Abstract
The effects of cognitive impairment on the occupational functioning, social activity, and economic life of patients with schizophrenia constitute major obstacles to recovery. Currently, the standard biological treatment for schizophrenia consists of antipsychotic medications, which results in significant improvements in psychotic symptoms such as delusions and hallucinations via a high affinity for numerous neurotransmitter receptors. However, the effects of antipsychotics on cognitive dysfunction appear very limited or minimal in clinical practice. In fact, according to recent clinical trials, newer antipsychotics, which have little effect on cholinergic receptors but potent antagonistic effects on the serotonin-7 receptor (5-HT7; e.g., lurasidone), may ameliorate cognitive defects in patients with schizophrenia. It has been consistently reported that both nicotinic and muscarinic receptors play crucial roles in cognition and, thus, that they may be considered potential therapeutic targets for new drugs designed to decrease cognitive deficits. Accordingly, cholinesterase inhibitors (ChEIs) may be effective in enhancing the cognitive functioning of patients with schizophrenia. Not surprisingly, such drugs have been utilized to treat cognitive deficits in patients with schizophrenia in a handful of clinical trials. This paper reviews a brief background information and discusses current clinical issues regarding the use of ChEIs in patients with schizophrenia.
1. Cholinergic system and schizophrenia
Schizophrenia is a prevalent, chronic, recurrent, and devastating mental illness with a number of clinical symptoms. Despite its longstanding history in medicine, a clear etiopathogenesis has not yet been elucidated. Therefore, the diagnosis of schizophrenia currently depends primarily on descriptive phenomenology established by clinicians' long-term observations and the classification of symptoms without any assessment of laboratory parameters. Although one must exhibit certain cardinal clinical features, such as delusions and hallucinations, to be diagnosed with schizophrenia, cognitive deficits may also contribute to decreased medication compliance, impaired global functioning, and refractoriness to current antipsychotic treatments Citation[1].
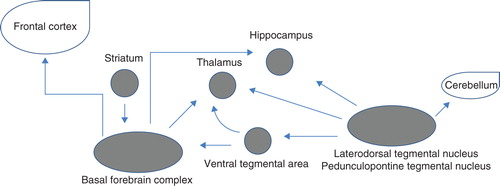
It is known that acetylcholine (ACh) plays an important role in the cognitive deficits of patients with schizophrenia. Seventeen different subunits of the nicotinic ACh receptor (AChR) and five different subtypes of the muscarinic (M) AChR have been cloned, and a majority of these are known to be expressed in the brain Citation[2]. A schematic of the major cholinergic systems is presented in Citation[3] and Citation[2-12]. ACh achieves its effects via two receptor classes: nicotinic receptors that are linked to a ligand-gated ion channel and M receptors that are coupled to a G-protein. Specific receptor subtypes within each class mediate cognitive functions in the brain Citation[2]. Cholinesterase inhibition leads to an enhancement of extracellular ACh.
Table 1. Major muscarinic and nicotinic receptors and their relevance to schizophrenia.
Substantial evidence supports an underlying role for the cholinergic system in the cognitive deficits experienced by patients with schizophrenia Citation[1,2,5,13-17]: i) a deficit in cholinergic neurotransmission may be similar in its effects and potentially clinically indistinguishable from an excess of dopaminergic transmission in the striatum (ACh has a modulating effect on dopamine neurotransmission in the striatum), ii) a decrease in brain choline acetyltransferase levels, iii) decreased density or availability of M or nicotinic cholinergic receptors, iv) high rates of cigarette smoking, v) favorable effect of clozapine, which acts as an agonist at M receptors, vi) differential neurocognitive effects of atypical antipsychotics which have a different affinity for AChRs compared with typical antipsychotics, and vii) worsening of psychosis via cholinergic antagonism. Therefore, we may assume that cholinergic agents with agonistic activity in synapses may improve cognitive deficits in patients with schizophrenia.
2. Cholinesterase inhibitors in the treatment of cognitive deficits in schizophrenia
It is well known that alterations in the cholinergic system may contribute to the neuropsychiatric manifestations of schizophrenia. However, patients with schizophrenia do not show neuronal cholinergic deficits, and their cognitive impairment is negatively correlated with choline acetyltransferase activity, indicating that the enhancement of cholinergic transmission may improve compromised cognition in schizophrenia Citation[18]. In fact, improvements in cognitive deficits and neuropsychiatric behavioral symptoms were profound in the treatment of patients with Alzheimer's disease (AD) with cholinesterase inhibitors (ChEIs) Citation[19]. The beneficial effects of ChEIs on emotion and affected behaviors are primarily dependent on influences in the limbic and paralimbic areas of the brain, which are also principal target sites for many different classes of antipsychotics Citation[20,21]. Furthermore, some of the atypical antipsychotics (e.g., quetiapine) significantly enhance the release of ACh in the prefrontal cortex, but not in the nucleus accumbens, through modulation of serotonin (5-HT)-1A receptors Citation[22].
Indeed, a number of open-label and randomized placebo-controlled clinical trials (RCTs) have extensively examined the potential role of ChEIs in the treatment of schizophrenia, especially with respect to cognitive deficits. The most commonly studied ChEIs included donepezil, galantamine, and rivastigmine. Donepezil is a centrally acting ChEI that leads to an increase in cortical ACh Citation[23]. Rivastigmine is a pseudo-irreversible ChEI that inhibits both acetylcholinesterase and butyrylcholinesterase Citation[23]. Galantamine is another ChEI that increases the concentration of ACh via reversible inhibition of its hydrolysis by acetylcholinesterase and also interacts with nicotinic AChR Citation[23].
Most ChEIs tested in RCTs function as augmentation agents when administered in conjunction with typical and/or atypical antipsychotics for the treatment of cognitive deficits in patients with schizophrenia. Several open-label add-on trials of rivastigmine demonstrated some improvement in cognitive function, whereas six small-scale RCTs (ranges: 12 – 24 weeks, 6 – 12 mg/d, n = 20 – 36) clearly failed to demonstrate significant clinical benefits with respect to the cognitive performance of patients with schizophrenia Citation[21,23]. In eight RCTs (ranges: 8 – 24 weeks, 4 – 32 mg/d, n = 16 – 86) Citation[21,23,24], galantamine was tested for its effects on cognitive impairment in patients with schizophrenia. However, the results were inconsistent in that some RCTs found beneficial effects on processing speed, attention, and verbal memory, whereas other RCTs failed to replicate such findings. In a recent 24-week study of 32 patients with schizophrenia, galantamine was administered in conjunction with long-acting risperidone (25 mg or 50 mg), which was delivered via injection, an established and stable antipsychotic-medication delivery system; however, galantamine augmentation failed to provide any significant cognitive improvements in this population Citation[24].
Among ChEIs, donepezil is the most studied. In 14 RCTs (ranges: 8 – 18 weeks, 5 – 10 mg/d, n = 6 – 245), donepezil failed to improve the cognitive impairments of patients with schizophrenia Citation[21,23]. Keefe et al. Citation[25] conducted a prospective 12-week RCT to investigate whether donepezil should be used to augment treatment for mild-to-moderate cognitive impairment in schizophrenia or schizoaffective disorder. This study was the first large-scale RCT among patients with schizophrenia/schizoaffective disorder to use a comprehensive neurocognitive battery to evaluate cognitive improvements of patients with stable symptoms and mild-to-moderate illnesses. The primary outcome measure was the composite score on the Clinical Antipsychotic Trials of Intervention Effectiveness neurocognitive battery. The placebo group showed either a significantly greater improvement or a trend toward greater improvement relative to the donepezil group at week 12 in complete and last-observation-carried-forward analyses. Interestingly, the cognitive improvement with donepezil was comparable to that of placebo at week 6; however, the effect of donepezil stopped after 6 weeks, whereas the improvement with placebo treatment continued until week 12. Despite the absence of a clear explanation of this finding, the differential effects of donepezil in AD and schizophrenia may stem from the different pathological changes in the cholinergic system associated with the two diseases. This also suggests that pro-cholinergic treatment will not be effective in schizophrenia.
In a recent RCT, coadministration of rivastigmine (3 – 4.5 mg/d) and electroconvulsive therapy (ECT) resulted in a significantly greater decrease in the cognitive subscale of the AD assessment scores compared with placebo (maximum period of 4 weeks). This finding suggests the possible involvement of the cholinergic system in the mediation of cognitive deficits following ECT and indicates a potentially beneficial effect for rivastigmine coadministration in minimizing some of these ECT-induced cognitive impairments in treatment-resistant schizophrenia Citation[26]. Interestingly, donepezil was also found to be useful in the treatment of the cognitive deficits associated with maintenance ECT, although this was found in a case report relying on a small sample Citation[27].
3. Interpretation of currently available data and future research directions
Several reasons can be offered to explain why ChEIs have failed to affect the cognitive deficits in schizophrenia despite their efficacy in AD. In general, the issue of small sample size is problematic when attempting to detect meaningful differences in RCTs. Likewise, most RCTs investigating ChEIs include fewer than 30 patients (only one included 245 patients; otherwise, fewer than 90 were included). However, this should not be the case with ChEIs given that the study conducted by Keefe and colleagues Citation[25] had a sufficient sample even though they found that donepezil was less effective than was placebo. The study conducted by Buchanan and colleagues Citation[28] was also moderately powered, but only a few post hoc analyses found significant results. The measurement tools used in such trials may have been inadequately sensitive to detect changes associated with ChEI treatment, and different end points were utilized in these RCTs, precluding unified meta-analytic results. Thus, the use of a standard set of neuropsychological batteries needs to be promoted. In fact, the joint academic and industry initiative known as Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) has developed guidelines and proposed the use of a cognitive battery (MCCB) addressing seven cognitive domains (speed of processing, attention/vigilance, working memory, verbal learning, visual learning, reasoning and problem solving, and social cognition) to improve the specificity of treatments for cognitive deficits Citation[29]. Although the study conducted by Keefe and colleagues investigating donepezil Citation[25] was designed and initiated before the establishment of the MATRICS guidelines and the MCCB, their study was consistent with these recommendations and may be considered representative of rigorous research in this field. Although the MCCB has excellent reliability, minimal practice effects, and significant correlations with measures of functional capacity, its sensitivity to changes during pharmacological treatment has yet to be confirmed Citation[30]. Additionally, there is a lack of sufficient information concerning the utility of the MCCB because relatively few studies have used the MCCB as primary endpoint thus far. This issue should be addressed in the next few years.
Most studies were only 12 weeks in duration, which is considered too short to detect differences between the effects of ChEIs and placebos on cognitive functioning. Different underlying pathological changes in the cholinergic system may result in differences between the cognitive dysfunction observed in AD and that observed in schizophrenia. In fact, patients with schizophrenia do not usually have typical AD neuropathology (i.e., neuronal degeneration vs. primarily neuronal dysfunction in the limbic system, respectively). It is also possible that multiple abnormalities in the cholinergic system in association with alterations in other neurotransmitter systems may be differentially involved in the development of cognitive deficits in schizophrenia; this may be reflected in the effects of donepezil (predominantly on M AChR) and galantamine (also stimulates the nicotinic system) found by some studies. Cognitive impairments may present at a very early age, often before the onset of other overt symptoms, in those with schizophrenia; however, they worsen as much as they do in AD Citation[31]. The ChEIs may benefit only a small subgroup of patients with decreased cholinergic activity, given that individual schizophrenia patients suffer from various types of cognitive impairment during their different clinical courses Citation[31]. The issue of dosing with ChEIs should also be taken into account because different dosing strategies have not been sufficiently studied in patients with schizophrenia. Because most studies were of short term, it is important that we conduct adequately powered long-term studies with uniquely designed and sensitive primary endpoints targeting the cognitive deficits in schizophrenia in the near future to ascertain whether ChEIs are beneficial to patients with this condition. Some evidence, albeit weak, has suggested that ChEIs may benefit different areas of cognition compared with placebo; this will also be further explored in the future.
Other cholinergic agents, such as selective M1/M4 agonists (e.g., xanomeline), were found to be effective for the improvement of psychotic symptoms according to total, verbal-learning, and short-term memory scores on the Brief Psychiatric Rating Scale and the Positive and Negative Syndrome Scale. This indicates that such agents may function as antipsychotics and cognitive enhancers Citation[6]. A specific and robust negative correlation was found between regional β2-nicotinic AChR availability and negative schizophrenic symptoms, which lends preliminary support to the development of medications targeting β2-nicotinic AChRs in the treatment of negative symptoms Citation[32]. Furthermore, the sigma-1 receptor, part of the N-Methyl-D-aspartate receptor-complex agonists, has a major impact on neurotrophic factors and can alter cognitive functioning. Following phencyclidine-induced cognitive impairment, which is utilized as an animal model of schizophrenia, significant improvement following the subchronic administration of sigma-1 receptor agonists was observed Citation[33]. It should be also noted that positron emission tomography has shown that donepezil exhibits an affinity for the sigma-1 receptor. Recent work with α-7 nicotinic agonists suggests that this AChR system may be also one of the promising candidates for improving cognition in patients with schizophrenia Citation[4,9]. Other novel neurotransmitter-related cognitive agents involving serotonin, dopamine, gamma-aminobutyric acid (GABA), and glutamate may also be considered. The inadequacy of proper animal models for the complex neurodevelopmental abnormalities underlying cognitive impairment in schizophrenia should be also kept in mind as an area for future research Citation[34].
4. Clinical implications and conclusion
The existing evidence, which supports the use of ChEIs for treating cognitive deficits in patients with schizophrenia, remains weak; thus, ChEIs should not yet be considered a viable and routine treatment option in practice. Newer antipsychotics, with different pharmacodynamic properties leading to putatively differential effects on cognitive dysfunction (related to psychosocial function) and partially differential effects on the cognitive functioning of patients with schizophrenia, are still being explored. Most importantly, typical antipsychotics are known to cause more cognitive impairment than atypical antipsychotics. Hence, instead of including ChEIs as a standard treatment for the cognitive impairment in schizophrenia, practitioners should prudently select and use proper antipsychotics during treatment. Nonetheless, despite the limited extant evidence, augmentative treatment with ChEIs may be a reasonable option for patients with schizophrenia following the failure of other presumably more adequate treatment options, given that ChEIs are tolerable and effective for improving different aspects of cognition.
Declaration of interest
The author states no conflict of interest and has received no payment in preparation of this manuscript.
Notes
Bibliography
- Money TT, Scarr E, Udawela M, Treating schizophrenia: novel targets for the cholinergic system. CNS Neurol Disord Drug Targets 2010;9:241-56
- Wallace TL, Ballard TM, Pouzet B, Drug targets for cognitive enhancement in neuropsychiatric disorders. Pharmacol Biochem Behav 2011;99:130-45
- Picciotto MR, Alreja M, Jentsch JD. Chapter 1. Acetylcholine. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors, Neuropsychopharmacology: the fifth generation of progress. Lippincott, Williams, & Wilkins, Philadelphia, Pennsylvania; 2002
- Leonard S, Gault J, Adams C, Nicotinic receptors, smoking and schizophrenia. Restor Neurol Neurosci 1998;12:195-201
- Radek RJ, Kohlhaas KL, Rueter LE, Treating the cognitive deficits of schizophrenia with alpha4beta2 neuronal nicotinic receptor agonists. Curr Pharm Des 2010;16:309-22
- Shekhar A, Potter WZ, Lightfoot J, Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry 2008;165:1033-9
- Martin LF, Freedman R. Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Int Rev Neurobiol 2007;78:225-46
- Martin LF, Leonard S, Hall MH, Sensory gating and alpha-7 nicotinic receptor gene allelic variants in schizoaffective disorder, bipolar type. Am J Med Genet B Neuropsychiatr Genet 2007;144B:611-14
- Olincy A, Harris JG, Johnson LL, Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry 2006;63:630-8
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523-39
- Ryan RE, Loiacono RE. Nicotinic receptor subunit mRNA in the thalamus of the rat: relevance to schizophrenia? Neuroreport 2000;11:3693-8
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci 2006;27:482-91
- Holt DJ, Herman MM, Hyde TM, Evidence for a deficit in cholinergic interneurons in the striatum in schizophrenia. Neuroscience 1999;94:21-31
- Davidson M, Keefe RS. Cognitive impairment as a target for pharmacological treatment in schizophrenia. Schizophr Res 1995;17:123-9
- Berman JA, Talmage DA, Role LW. Cholinergic circuits and signaling in the pathophysiology of schizophrenia. Int Rev Neurobiol 2007;78:193-223
- Scarr E, Dean B. Role of the cholinergic system in the pathology and treatment of schizophrenia. Expert Rev Neurother 2009;9:73-86
- Brunzell DH, McIntosh JM. Alpha7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia. Neuropsychopharmacology 2012;37:1134-43
- Powchik P, Davidson M, Haroutunian V, Postmortem studies in schizophrenia. Schizophr Bull 1998;24:325-41
- Rees TM, Brimijoin S. The role of acetylcholinesterase in the pathogenesis of Alzheimer's disease. Drugs Today (Barc) 2003;39:75-83
- Cummings JL. Cholinesterase inhibitors: a new class of psychotropic compounds. Am J Psychiatry 2000;157:4-15
- Ribeiz SR, Bassitt DP, Arrais JA, Cholinesterase inhibitors as adjunctive therapy in patients with schizophrenia and schizoaffective disorder: a review and meta-analysis of the literature. CNS Drugs 2010;24:303-17
- Ichikawa J, Li Z, Dai J, Atypical antipsychotic drugs, quetiapine, iloperidone, and melperone, preferentially increase dopamine and acetylcholine release in rat medial prefrontal cortex: role of 5-HT1A receptor agonism. Brain Res 2002;956:349-57
- Singh J, Kour K, Jayaram MB. Acetylcholinesterase inhibitors for schizophrenia. Cochrane Database Syst Rev 2012;1:CD007967
- Lindenmayer JP, Khan A. Galantamine augmentation of long-acting injectable risperidone for cognitive impairments in chronic schizophrenia. Schizophr Res 2011;125:267-77
- Keefe RS, Malhotra AK, Meltzer HY, Efficacy and safety of donepezil in patients with schizophrenia or schizoaffective disorder: significant placebo/practice effects in a 12-week, randomized, double-blind, placebo-controlled trial. Neuropsychopharmacology 2008;33:1217-28
- Stryjer R, Ophir D, Bar F, Rivastigmine treatment for the prevention of electroconvulsive therapy-induced memory deficits in patients with schizophrenia. Clin Neuropharmacol 2012;35:161-4
- Rao NP, Palaniyappan P, Chandur J, Successful use of donepezil in treatment of cognitive impairment caused by maintenance electroconvulsive therapy: a case report. J ECT 2009;25:216-18
- Buchanan RW, Conley RR, Dickinson D, Galantamine for the treatment of cognitive impairments in people with schizophrenia. Am J Psychiatry 2008;165:82-9
- Buchanan RW, Davis M, Goff D, A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr Bull 2005;31:5-19
- Keefe RS, Buchanan RW, Marder SR, Clinical trials of potential cognitive-enhancing drugs in schizophrenia: what have we learned so far? Schizophr Bull 2011; In press
- Risch SC. Do cholinesterase inhibitors enhance cognition in schizophrenia? Curr Psychiatry 2008;7:96-100
- D'Souza DC, Esterlis I, Carbuto M, Lower ss2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia. Am J Psychiatry 2012;169:326-34
- Niitsu T, Iyo M, Hashimoto K. Sigma-1 receptor agonists as therapeutic drugs for cognitive impairment in neuropsychiatric diseases. Curr Pharm Des 2012;18:875-83
- Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav 2011;99:245-53