the other with genes” (CitationShimizu 2000: 204). In a book published in the same month the first phase of decoding the human genome was completed, the journalist Yuri Aono regards that gene therapy will be “an inevitable problem for everybody in 21st century” (CitationAono 2000: 232).
After 2000, the Human Genome Project entered the public consciousness, and gene therapy was associated with the celebrated project. In many books on genetics that were published after 2000, gene therapy was mentioned (CitationMori 2001; CitationNakahara 2001). In 2002, a book entitled The Human Genome and Gene Therapy (CitationMotohashi 2002) appeared at last.
It is true that early in the new millennium the Human Genome Project was not expected to immediately lead to the identification of disease genes, but the association between the two did not weaken. Not only the mass media and the public but also many doctors and scientists produced hopeful messages that cultivated the links between them.
However, the final reports from the clinical trials did not yield favorable results. According to reporting by Eliot Marshall (Citation1995) in Science, although more than 1,000 patients took part in trials, none of the results proved beyond a doubt that the method worked. Yes, French trials involving X-linked severe combined immune deficiency (SCID) did offer hope (CitationCavazzana-Calvo et al. 2000), as scientists (e.g., CitationSakiyama and Ariga 2001) and journalists (e.g., CitationTerakado 2000) agreed. But then came Jesse Gelsinger and, in 2002, the two children with SCID who developed leukemia as a result of the genetic manipulation of their own cells (CitationHacein-Bey-Abina et al. 2003; CitationKaiser 2003).
Despite promising early signs, Anderson, “the father of gene therapy,” was obliged to offer a grim prognosis: “Several hundred [gene therapy trials] were approved over the next several years. However, by the mid-1990s, it became apparent that there were problems. Although patients' laboratory values improved, and a few patients were significantly helped, the efficiency of gene delivery was not sufficient to provide ‘success’ in the treatment of populations of patients” (CitationAnderson 2002: 1,261).
The current picture remains unpromising. It is said that primary immunodeficiencies may be treated successfully, but there are significant problems with severe side effects (CitationSantilli et al. 2008). The number of target diseases has been increasing, and the number of protocols and trials has grown. However, it has become obvious that virus vector-mediated gene transfer has serious issues in terms of the side effects involved.
In newspapers, the feasibility of medical application for the HGP is often discussed along the following lines: “We hear that complete sequencing of the human genome has been announced…In the not-too-distant future, gene therapy will be used for the treatment of severe diseases and the prevention of disabilities.” (Asahi Shimbun 2003, April 26, p. 30)
In ethical thinking, expectations for medical applications have not decreased. In 2001, the American ethicist Gerard Magill wrote:
It is almost impossible to grasp the significance of this virtual blueprint of the human condition that we can now see, read, study, and apply. Some have referred to this map as the so-called book of life (a sort of “language” used by God to created [sic] life), or the “holy grail” of molecular medicine, that will enable us to develop treatments for a vast array of diseases at their genetic roots. Hopefully, help will be on the way for so many in the United States afflicted with gene related dysfunction, such as cardiovascular disease (50 million), diabetes (15 million), cancer (8 million), or Alzheimer's disease (8 million)—not to mention so many other costly ailments, such as psychiatric disorders, multiple sclerosis, and obesity. There is little doubt that we will have effective genetic therapies within a few years. (CitationMagill 2001)
In Japan there were still high expectations for gene therapy. Biomedical researchers Toshio Ogihara and Ryuichi Morishita offered a rosy picture of post-genomic research: “Genes that lead to cures for diseases will be discovered once the function of genes has become apparent. Then, the treatment known as gene therapy will be realized” (CitationOgiwara and Morishita 2001: 4).
On the other hand, another tendency appeared. The release of the complete sequence of the human genome in 2003 failed to rate many headlines. The deterministic view of genes seemed to start losing momentum, as shown by the following quotation: “The decoding of the genome will not quickly lead to the treatment of disease” (Asahi shimbun, February 13, 2001 [evening ed.]: 16).
At the beginning of the twenty-first century, personalized medicine has become the main medical beneficiary of the Human Genome Project (CitationNakamura and Nakamura 2001), and genome-based drug discovery is also coming on strong (CitationEmilien et al. 2000; CitationHattori 2005). Genomic research has shifted from monogenic to polygenic diseases—the category for most common ailments (CitationItakura and Moritani 2000). Genomic research may end up benefiting many areas of medical treatment besides gene therapy.
The failure of many trials, in combination with scientific discoveries such as the function of m-RNA, has also shaken the deterministic view of genes. Biological historian Shohei Yonemoto described the situation: “For researchers, the image of a genome depicted by high-speed DNA sequencers is like a limitless expanse of water. The metaphor of ‘DNA as a blueprint of life’ has already lost its reality for them. Some say that we must not intrude on the realm of the divine, but those who have learned that DNA is something special would find this puzzling, and might ask what sacred realm this is” (Asahi shimbun, December 22, 2000 [evening ed.]: 17). Whatever the merits of Yonemoto's other comments, the blueprint metaphor continued to appear in articles written by scientists (e.g., CitationShimizu 2001), and many say that their hopes for gene therapy remain undimmed. The association of gene therapy with the success of the Human Genome Project continued into the early twenty-first century.
The prime minister's Biotechnology Strategy Council released an outline plan in December 2002. It stipulated that biotechnology must be promoted for economic and industrial reasons, and that a national understanding of biotechnology is necessary for a developing society.
7 Conclusion and Considerations
In the preceding pages, I have investigated various Japanese discourses on gene therapy, especially those associating it with the Human Genome Project.
At the beginning of the 1980s, it was supposed that there were ethical problems specific to gene therapy, but these tended to be subsumed within worries provoked by the Human Genome Project. That was the beginning of an association that remained strong. Subsequently, serious ethical concerns related to gene therapy gradually disappeared. Most public voices advocated the pursuit of what seemed a promising medical technique.
The newspapers and popular magazines of the 1990s, translated an underlying genetic determinism into a discourse pairing research on the human genome and applications in gene therapy, the latter better known than the former at this time. It was repeatedly insisted that, thanks to advances in the robust genome research project, gene therapy would be realized in the near future and would become the ultimate treatment for many severe diseases. Many scientists cast the Human Genome Project as basic research, but its practical side was foregrounded in popular books, even written by scientists throughout the duration of the project. In due course, as genomic research attracted significant publicity, gene therapy benefited from the association, drawing massive corporate investments in spite of mixed results.
This does not necessarily mean that scientists and journalists intentionally circulated an excessively optimistic view of gene therapy, nor does it mean that the media and the public necessarily misunderstood the relevant scientific knowledge. The public interpretation of gene therapy is to be seen as a result of interactions among researchers, media, and laypeople.
Human gene therapy seemed to be promising remarkable advances in the treatment of serious illnesses. Meanwhile, many trials were conducted in Japan, many subjects participated, many papers were written, and several joint ventures were established—but few patients got better. Is there still hope that human gene therapy may offer an attractive alternative to other options? Once a system is in place, it is not easily dislodged. One has to ask: Why did Japanese society accept human gene therapy despite so much uncertainty?
This question can be answered in a variety of ways. The first Japanese clinical trial was conducted after two separate ministries had reviewed and approved it; the general opinion was that there were no specific ethical issues related to gene therapy, and as such the trial should not be held up on ethical grounds. Indeed, Japanese researchers conducted the early trials carefully. The “success” of the first Japanese gene therapy trial might have a great influence. Yet risks may have been underestimated. Biomedicine was expected to create business opportunities: there was not only governmental support but also enthusiasm from industrial circles. Groups who had opposed biotechnology in general lost power in the 1990s. In addition to these factors, the public interpretation of gene therapy may have played an important role.
The results of a Japanese nationwide opinion survey conducted in 2005 indicated that 69.4% of respondents favored the promotion of genomic studies related to medicine (CitationIshiyama et al. 2008). This rate is much higher than that for studies related to agriculture and pure science, and it suggests that most people expected the principal payoff for genetic research to be medical. Similar opinions may have prevailed a decade ago. The first small trial of a pTA, consensus conference on gene therapy, was conducted by STS researchers in 1998 (CitationKobayashi 2004), but this seems to have taken place too late to have an impact on public opinion, which had already been cast.
To finish let us consider two additional points.
This case study may have important implications for research on the public understanding of science and how information about science is communicated. It obliges us to ask what a “proper understanding” of science might be. It has been shown that public acceptance does not mean that people are armed with accurate information, nor does it mean that they consent to the general application of a novel technique; the case of Japanese gene therapy suggests that interactions among researchers, the media, and the public bring about a new interpretation of technology.
The advent of gene therapy has obliged people to confront a number of ethical problems, but its historical and sociological evaluation is not yet complete. The Japanese journalist Eiko Fukumoto stated that most gene therapy trials are nothing more or less than human experiments—corporate research approved by the government for industrial application but with no merit for patients (CitationFukumoto 2002). If this is even partly true, then the question of why these experiments have been so readily accepted by Japanese society becomes much more important.
Notes
All URLs are browsed in 5 May 2010 (JST).
References
- Anderson W. F. (1992). Human gene therapy. Science, 256, 808–813.
- Anderson W. F. (2002). The current status of clinical gene therapy. Human Gene Therapy, 13(11), 1261–1262.
- Anon (1997). (Gene therapy: can it be a good example of clinical medicine?). Scias, 2(9), 56–60.
- Aono Y. (2000). (What is the gene problem?). Tokyo: Shin'yosha.
- Bush V. (1945). Science: The endless frontier. Washington: United States Government Printing Office.
- Cantor C. R. (1990). Orchestrating the human genome project. Science, 248, 49–51.
- Cavazzana-Calvo M. (2000). Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science, 288, 669–672.
- Cohen D. (1999). (Genes of hope: The Human Genome Project and gene therapy). Tokyo: Kosakusha. Translation of Cohen (1993) Les genes de l'espoir. Paris: Robert Laffont.
- Collins H. M. Evans R. (2002). The third wave of science studies: Studies of expertise and experience. Social Studies of Science, 32(2), 235–296.
- Conrad P. Gabe J. (Eds.). (1999). Sociological perspectives on the new genetics. Oxford: Blackwell.
- Council for International Organizations of Medical Sciences (CIOMS) (1990). The Declaration of Inuyama. http://www.cioms.ch/frame_1990_texts_of_guidelines.htm
- DNA Mondai Kenkyukai (1994). (Gene therapy). Tokyo: Shakai hyoronsha.
- Emilien G. (2000). Impact of genomics on drug discovery and clinical medicine. QJM: Monthly journal of the Association of Physicians, 93(7), 391–423.
- Eurotransplant International Foundation (2009). Annual Report 2008. http://www.eurotransplant.org/files/annual_report/ar_2008.pdf
- Friedmann T. (1986). (Gene therapy: A giant step or a small step). Tokyo: Shujunsha. Translation of Friedmann (1983) Gene therapy: Fact and fiction in biology's new approaches to disease. Cold Spring Harbor Laboratory Press.
- Fukami T. (1996). (Gene therapy of cancer). Tokyo: Kodansha.
- Fukumoto E. (1987). (The age of gene therapy). Gijutsu to ningen 技術と人間 (Technology and Human Beings), 16(9), 115–127.
- Fukumoto E. (1988). (Gene therapy and eugenic policy). Gijutsu to ningen 技術と人間 (Technology and Human Beings), 17(3), 10–13.
- Fukumoto E. (2002). (Slippery slope to the capitalization of human body). Tokyo: Gendai shokan.
- General Administrative Agency of the Cabinet (1986). The Public Survey on Lifescience . http://www8.cao.go.jp/survey/s60/S60-12-60-14.html
- Grobstein C. Flower M. (1984). Gene therapy: Proceed with caution. The Hastings Center Report, 14 (2), 13–17.
- Hacein-Bey-Abina S. (2003). LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science, 302, 415–419.
- Hattori S. (2005). (From complete decoding of human genome to the understanding of human beings). Tokyo: Toyoshoten.
- Hayashi M. (2002). (Manipulating life: Social construction of biological science and technology). Tokyo: NTT publishing.
- Hayashi M. (2003). (What does “successful” mean in advanced medicine? An example of Japanese gene therapy applied to ADA deficiency). Kagaku Gijutsu Shakairon Kenkyu 科学技術社会論研究 (Journal of Science and Technology Studies), 2, 57–67.
- Hayashi M. (2004). (Whose misunderstanding of gene?). In Kuwako T. (Ed.), Inochi no Rinrigaku いのちの倫理学 (Ethics of life) (pp. 190–208). Tokyo: Corona Publishing.
- Heath D. Rapp R. Taussig K.-S. (2004). Genetic citizenship. In Nugent D. Vincent J. (Eds.), A companion to the anthropology of politics (pp. 152–167). Malden: Blackwell.
- Irwin A. (2001). Constructing the scientific citizen: Science and democracy in the biosciences. Public Understanding of Science, 10(1), 1–18.
- Irwin A. Wynne B. (1996). Misunderstanding science? The public reconstruction of science and technology. Cambridge: Cambridge University Press.
- Ishiyama I. (2008). Relationship between public attitudes toward genomic studies related to medicine and their level of genomic literacy in Japan. American Journal of Medical Genetics. Part A, 13, 1696–1706.
- Isono N. (1981). (Gene “therapy” that concerns you). Kagaku asahi 科学朝日 (Scientific Asahi), 1, 32–34.
- Itakura M. Moritani M. (2000). (Medicine in the age of “genome science”). Kagaku (Science Journal), 70(4), 312–317.
- Juengst E. T. (1991). The human genome project and bioethics. Kennedy Institute of Ethics Journal, 1, 71–74.
- Kaiser J. (2003). Gene therapy: Seeking the cause of induced leukemias in X-SCID trials. Science, 299, 495.
- Kato H. (Ed.). (1996). (Studies on points of contact of human genome research with society). Kyoto: Kyoto Daigaku Rinrigaku Kenkyu-shitsu.
- Kayukawa J. (2010). (Power that permeates stem cells: Human beings in biocapitalism). Tokyo: Meiji Gakuin University. Ph.D. diss.
- Kobayashi T. (2004). (Who considers science and technology policy?). Nagoya: Nagoya University Press.
- Lock M. (2002). Twice dead: Organ transplants and the reinvention of death. Berkeley: University of California Press.
- Lyon J. Gorner P. (1998). (The birth of gene therapy: Here started the dramas which startle the world). Translation of Lyon & Gorner (1995) Altered fates: Gene therapy and the retooling of human life. New York: Norton.
- Magill G. (2001). Ethical Perspectives on Mapping The Human Genome. Health Care Ethics USA, 9(1), http://chce.slu.edu/hceusa/1_2001_1.html
- Marshall E. (1995). Gene therapy's growing pains. Science, 269, 1050–1055.
- Matsubara Y. (2007). (genes, patients and citizens) In Tsuge A. Kato S. (eds.), Idenshi gijutsu no shakaigaku 遺伝子技術の社会学 (Studies of technosociety; Sociology of genomics). Tokyo: Bunka shobo hakubunsha. 63-77.
- McKie R. (1992). (The Front Line of Gene Therapy). Translation of McKie (1988) The genetic jigsaw: The story of the new genetics. New York: Oxford University Press.
- Mori K. (2001). (The age of remodeling human beings). Tokyo: Kodansha.
- Motohashi N. (2002). (Human genome and gene therapy). Tokyo: Maruzen.
- Murray T. H. (1990). Human gene therapy, the public, and public policy. Human Gene Therapy, 1, 49–54.
- Nakahara H. (2001). (Overview of human genome). Tokyo: PHP publishing.
- Nakamura Y. Nakamura M. (2001). (The genome century: Socioeconomic change in the era of biotechnology). Tokyo: Kodansha.
- National Institute of Science and Technology Policy (2001). (The 2001 Survey of Public Attitudes Toward and Understanding of Science & Technology in Japan). http://www.nistep.go.jp/achiev/ftx/jpn/rep072j/pdf/rep072j.pdf
- Nelkin D. Lindee M. S. (1995). The DNA mystique: The gene as cultural icon. New York: Freeman.
- Nichols E. K. (1992). (What is gene therapy? Challenging fatal diseases). Tokyo: Kodansha. Translation of Nichols(1988) Human gene therapy. Cambridge, MA: Harvard University Press.
- Nudeshima J. (1995). (Principle is needed in the ethics of advanced medicine). Asahi Shimbun, April 21, 1995.
- Nukaga Y. (2008). The President's commission and somatic cell gene therapy: An analysis of regulatory science in the United States. Seibutsugakushi kenkyu 生物学史研究 (The Japanese Journal of the History of Biology), 80, 1–16.
- Office of Technology Assessment (1988). Mapping our genes: Genome projects: How big? How fast?
- Ogiwara T. Morishita R. (2001). (Genes save lives: Cardiovascular disease and gene therapy). Suita: Osaka University Press.
- Onodera M. (1998). Successful peripheral T-lymphocyte-directed gene transfer for a patient with severe combined immune deficiency caused by adenosine deaminase deficiency. Blood, 1, 30–36.
- Ozawa K. (1994). (Gene therapy to treat cancers and incurable diseases). Tokyo: Houken.
- President's Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research (1982). Splicing life: A report on the social and ethical issues of genetic engineering with human beings.
- Rose N. Novas C. (2004). Biological citizenship. In Ong A. Collier S. J. (Eds.), Global assemblages: Technology, politics, and ethics as anthropological problems (pp. 439–463). Oxford: Blackwell.
- Sakaki Y. (1995). (Human genome: The goal of the human genome project). Tokyo: Iwanami shoten.
- Sakaki Y. (2001). (Human genome: From decoding to application and understanding of people). Tokyo: Iwanami shoten
- Sakiyama Y. Ariga T. (2001). (Gene therapy for immune deficiency). In Tani S. Asano S. (Eds.), Idenshi-chiryo no shintenkai 遺伝子治療の新展開 (New development of gene therapy) (pp. 75–85). Tokyo: Yodosha.
- Santilli G. Thornhill S. I. Kinnon C. Thrasher A. J. (2008). Gene therapy of inherited immunodeficiencies. Expert Opinion on Biological Therapy, 8(4), 397–407.
- Savill J. (1997). Science, medicine, and the future: Molecular genetic approaches to understanding disease. British Medical Journal, 314, 126–129.
- Shapiro R. (1993). (Reading the human blueprint, genome). Tokyo: Kodansha. Translation of Shapiro (1991) The human blueprint: The race to unlock the secrets of our genetic script. New York: St. Martin's.
- Shimizu N. (2000). (Japanese front runner Shimizu Nobuyoshi talks on the truth and falsehood of the human genome project). Tokyo: Bizinesu-sha.
- Shimizu N. (2001). (Reading the human genome, the blueprint of life). Tokyo: Iwanami shoten.
- Stockdale A. (1999a). Waiting for the cure: Mapping the social relations of human gene therapy research. Sociology of Health & Illness, 21(5), 579–596.
- Stockdale A. (1999b). Public understanding of genetics and Alzheimer disease. Genetic Testing, 3(1), 139–145.
- Takaku F. (1994). Foreword. In Ozawa ( 1994) 1–2.
- Terakado K. (2000). (Breakthrough of gene therapy: French team's world first success for inborn immune deficiency). Newton, 20(7), 8–15.
- Thompson L. (1995). (Gene therapy revolution: Footsteps of the scientists who fought against genes). Translation of Thompson. (1994). Correcting the code: Inventing the genetic for the human body. New York: Simon & Schuster.
- Tsuge A. (1999). (Reproductive technology as culture: Discourse analysis of doctors who are involved with fertility treatment). Kyoto: Shoraisha.
- Watson J. D. (1990). The human genome project: Past, present, and future. Science, 248, 44–49.
- Yamaguchi H. (1989). (Gene therapy putting into practical use). Zaikai Kansoku 財界観測 (Observation of business world)., 5, 72–75.
- Yonemoto S. (1982). (Gene manipulation and human rights: Test-tube baby and gene therapy). Gijutsu to ningen 技術と人間 (Technology and Human Beings), 11(7), 12–27.
- Yonemoto S. (1985). (Bioethics). Tokyo: Kodansha.