Abstract
Introduction: A novel coronavirus (CoV), unlike previous typical human coronaviruses (HCoVs), was identified as causative agent for severe acute respiratory syndrome (SARS). SARS first surfaced as a pandemic in late 2002 and originated in southern China. SARS-CoV rapidly spread to > 30 countries by 2003, infecting nearly 8,000 people and causing around 800 fatalities. After 10 years of silence, a 2012 report alarmed researchers about the emergence of a new strain of CoV causing SARS-like disease.
Areas covered: To combat SARS, scientists applied for patents on various therapeutic agents, including small-molecule inhibitors targeting the essential proteases, helicase and other proteins of the virus, natural products, approved drugs, molecules binding to the virus, neutralizing antibodies, vaccines, anti-sense RNA, siRNA and ribozyme against SARS-CoV. In this article, the patents published from 2008 to the present for the new therapeutics that could potentially be used in the prophylaxis and treatment of SARS are reviewed.
Expert opinion: The therapeutic interventions or prophylaxis discussed in this review seems to offer promising solutions to tackle SARS. Rather than being complacent about the results, we should envisage how to transform them into drug candidates that may be useful in combating SARS and related viral infections in the future.
1. Introduction
A life-threatening form of atypical pneumonia threatened the world with a sporadic outburst in late 2002, which was later designated ‘severe acute respiratory syndrome' (SARS) in March 2003 Citation[1-6]. It was first reported in Guangdong province of China and by July 2003 SARS spread to > 30 countries infecting 8,000 people and killing nearly 800 before being contained through public health measures Citation[7]. SARS is caused by a novel coronavirus (CoV)–SARS coronavirus (SARS-CoV). The first isolated strain was named the Urbani strain after the WHO scientist Carlo Urbani, who first alerted the world about the existence of SARS before he succumbed to the disease in Bangkok on 29 March 2003 Citation[4].
SARS-CoV is a virus from genus Coronaviridae, the family of CoVs, which are enveloped, positive-stranded viruses with ∼ 30,000 nucleotides Citation[8]. These largest RNA viruses are composed of three groups: Group 1 contains transmissible gastroenteritis coronavirus (TGEV), porcine gastroenteritis virus etc.; Group 2 consists of SARS-CoV, mouse hepatitis virus (MHV) etc. and Group 3 contains avian infectious bronchitis virus (AIBV) etc. Citation[9].
Two-thirds of SARS-CoV genome encode viral replicase gene which is translated into two overlapping replicase polyproteins pp1a (∼ 490 kDa) and pp1ab (∼ 790 kDa). The polyproteins are later cleaved by two viral proteinases, 3C-like protease (3CLpro) and papain-like protease (PLpro), to yield non-structural proteins essential for viral replication Citation[10,11]. The remaining one-third encode structural proteins of virus, namely spike (S), envelope (E), membrane (M) and nucleocapsid (N) proteins Citation[12,13], of which antibodies against the S protein are capable of neutralizing SARS-CoV Citation[14].
Evolutionary studies have suggested SARS-CoV originated from non-humans, most likely bats, based on evidence of phylogenic analyses on the lineage of SARS-like CoVs. It is believed to be transmitted to humans by aerosols through intermediate hosts like palm civets infected by the virus Citation[15-17]. Therefore, zoonotic transmission, due to CoV's ability of interspecies transfer, into human population is still a viable threat. SARS is identified by symptoms like malaise, rigors, fatigues and high fevers, indications similar to influenza, but later progresses to atypical pneumonia in most cases Citation[18].
Since 2005, there have been no reported cases of SARS. Recently there was report of less lethal human CoV HCoV-NL63 and HCoV-HKU1 Citation[19,20]. In early 2012, there were two reported cases of a new type of HCoV, now called Middle East respiratory syndrome coronavirus (MERS-CoV), a sixth CoV that could infect humans, reported from Saudi Arabia, which claimed one life Citation[21,22]. As of 25 April 2013, the WHO was notified of 17 cases of SARS-like disease caused by MERS-CoV collectively from Jordan, Qatar, Saudi Arabia, UK and United Arab Emirates, among which two reported cases from Jordan were confirmed fatalities. Cases reported from UK support human-to-human transmission, while the search for the sources of zoonotic transmission is still ongoing Citation[23]. The WHO reported nine deaths in the eastern part of Saudi Arabia within a fortnight in May. This increases the HCoV-caused death toll since September 2012 from 20 to 38 confirmed cases Citation[24]. In spite of a persisting threat, there is no therapy available except for symptomatic control.
Although active research to identify antiviral agents for SARS had been pursued since the outbreak, under the present scenario there are neither effective antiviral agents nor vaccines available in market to tackle future outbreaks. Popularly, 3CLpro and PLpro are still considered as a viable targets, along with some new alternatives, such as E protein (Orf4), M protein (Orf6), N protein (Orf9), Orf3a, RNA-dependent RNA polymerase (RdRp) and 5′–3′ helicase Citation[7]. Vaccine development using polypeptides derived from structural or non-structural proteins to induce humoral or cell-mediated immunity has also been pursued. Efforts made towards drug development have been summarized in previous reviews, which include inhibitors against main protease Citation[25-27], helicase Citation[28], using S protein for vaccine and therapeutic development Citation[29] and computational approaches Citation[30]. In this review, the authors have recapitulated the patents published since 2008 for the prophylaxis and treatment of SARS in the following order: protease inhibitors, helicase inhibitors, peptides, RNA products, immunotherapy, inhibitors with unknown targets and miscellaneous.
2. Protease inhibitors
The main protease in SARS-CoV is a chymotrypsin-like cysteine protease, which is also called 3C-like protease (3CLpro), because it is analogous to the 3C protease (3Cpro) of the picornavirus. 3Cpro and 3CLpro play pivotal roles in these viruses' lifecycles and hence have been used as vital targets for drug development. Kuo et al. were the first to prepare a recombinant SARS-CoV 3CLpro without any tag, which is the most active form (fully dimeric), and developed a fluorogenic assay using the substrate peptide containing a fluorescence quenching pair, Dabcyl-KTSAVLQSGFRKME-Edans, for measuring the kinetics and inhibition of the protease Citation[31]. Yang et al. solved the first crystal structure of SARS-CoV 3CLpro complexed with a substrate-like hexapeptidyl chloromethylketone Citation[32], which is analogous to the previously solved structure of TGEV main protease Citation[33], thus providing the basis for catalytic mechanism and inhibitor design. Hsu et al. solved an unsymmetric structure for the two protomers of the dimeric SARS-CoV 3CLpro with one of the active sites occupied by the C-terminal six amino acids from a protomer of another dimeric protein, giving a clue to its maturation processing Citation[34].
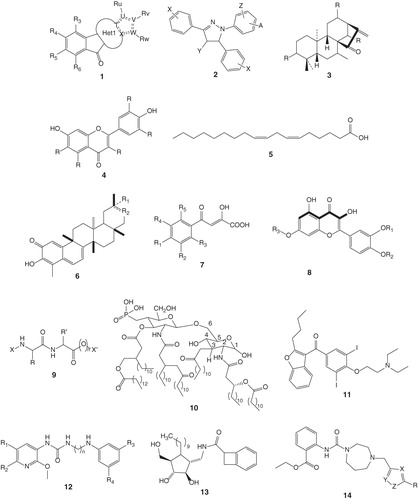
Most of the compounds discussed under protease inhibitors elicit their action by inhibiting 3CLpro. SARS-CoV 3CLpro induces autolytic cleavage of polyproteins pp1a/1ab and thus assists in the maturation process of SARS-CoV. It cleaves the polyprotein at 11 sites with conserved Gln at the P1 position linked to a small amino acid (Ser, Ala or Gly) at the P1' position. Due to its pivotal role in viral maturation, 3CLpro has become an important target for the therapeutic intervention. On the other hand, Compound () targets SARS-CoV PLpro which is involved in processing the viral polyprotein and also a deubiquitinating enzyme Citation[35,36]. Although the exact function is not known, it is believed that deubiquitination might protect replicase subunit against proteosomal degradation Citation[37]. In their patents Citation[38], Boissy et al. at Hybergenics disclosed new pentaaza-cyclopenta[b]fluoren-9-one compounds with general structure (), which is effective against a group of cysteine proteases for ubiquitin-specific processing (USP), a de-ubiquitinylating enzyme family. Compounds were tested against different USP family members, including USP-5, USP-7 and USP-8. In the formula, Het1 and Ru are defined as follows: T, U, V, W and X are made of either C or N while R3, R4, R5, R6 could be H, OAlk, Alk, Hal, NRR′, CN, OH, OCF3, Aryl, Heteroaryl. Their claims also extend to pharmaceutically acceptable salts, hydrates or hydrated salts, or their optical isomers, racemates, diastereomers or enantiomers. Inhibitor with alkyl chain at substituent Ru seems to inhibit all USP enzymes effectively at low nanomolar and could also inhibit SARS-CoV PLpro for suppressing viral replication.
Inhibitors of SARS-CoV 3CLpro reported prior to 2008 in various patents and research papers were reviewed earlier Citation[39]. Here, only the patents published since 2008 are included. As published in the paper Citation[40] and patented in Republic of Korea Citation[41], by screening a library of ∼ 6,800 compounds from Korea Chemical Bank (Daejeon, Korea), a general structure containing a dihydropyrazole ring with three substituents as shown in (), inhibited both 3Cpro and 3CLpro from picornaviruses and CoVs. Picornaviruses are small non-enveloped RNA viruses with a single strand of genomic RNA of 7,500 – 8,000 nucleotides Citation[42]. The members of picornaviruses include rhinoviruses, enteroviruses, coxsackieviruses, polioviruses, echoviruses, encephalomyocarditis viruses, meningitis virus, foot and mouth viruses, hepatitis A virus and so on. Among them, rhinoviruses are the major cause of the common cold, whereas enterovirus and coxsackievirus infections can cause hand, foot, and mouth diseases in humans and animals. In severe cases, enterovirus can damage the central nervous systems leading to viral meningitis, encephalitis and severe myocarditis, as well as fatal pulmonary edema Citation[43-46]. Coxsackievirus strain B3 is a major human pathogen that causes meningitis and mycocarditis leading to heart failure in young adults and congestive heart failure Citation[47]. As also mentioned above, in these viruses, a chymotrypsin-like protease (named 3Cpro) is required to process polyproteins into mature proteins for viral replication, thus representing a promising anti-viral drug target Citation[48]. Specifically, when the three substituents are two phenyl rings and one lengthy N-butyl-benzimidazolylamino-toluene, the compound inhibited both 3Cpro and 3CLpro with IC50 values of 10.3, 5.4, 3.3 and 5.2 μM, respectively, against CoV-229E 3CLpro, Coxsackievirus B3 3Cpro, Enterovirus 71 3Cpro and Rhinovirus 14 3Cpro. Therefore, this kind of inhibitors could be used as dual inhibitors of 3CLpro and 3Cpro against a wide spectrum of CoVs and picornaviruses.
As described in a Chinese patent Citation[49], a natural product was found to co-crystallize with the SARS-CoV 3CLpro. From the X-ray diffraction electron density map, the natural product was identified with the general structure as shown in () with substitution R being either H, OH, OAc, OCH3, OCH2CH3, OCH(CH3)2 or carbonyl group. There are several natural products in accordance with the general structure, including Pseurata A, B and C, Leukamenin E, Glaucocalyxin B and D, Liangshanin A. They claim that these compounds as effective 3CLpro inhibitors could be used as therapeutic agents against SARS-CoV, AIBV and others.
It is claimed in the Chinese patent Citation[50], the Chinese medicines with a general structure shown in () inhibited SARS 3CLpro activity in fluorescent assay. The claimed compounds include those with varying R as H, OH, OSO3H, OPO3H2, OAc, OCH3, OCH2CH3, OCH2CH2CH3, OCH2CH2CH2CH2CH3, Oglu and Ogal. The natural products with this general structure including scutellarein, quercetagetin, myricetin and robinetin showed different enzyme inhibitory activities at 25 μM. As described in the patent Citation[51], unsaturated fatty acid as shown in () such as isolinoleic acid at 50 μM inhibited SARS 3CLpro activity.
As described in a Korean patent Citation[52], Celastrus orbiculatus extract or Brown seaweed line tree extract contains a composition for the prevention or the treatment of SARS-CoV infection. The fractions of extracts or hydropicenone compounds that are extracted from Celastrus orbiculatus extract or Brown seaweed line tree inhibit 3CLpro activity. IC50 are 19.4 μg/ml for EtOH extract of Celastrus orbiculatus, 17.8 μg/ml for EtOAc fraction of Celastrus orbiculatus, 38.7 μg/ml for water fraction of Celastrus orbiculatus, 14.7 μg/ml for EtOH extract of Brown seaweed line tree, 8.5 μg/ml for EtOAc fraction of Brown seaweed line tree and 20.0 μg/ml for water fraction of Brown seaweed line tree. IC50 of the isolated single compounds as shown in () are 10.3 mM for the Compound 1 (Celastrol, R1 is CO2H, R2 is CH2), 5.5 mM for the Compound 2 (Pristimerin, R1 is CO2CH3, R2 is CH2), 9.9 mM for the Compound 3 (Tingenone, R1 is H, R2 is C=O) and 2.6 mM for the Compound 4 (Iguesterin, R1 is nothing, R2 is CH).
3. SARS-CoV helicase inhibitors
SARS-CoV helicase is a non-structural protein, a cleavage product of pp1ab, which binds to 5′ overhang and moves in 5′→3′ polarity to bring about negative supercoiling of double-stranded RNA Citation[53,54]. Its role in unwinding of DNA or RNA makes it indispensable for viral replication and is, therefore, an attractive target for antiviral agents. The compounds discussed below are assumed to elicit their action by obstructing negative supercoiling.
As described in a Korean patent Citation[55], Chung et al. have patented their aryl diketoacids (ADK), also known as a pyrophosphate analog, as an inhibitor of the viral helicase. ADK is known to inhibit HIV-1 integrase and HCV RdRp by sequestering the metal at the active site. Their claim includes compounds with the chemical formula of , in which R1 is H, R2, R3, R4, or R5 is H or R6-(CH2)n-R7, where R6 is O or NH, n is 1 – 3, and R7 is phenyl or halogenated phenyl, along with their pharmaceutically acceptable salts as active ingredients. A compound, where R1 = H, R2 = NHCH2(4-ClPh), and R3 = H, is the best inhibitor with IC50 value of 0.96 µM and its EC50 value being 0.82 µM against the virus. In another Korean patent Citation[56], Chung et al. has extended their work by modifying flexible diketoacid of ADK to rigid dihydroxychromone derivatives as SARS-CoV helicase inhibitors. The general structure is shown in of . The best inhibitor where R1 = R2 = H and R3 = 3-CN-Bn could inhibit the helicase with IC50 value of 2.7 µM.
4. Peptides
As described in the patent Citation[57], Rudolph et al. have patented Thymosin α1 (TA1) peptide, a synthetic 28-amino acid peptide, for prevention or treatment of SARS-CoV infection. Their claim also includes peptides derived by substitution, deletion, elongation, replacement or with a modified sequence that possesses activity substantially similar to TA1. This synthetic peptide has shown to possess immunomodulatory properties to significantly reduce viral titer value in lungs. These peptides could either be the ones obtained from natural sources or recombinant peptides with their sequence the same or similar to natural ones. Administration of TA1 is preferred as injection, periodic infusion, continuous infusion or other forms at a dose of 0.001 – l0 mg/kg/day, because the plasma half-life of subcutaneously administered TA1 is only about 2 h. Their claims also include a form where TA1 is conjugated with non-antigenic polymer within a molecular weight range of 200 – 300,000 Da with water solubility. This could be administered along with other immune stimulators or antiviral agents.
Lee et al. have patented their designed dipeptide compound with dual purpose of detection and inhibition of SARS-CoV Citation[58]. The major focus is on the development of assay platform for the rapid detection of SARS-CoV in systemic circulation as an existing ELISA method could detect the virus only about 7 days after the symptoms first appeared. Compounds used for detection and inhibition is claimed to have general structural features as in () where X and X′ are independently H to facilitate linking of compound with biotin, streptavidin and avidin and n is 0 or 1. R and R′ are comprised of different amino acid side chains. These dipeptides were selected based on their docking with SARS-CoV envelope proteins (PDB ID 2AMQ). Compounds that could be readily synthesized and cost effective were selected, synthesized using known methods. These compounds were tested against commercially available SARS-CoV peptides namely SARS envelope peptide (3533P) and a SARS matrix peptide (3529P). The synthesized compounds were remodeled to be applicable to sensors, tested with SARS-CoV antigens and with human plasma to determine their selectivity.
The invention in the patent Citation[59] relates to isolated polypeptide sequence of Spike protein present on the surface of SARS-CoV, which interacts with receptor-binding domain on the host cell to initiate infection. They have disclosed the nucleic acid sequence of immunogenic fragment that could elucidate the above-mentioned action along with related expression with a suitable vector and purification from the host cell. The peptides obtained apart from being used to treat SARS-CoV infection, are claimed to be useful in the diagnosis of SARS-CoV infection and screening of drugs which can block interaction of S protein with the receptor-binding domain.
5. RNA products
It was reported in 2003 that angiotensin-converting enzyme 2 (ACE2) is a functional receptor for SARS-CoV in mediating virus entry into the cell while the soluble form of ACE2 works contrarily Citation[60]. The claim in the patent Citation[61] defines administration of ACE2 in the form of encoded DNA molecule using nucleic acid shuttles instead of direct administration of ACE2 facilitated by any gene therapy method. The therapy targets acute lung failures arising from acid aspiration or sepsis, lung oedemas, acute respiratory distress syndrome and lung failures associated with SARS-CoV infections. The invention also encompasses use of ACE2 in combination with an AT1-inhibitor or its pharmaceutically acceptable salt, with a special emphasis on telmisartan (an angiotensin II receptor antagonist) and its pharmaceutically acceptable salt and also in combination with ACE inhibitors and bradyikinin receptor (a G-protein coupled receptor) inhibitors.
Fukuda et al. in their patent Citation[62] and paper Citation[63] describe an invention of a therapeutically useful ribozyme, an antisense RNA molecule with catalytic activity, for the treatment of infections by SARS-CoV and other CoVs like MHV. The basic design of their ribozyme claimed to target SARS-CoV mRNA to elicit a therapeutic effect is shown in . This ribozyme specifically recognizes the base sequence, namely GUC, present in the loop region, on the mRNA of SARS-CoV or other HCoVs. The complementary base sequence on the ribozyme is derived by deleting, adding or modifying bases without altering its binding affinity. This invention also extends to a method adopted to prevent its in vivo decomposition by designing it as RNA/DNA chimeric structure containing RNA structure in the conserved region and DNA structure in other regions along with phosphothioate modification at the 3′ end. The results were compared experimentally by preparing mismatch-ribozyme which was ineffective in cleaving mRNA of SARS-CoV or MHV. This confirmed the importance of sequence specificity of ribozyme to be therapeutically effective.
Figure 2. (A) Basic structure of ribozyme and (B) Ribozyme designed against SARS-CoV. The ribozyme structures are adopted from Ref Citation[62].
![Figure 2. (A) Basic structure of ribozyme and (B) Ribozyme designed against SARS-CoV. The ribozyme structures are adopted from Ref Citation[62].](/cms/asset/6b7cbeeb-1853-47fc-b529-59445a4b0182/ietp_a_823159_f0002_b.jpg)
A Chinese patent Citation[64] has claimed to use small interference RNA to inhibit SARS-CoV's M protein expression. The designed double-stranded RNA, named siRNA-M1, has sequence of 5′-gggugacuggcgggauugcgau-3′, complementary to the sequence of M protein mRNA 220 – 241 nucleotides. Another siRNA-M2, 5′-gggcgcugugacauuaaggac-3′, is complementary to the 460 – 480 nucleotides of M protein mRNA. These two siRNAs were shown to inhibit the expression level of M protein mRNA.
6. Immunotherapy – vaccines, antibodies etc.
Vaccination is the preferred line of defense against diseases due to its specificity compared to chemotherapeutic agents. Antigenic peptides, either recombinant or derived from pathogens, are used to induce either humoral- or cell-mediated immunity. Direct administration of monoclonal antibodies was sought because it was observed that patients recovering from SARS had a neutralizing antibody effective in preventing infection Citation[65]. It was also observed that the presence or absence of other structural proteins did not affect immunogenicity of S protein Citation[66] and the binding of the Spike protein to ACE2 is a vital step for virus to access into host cell Citation[60]. All these make S protein a putative fragment to be considered as an antigen to deliver effective therapy. Focus on works in recent patents also substantiates the importance of S protein as a valid antigenic source.
A vaccine as described in the patent Citation[67], is claimed as an effective treatment or prevention of SARS. It is composed of immunogenic S-polypeptide or any portion of it which has an epitope capable of triggering an immune response. This S-polypeptide comprises an amino acid sequence from 14 to 1,193 of SARS-CoV's S-protein, which is fused at the C-terminal to an octapeptide Flag (DYKDDDDK) through a Serine-Glycine linker. It is claimed to be therapeutically effective at a dose of 1 – 5 μg per human dose. It also consists of adjuvant 3-deacylated monophosporyl lipid-A (3D-MPL), in , which is a non-toxic derivative of lipid A, as well as saponins QS21, which is derived from Quillaja Saponaria Molina in the form of a liposome and known to stimulate CD8+ cytotoxic T-cells, in the formulation. 3D-MPL and QS21 are present in 1:1 ratio in their vaccine composition. In another patent, they extend the work on S-protein with the same amino acid sequence and claim that alternative adjuvant comprising oil-in-water emulsion would be an effective treatment Citation[68]. In this case, the oil used is metabolizable or biodegradable and is usually squalene, TWEEN 80 or α-tocopherol.
The focus in patent Citation[69] is to develop a vaccine to induce cytotoxic T-lymphocytes (CTL) specifically against SARS-CoV for therapy or prophylaxis. Some non-patented works have used partial peptides from Spike protein as CTL epitope peptides. Use of CTL epitope peptide pp1a, a non-structural protein, for inducing this cell-based immunity defines their novelty. Thirty different peptides were initially selected and screened for binding affinity towards the HLA-A2 molecule, which is MHC class I molecule. Nine designed peptides had significant CTL-inducing activity, amongst which the sequence SMWALVISV was considered the most suitable one. Prior knowledge about the ability of peptide-bound liposomes containing peptides to induce and initiate CTL response was applied successfully by placing CTL epitope derived from pp1a. The liposome used is comprised of phospholipid made from C14 to C24 acyl groups with one unsaturated bond along with cholesterol as a stabilizer. Interestingly, since non-structural proteins are produced earlier than structural proteins, viral elimination can be achieved at initial stage of infection by using CTL epitope derived from pp1a to induce immune response.
A patent Citation[70] based on their paper Citation[71] describes the use of antibodies and their compositions for the diagnosis and treatment of SARS. This invention describes how isolated heavy- and light-chain immunoglobulins derived from human anti-SARS-CoV's S protein antibodies and nucleic acid molecules encoding such immunoglobulins are used to obstruct attachment and/or fusion of virus to host cells. It also provides gene therapy methods to deliver nucleic acid molecules which encode the heavy and/or light immunoglobulin molecules along with a suitable vector to express desired S protein antibodies.
Based on their studies Citation[72], a Chinese patent Citation[73] claims the use of immuoglobulins, including polyclonal and monoclonal antibodies, for diagnosis, prevention and therapy of SARS by targeting S protein's 318 – 510 amino acid residues with number 479 being any amino acid except Asn. Another Chinese patent Citation[74] with details already reviewed Citation[29] claims the use of monoclonal antibodies against S protein's receptor-binding domain, a 193-amino acid fragment, prevents the binding of virus to its human host through the S protein with ACE2. These monoclonal antibodies can be used as therapeutic and diagnostic agents in emergencies and also for studying immunogenesis of S protein.
Disclosed in the Korean patent Citation[75] is an SARS vaccine nano-delivery system in which a SARS-CoV DNA vaccine (psi-S) encoding the spike protein is complexed with a polymer polyethylenimine (PEI) to effectively deliver pci-S into cells. PEI has been widely used as a non-viral vector since it was evaluated for its capacity in drug delivery systems including DNA vaccines. The inventors found that when complexed with PEI, a SARS DNA vaccine can be effectively delivered into cells and mouse body as shown by the elevated number of B220+ cells found in PEI/pci-S vaccinated mice. In addition, increased levels of co-stimulatory molecules (CD80 and CD86) and class II major histocompatibility complex molecules (I-Ad) were found in CD11c+ dendritic cells from cervical lymph nodes of the mice after PEI/pci-S vaccination. The percentages of IFN-gamma-, TNF-alpha- and IL-2-producing cells were higher in PEI/pci-S vaccinated mice than in pci-S- or pci-mock-treated mice. The results showed that intranasal immunization with PEI/pci-S nanoparticles induces antigen-specific humoral and cellular immune responses.
7. Inhibitors with unknown targets
A patent Citation[76] as detailed in paper Citation[77] owns the idea for treatment and prophylaxis of viral diseases using diiodobenzoyl-benzofuran derivatives like amiodarone ( of ), dronedarone, mono-desethyl-amiodarone, and di-desethyl-amiodarone. These compounds are claimed to be effective when administered in forms of suitable pharmaceutical preparations and administered either as tablets, capsules (soft, hard, oily, operculate), dragees or powders (as suspension or emulsion). Although the exact mechanism is not revealed, efficacy of this compound could be due to its effect on the host cell membrane to disrupt the late endocytic pathway, which is essential for budding of virus, to curb spread of virus. It is also speculated that inhibition could be due to interference with assembly of structural proteins and genomic RNA into new virions. Experiments showed amiodarone to be effective in preventing viral multiplication in Vero cells at 50 µM without toxicity.
A WO patent Citation[78] and a Canadian patent Citation[79] as well as their paper Citation[80] published by inventors from Hong-Kong Pasteur Research Center, China, relates to the identification and/or use of anti-CoV molecules that inhibit or disrupt viral protein-cellular protein interactions, important or essential in the viral life cycle of a CoV, for example, a method for identifying inhibitors of early stages of SARS-CoV replication cycle by using lentiviral Spike pseudotyped particles. Using a yeast two-hybrid screening strategy, the inventors have identified cellular factors that interact with the SARS-CoV's E, S and M proteins, in particular, ezrin (ERM protein) and PALS1 (PDZ-domain containing protein) for interaction with S and E proteins, respectively.
A Chinese patent Citation[81] based on their studies Citation[82] claims preparing Thymosin α 1 (Ta1) with polyethylene glycol (PEG) modification (Ta1-PEGs) as a treatment for SARS and other viral diseases. Ta1, an immunostimulant of T-lymphocyte, composed of 28 amino acids, can stimulate secretion of IL-2 and γ-interferon etc. by T-cells, thereby enhancing immunity. A general formula is Z-[Cysx(PEG-MAL)]-(Aa)n-T, where Z = H, methyl, ethyl, acetyl, etc., MAL is 1,3-dimethylpyrrolidine-2,5-dione (a Malemide derivative), –SO2CH2CH2–, PEG = RO(CH2CH2O)mCH2CH2– (R = H or CH3, m = 5 – 2,000). The side chain, carboxylate or amino end of Cys is covalently linked with MAL, T's (Ta1 with one residue substituted by Cys) N-terminus, or T's C-terminus, respectively. X represents the position of Cys in T, Aa could be any amino acid and n = 0 – 10. An example is Ac-[Cys5(mPEG5000-MAL)]Ta1, named BTJB005 in the patent.
A Korean patent Citation[83] claims the use of urea derivatives (general structure shown in of ) as a treatment for the diseases caused by CoVs, including SARS-CoV. EC50 against feline CoV and feline infectious peritonitis virus are 2.87 and 1.98 μg/ml for Compound 2 (R1 = R2 = R3 = R4 = CH3, n = 2), 3.61 and 2.39 μg/ml for Compound 3 (R1 = CH3C(O)OEt, R2 = R3 = R4 = CH3, n = 2), 7.11 and 8.19 μg/ml for Compound 4 (R1 = CH3C(O)OEt, R2 = CH3, R3 = R4 = OCH3, n = 2), 3.29 and 2.51 μg/ml for Compound 6 (R1 = CH3C(O)OEt, R2 = CH3CH2CH2, R3 = R4 = CH3, n = 2), 9.49 and 3.038 μg/ml for Compound 7 (R1 = CH3C(O)CH3, R2 = R3 = R4 = CH3, n = 2), and 87.46 μg/ml, respectively, as compared to 61.95 μg/ml against infectious peritonitis virus for ribavirin. CC50 values of the compounds 2, 3, 4, 6, and 7 are larger than 100 μg/ml.
A Taiwanese patent Citation[84] applied by Japanese inventors from a Japanese company (POKKA Corp.) claims using extract from Psidium Guajava Linn's leaves or stems and ascorbic acid as a treatment for SARS. No compound structure was specified.
8. Miscellaneous
Invention describes use of five-membered iminocyclitol derivatives in the treatment of osteoarthritis, Japanese encephalitis virus, dengue virus serotype 2 (DEN-2) and SARS-CoV. Amongst the list of claimed molecules, in is the representative compound with SARS-CoV inhibitory activity of 3.3 – 10 µM in a cell-based assay using Vero E6 cells incubated with SARS-CoV (H.K. strain). As these molecules are glycosidase inhibitors designed against osteoarthritis, it is speculated that inhibition of SARS-CoV could be through the inhibition of ER-α-glucosidase, while other possible mechanisms cannot be ruled out Citation[85,86].
Invention in the patent Citation[87] claims the effective usage of a type I or type III interferon receptor agonist alone or in combination with a type II interferon receptor agonist in the treatment of SARS-CoV infection. The claimed intervention is effective in reducing either viral load, or time for viral clearance, or morbidity or mortality in patients suffering from a SARS-CoV infection apart from being effective in prophylaxis for high-risk individuals. A type I or type III interferon receptor agonist is claimed to be effective at dosages ranging from 1 µg to about 300 µg while a type II interferon receptor agonist at dosages ranging from 25 to 500 µg when it is either given alone or in combination with each other, or along with other nucleotide or nucleoside analogs. It is claimed to be effective therapeutically when administered within a time period of 24 h to 35 days of exposure to SARS-CoV.
Invention in the patent Citation[88] claims to overcome the shortcomings of using a single drug for treating viral diseases caused by influenza virus and CoV which are known to induce a cytokine storm. Antiviral drugs typically reduce viral load but are incapable of preventing tissue damage resulting from the release of pro-inflammatory cytokines which in turn could effectively be overcome by an EP4 receptor agonist. However, when administered alone, this leads to partial treatment. Synergism was observed when these two therapeutic agents were given in combination with suitable dosage adjustment as exemplified by treating mice bearing H5N1 with a combination of oseltamivir (anti-influenza) and beraprost (EP4 receptor agonist). Dose adjustment was made either using an optimal dose of antiviral agent along with a suboptimal dose at 10 – 80% of optimal dose of EP4 receptor agonist, or using 10 – 50% of optimal dose of the antiviral agent along with an optimal dose of EP4 receptor agonist. Antiviral agents used could be neuraminidase, M2 channel, RNA replication and translation, or polymerase inhibitors, whereas EP4 receptor agonists could be selected from 5-cyano-prostacyclin, prostacyclin, carbacyclin and many more derivatives. The claim also includes an addition of non-steroidal anti-inflammatory drugs or steroids to the regimen to alleviate inflammation.
A Chinese patent Citation[89] claims using a JAK3 inhibitor to treat SARS. As already known, SARS-CoV's S protein can stimulate phosphorylation of JAK3, a member of the JAK/STAT family, thereby activating IP-10 for gene activation. They showed that targeting JAK3 indeed inhibits the S protein-mediated IP-10 expression.
As described in the Japanese patent Citation[90] applied by researchers from Republic of Korea and their paper Citation[91], homopiperazine compounds, with a general structure shown in of , can bind to the RNA pseudo-knot structure of SARS-CoV. Binding of this inhibitor obstructs attachment of ribosome to stable upper RNA pseudoknot during ‘-1' ribosomal frame-shift Citation[92]. This alters ratio of gag to gag-pol nucleocapsid proteins. While increased amount of gag-pol leads to the formation of defective viral particles by inhibiting the proteolytic processing of the viral polyprotein, decrease results in inefficient dimerization of the gag-pol fusion protein and failure of packaging Citation[93]. Therefore, ‘-1' ribosomal frame-shift is a viable target for the prevention of viral propagation.
9. Conclusion
We have summarized here the patents published since 2008 till now for agents developed against SARS-CoV. These include small molecules inhibiting 3CLpro, PLpro and helicase, Thymosin a1 peptide, dipeptides which can be docked into envelop proteins, peptides derived from S protein sequence which can interrupt the binding of SARS-CoV to humans, ACE2 in the form of DNA molecule or ACE2 inhibitors, also for preventing virus attachment, ribozyme targeting viral mRNA, small interference RNA to inhibit viral M protein expression, therapeutic antibodies and vaccine using S protein as an antigen, a nano-delivery system to facilitate DNA vaccine, inhibitors with unknown targets and others including ER-α-glucosidase inhibitor, interferon receptor agonist, EP4 receptor agonist, JAK3 inhibitor and the agents that bind to RNA pseudo-knot structure of SARS-CoV to inhibit ‘-1' frame-shift. These agents have been initially tested to have great potential in treating or preventing SARS-CoV infection, or diagnosing the virus.
10. Expert opinion
Since the discovery of the causative agent of SARS, the SARS-CoV, sequencing of its whole genome has provided a clue for viral replication machinery and several valuable protein and nucleic acid targets for developing potential anti-SARS therapy. Efforts on characterizing these targets including studies of their functions, properties, structures, etc. have enhanced our understanding on these targets and facilitated therapeutic agent development for SARS. Some of these important efforts have been indicated in Bibliography, particularly, the identification of SARS-CoV, its genome sequencing to reveal the functional and structural proteins, the preparation of the fully active recombinant 3CLpro for identifying the inhibitors using a convenient FRET assay, the crystal structures of 3CLpro for facilitating structure-aided drug discovery, the function of SARS-CoV helicase, the identification of Spike protein antibodies recovered from SARS-CoV patients, the identification of S protein's receptor ACE2 in human, the development of monoclonal antibodies for neutralizing the virus, and vaccine to stimulate immunity against SARS-CoV, as well as the isolation of the recently emerged MERS-CoV, etc.
In addition to the recent patents, the focus of this review article, there are papers describing potential therapeutic agents such as nucleic acids aptamers targeting helicase Citation[94,95] and inhibitors of RdRp Citation[96], which should not be ignored. However, most of the patents describe the agents for treatment or prevention of SARS based on in vitro and/or cell-based experiments, but few of them have been actually tested in animals and none in humans. This is because SARS-CoV is extremely dangerous and the experiments involving live SARS-CoV should be performed in a BSL-3 laboratory. Furthermore, there are currently no patients as human subjects to test these agents. Therefore, it is unsure there will be any cure for SARS if it re-emerges. However, if the disease unfortunately reoccurs, the potential agents can be quickly moved forward to clinical trials if their bioavailability and safety profiles have been approved. Initiative should be taken now at least to finish evaluating these profiles of the promising leads in animal models. These could be done without using the live SARS-CoV to avoid its accidental leakage. In the next stage, the carefully selected drug candidates could be tested for efficacy in combating the virus in animal models using live virus with necessary precautions. Among the efforts, developing immunotherapy such as neutralizing monoclonal antibodies and vaccines may be more feasible since the inactivated virus or replicon with only the surface proteins (e.g., spike protein) can be used for most of the studies. These technologies and developed agents accordingly may be also useful for treating the SARS-like disease caused by the newly emerged MERS-HCoV, which has killed 33 people in the 58 cases till 14 June 2013 according to WHO. Gene sequencing of their RdRp, S and N proteins reveals that MERS-CoV is closely related to Tylonycteris bat CoV HKU4 and Pipistrellus bat CoV HKU5 in bats from Hong Kong Citation[97], and other potential lineage C betacoronaviruses in bats from Africa, Europe and America. SARS-CoV also originated from non-humans, most likely bats. It is not known yet whether the inhibitors of the molecular targets in SARS-CoV can act as inhibitors for MERS-CoV. Anyhow, we hope to see approved therapeutic agents available for SARS and SARS-like diseases in the near future.
Article highlights.
SARS with atypical pneumonia symptom by SARS-CoV caused high mortality during 2003 epidemic.
Enzymes essential for SARS-CoV replication include 3CLpro, PLpro, helicase and RdRp and inhibitors against these potential targets have been discovered.
RNA molecules for gene therapy, ribozyme and interference RNA have also been tried to inhibit SARS-CoV replication.
Since patients recovering from SARS had a neutralizing antibody effective in preventing infection, vaccination or direct administration of monoclonal antibodies is promising.
The newly emerged MERS-CoV likely originated from the same immediate host and the available anti-SARS agents might be worth trying on MERS-CoV.
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Notes
This box summarizes key points contained in the article.
Bibliography
- Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003;348:1967-76
- Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 2003;348:1953-66
- Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 2003;348:1986-94
- WHO. Coronavirus never before seen in humans is the cause of SARS 2003
- Peiris JSM, Lai ST, Poon LLM, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 2003;361:1319-25
- Fouchier RAM, Kuiken T, Schutten M, et al. Aetiology - Koch's postulates fulfilled for SARS virus. Nature 2003;423:240-0
- Barnard DL, Kumaki Y. Recent developments in anti-severe acute respiratory syndrome coronavirus chemotherapy. Future Virol 2011;6:615-31
- Rota PA, Oberste MS, Monroe SS, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 2003;300:1394-9
- Spaan WJM, Cavanagh D. Coronaviridae, in virus taxonomy, VIIIth Report of the ICTV. Elsevier Academic Press, London; 2004. p. 945-62
- Thiel V, Ivanov KA, Putics A, et al. Mechanisms and enzymes involved in SARS coronavirus genome expression. J Virol 2003;84:2305-15
- Thiel V, Herold J, Schelle B, Siddell SG. Viral replicase gene products suffice for coronavirus discontinuous transcription. J Virol 2001;75:6676-81
- Narayanan K, Maeda A, Maeda J, Makino S. Characterization of the coronavirus M protein and nucleocapsid interaction in infected cells. J Virol 2000;74:8127-34
- Opstelten DJ, Raamsman MJ, Wolfs K, et al. Envelope glycoprotein interactions in coronavirus assembly. J Cell Biol 1995;131:339-49
- Buchholz UJ, Bukreyev A, Yang L, et al. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc Natl Acad Sci USA 2004;101:9804-9
- Hon CC, Lam TY, Shi ZL, et al. Evidence of the recombinant origin of a bat severe acute respiratory syndrome (SARS)-like coronavirus and its implications on the direct ancestor of SARS coronavirus. J Virol 2008;82:1819-26
- Li W, Shi Z, Yu M, et al. Bats are natural reservoirs of SARS-like coronaviruses. Science 2005;310:676-9
- Wang LF, Eaton BT. Bats, civets and the emergence of SARS. Curr Top Microbiol Immunol 2007;315:325-44
- Peiris JS, Yuen KY, Osterhaus AD, Stohr K. The severe acute respiratory syndrome. N Engl J Med 2003;349:2431-41
- Fielding BC. Human coronavirus NL63: a clinically important virus? Future Microbiol 2011;6:153-9
- Cui LJ, Zhang C, Zhang T, et al. Human coronaviruses HCoV-NL63 and HCoV-HKU1 in hospitalized children with acute respiratory infections in Beijing, China. Adv Viro 2011;2011:129134
- Zaki AM, van Boheemen S, Bestebroer TM, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012;367:1814-20
- de Groot RJ, Baker SC, Baric RS, et al. Middle east respiratory syndromecoronavirus (MERS-CoV); Announcement of the coronavirus study group. J Virol 2013. [Epub ahead of print]
- Available from: http://www.who.int/csr/disease/coronavirus_infections/update_20130425/en/
- Available from: http://www.who.int/csr/don/2013_05_14_ncov/en/index.html
- Liang PH. Characterization and inhibition of SARS-coronavirus main protease. Curr Top Med Chem 2006;6:361-76
- Yanh H, Bartlam M, Rao Z. Drug design targeting the main protease, the Achille's heel of coronavirus. Curr Pharm Des 2006;12:4573-90
- Ramajayam R, Tan KP, Liang PH. Recent development of 3C and 3CL protease inhibitors for anti-coronavirus and anti-picornavirus drug discovery. Biochem Soc Trans 2011;39:1371-5
- Keum YS, Jeong YJ. Development of chemical inhibitors of the SARS coronavirus: viral helicase as a potential target. Biochem Pharmacol 2012;84:1351-8
- Du L, He Y, Zhou Y, et al. The spike protein of SARS-CoV–a target for vaccine and therapeutic development. Nat Rev Microbiol 2009;7:226-36
- Chou KC, Wei DQ, Du QS, et al. Progress in computational approach to drug development against SARS. Curr Med Chem 2006;13:3263-70
- Kuo CJ, Chi YH, Hsu JT, et al. Characterization of SARS main protease and inhibitor assay using a fluorogenic substrate. Biochem Biophys Res Commun 2004;318:862-7
- Yang H, Yang M, Ding Y, et al. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc Natl Acad Sci USA 2003;100:13190-5
- Anand K, Ziebuhr J, Wadhwani P, et al. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science 2003;300:1763-7
- Hsu MF, Kuo CJ, Chang KT, et al. Mechanism of the maturation process of SARS-CoV 3CL protease. J Biol Chem 2005;280:31257-66
- Lindner HA, Fotouhi-Ardakani N, Lytvyn V, et al. The papain-like protease from the severe acute respiratory syndrome coronavirus is a deubiquitinating enzyme. J Virol 2005;79:15199-208
- Barretto N, Jukneliene D, Ratia K, et al. The papain-like protease of severe acute respiratory syndrome coronavirus has deubiquitinating activity. J Virol 2005;79:15189-98
- Lindner HA. Deubiquitination in virus infection. Virology 2007;362:245-56
- Boissy G, Peretto I, Delansorne R, et al. Inhibitors of cysteine proteases, the pharmaceutical compositions thereof and their therapeutic applications. CA2632635A1; 2007
- Wang HM, Liang PH. Pharmacophores and biological activities of severe acute respiratory syndrome viral protease inhibitors. Expert Opin Ther Patents 2007;17:533-46
- Kuo C-J, Liu H-G, Lo Y-K, et al. Individual and common inhibitors of coronavirus and picornavirus main proteases. FEBS Lett 2009;583:549-55
- Liang PH, Jung KC, Ge LH, et al. Composition for preventing or treating picornavirus and coronavirus induced diseases containing 3,5-diaryl-4,5-dihydro pyrazol derivatives or pharmaceutically acceptable salts thereof as an active ingredient. KR20100066142A; 2010
- Melnick JL. Enterovirus: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses in virology. Lippincott-Raven; Philadephia, PA: 1996
- Blomberg J, Lycke E, Ahlfors K, et al. Letter: new enterovirus type associated with epidemic of aseptic meningitis and-or hand, foot, and mouth disease. Lancet 1974;2:112
- Sperber SJ, Hayden FG. Chemotherapy of rhinovirus colds. Antimicrob Agents Chemother 1988;32:409-19
- Lum LC, Wong KT, Lam SK, et al. Neurogenic pulmonary oedema and enterovirus 71 encephalomyelitis. Lancet 1998;352:1391
- Ho M, Chen ER, Hsu KH, et al. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med 1999;341:929-35
- Lee CK, Kono K, Haas E, et al. Characterization of an infectious cDNA copy of the genome of a naturally occurring, avirulent coxsackievirus B3 clinical isolate. J Gen Virol 2005;86:197-210
- Krausslich HG, Wimmer E. Viral proteinases. Annu Rev Biochem 1988;57:701-54
- Rao Z, Lou Z, Sun Y, et al. Diterpenes diterpenoids natural product inhibitor for main protease of coronaviruses such as SARS and screen method thereof. CN101418334A; 2009
- Rao Z, Ma M, Lou Z, et al. Method for separating SARS coronavirus main proteinase inhibitor from traditional Chinese medicine. CN101701245A; 2010
- Rao Z, Ma M, Lou Z, Li X. Method for screening SARS corona virus major protease inhibitor from traditional Chinese medicine and screened SARS corona virus major protease inhibitor. CN101921823A; 2010
- Lee WS, Rho MC, Park SJ, et al. Composition for prevention or treatment of coronavirus and composotion for inhibiting the activity of 3C-like protease. KR20110068191A; 2011
- Adedeji AO, Marchand B, te Velthuis AJW, et al. Mechanism of nucleic acid unwinding by SARS-CoV helicase. PLoS ONE 2012;7
- Tanner JA, Watt RM, Chai YB, et al. The severe acute respiratory syndrome (SARS) coronavirus NTPase/helicase belongs to a distinct class of 5′ to 3′ viral helicases. J Biol Chem 2003;278:39578-82
- Chung YH, Jeong YJ, Lee CY. Composition for inhibiting SARS coronavirus comprising aryl diketoacid derivatives. KR20100029528A; 2010
- Chung YH, Jeong YJ, Lee CY. Pharmaceutical compositions comprising dihydroxychromone derivatives as an active ingredient for treating and preventing diseases caused by coronaviruses. KR20110006083A; 2011
- Rudolph AR, Tuthill CW. Treatment or prevention of respiratory viral infections with alpha thymosin peptides. US2010311656A1; 2010
- Lee SH, Hong HB, Kim TW, et al. Peptide compounds for detecting or inhibiting SARS coronavirus and application thereof. US2010304363; 2010
- Chong PCS, Hsieh SL. Receptor binding polypeptides. US2009022735A1; 2009
- Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426:450-4
- Penninger JM, Imai Y, Kuba K, et al. Use of inhibitors of the renin-angiotensin system for the treatment of lung injuries. US2008159962A1; 2008
- Fukuda N, Ueno T, Fukushima A, Kuroda K. Ribozyme to cleave coronavirus gene. US2010273997A1; 2010
- Fukushima A, Fukuda N, Lai Y, et al. Development of a chimeric DNA-RNA hammerhead ribozyme targeting SARS virus. Intervirology 2009;52:92-9
- Wang Y, Liu L, Wang S, et al. Small interfering RNA for restraining SARS corona virus M protein gene expression, encoding gene and application thereof. CN101173275; 2008
- Yang ZY, Kong WP, Huang Y, et al. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature 2004;428:561-4
- Buchholz UJ, Bukreyev A, Yang L, et al. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc Natl Acad Sci USA 2004;101:9804-9
- Benoit B, Benoit C, Nicolas E, et al. Vaccine. US2010233250A1; 2010
- Benoit B, Benoit C, Nicolas E, et al. Vaccine. US2012045469A1; 2012
- Masanori M, Tetsuya U, Hiroshi O. Cytotoxic T cell epitope peptide for SARS coronavirus, and use thereof. US2011262529A1; 2011
- Babcook JS, Prabhakar BS, Coughlin M. Antibodies to SARS coronavirus. WO2008060331A2; 2008
- Coughlin M, Lou G, Martinez O, et al. Generation and characterization of human monoclonal neutralizing antibodies with distinct binding and sequence features against SARS coronavirus using XenoMouse. Virology 2007;361:93-102
- van den Brink EN, Ter Meulen J, Cox F, et al. Molecular and biological characterization of human monoclonal antibodies binding to the spike and nucleocapsid proteins of severe acute respiratory syndrome coronavirus. J Virol 2005;79:1635-44
- Ter Meulen JH, van den Brink EN, de Kruif CA, et al. Compositions against SARS-coronavirus and uses thereof. CN101102794A; 2008
- Jiang S, He Y. Neutralizing monoclonal antibodies against severe acute respiratory syndrome-associated coronavirus. CN101522208; 2009
- Yoon CH, Cho JS. SARS vaccine nano-delivery system. KR20100120473A; 2010
- Baritussio A. Use of amiodarone and amiodarone analogues as antiviral agents. WO2008044261A1; 2008
- Stadler K, Ha HR, Ciminale V, et al. Amiodarone alters late endosomes and inhibits SARS coronavirus infection at a post-endosomal level. Am J Respir Cell Mol Biol 2008;39:142-9
- Nal B, Altmeyer RM, Kien F, et al. Anti-coronavirus molecules and their use in compositions and methods for treating and/or preventing infection caused by a coronavirus. WO2008062309A2; 2008
- Altmeyer RM, Kien F, Jean M, et al. Anti-coronavirus molecules and their use in compositions and methods for treating and/or preventing infection caused by a coronavirus. CA2611106A1; 2008
- Millet JK, Kien F, Cheung CY, et al. Ezrin interacts with the SARS coronavirus Spike protein and restrains infection at the entry stage. PLoS One 2012;7:e49566
- Dong S, Liu K, Ma J, et al. Long-acting thymosin alpha1-polyethylene glycol modifiers. CN101759805A; 2010
- Qie J, Ma J, Wang L, et al. Studies of bioactivity, conformation and pharmacokinetic profiles of site-specific PEGylated thymosin alpha 1 derivatives. Drug Metab Lett 2007;1:232-40
- Cho EH, Chung SG, Myung HN, et al. Urea derivatives, process for the preparation thereof, and antiviral composition containing the same. KR20080020862A; 2008
- Fukumoto S, Goto T, Hayashi S, et al. Anti-SARS coronavirus agent, and product containing anti-SARS coronavirus agent. TW201018473A; 2010
- Liang PH, Cheng WC, Lee YL, et al. Novel five-membered iminocyclitol derivatives as selective and potent glycosidase inhibitors: new structures for antivirals and osteoarthritis. ChemBioChem 2006;7:165-73
- Liang PH, Lee YL, Wong CH. Novel five-membered iminocyclitol derivatives as selective and potent glycosidase inhibitors: new structures for antivirals and osteoarthritis therapeutics. US2012046337A1; 2012
- Blatt LM. Compositions and methods for treating coronavirus infection and SARS. US2009068142A1; 2009
- Guilford JW, Faulds HD. Combination therapy treatment for viral infections. WO2011047048A1; 2011
- Jun X. Uses of selective JAK3 restrainer VI in antiviral mediated acute lung damnification. CN101401807A; 2009
- Park HJ, Park SJ, Kim YG. Homopiperazine-based compound suppressing ribosomal frame shift by binding to RNA pseudoknot structure of SARS coronavirus. JP2008156357A; 2008
- Park SJ, Kim YG, Park SJ. Identification of RNA pseudoknot-binding ligand that inhibits the -1 ribosomal frameshifting of SARS-coronavirus by structure-based virtual screening. J Am Chem Soc 2011;133:10094-100
- Plant EP, Rakauskaite R, Taylor DR, et al. Achieving a golden mean: mechanisms by which coronaviruses ensure synthesis of the correct stoichiometric ratios of viral proteins. J Virol 2010;84:4330-40
- Dinman JD, Wickner RB. Ribosomal frameshifting efficiency and gag/gag-pol ratio are critical for yeast M1 double-stranded RNA virus propagation. J Virol 1992;66:3669-76
- Shum KT, Tanner JA. Differential inhibitory activities and stabilisation of DNA aptamers against the SARS coronavirus helicase. ChemBiochem 2008;9:3037-45
- Jang KJ, Lee NR, Yeo WS, et al. Isolation of inhibitory RNA aptamers against severe acute respiratory syndrome (SARS) coronavirus NTPase/Helicase. Biochem Biophys Res Commun 2008;366:738-44
- te Velthuis AJ, van den Worm SH, Sims AC, et al. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog 2010;6:e1001176
- Lau SK, Li KS, Tsang AK, et al. Genetic characterization of betacoronavirus lineage C viruses in bats revealed marked sequence divergence in the spike protein of Pipistrellus bat coronavirus HKU5 in Japanese pipistrelle: implications on the origin of the novel Middle East Respiratory Syndrome Coronavirus. J Virol 2013. [Epub ahead of print]