Abstract
Hereditary angioedema (HAE) through C1 inhibitor deficiency is a rare but important disease. It is characterized by recurrent episodes of angioedema, which commonly affects the skin (in the form of swelling in the extremities, face and genitals) as well as the gastrointestinal tract (abdominal pain attacks). In approximately 1% of cases of angiodema-related swelling, there is obstruction of the upper airway, which is potentially life-threatening. Therefore, HAE due to C1 inhibitor deficiency may be associated with significant morbidity and mortality. Recent research has added to our ever-increasing understanding of the pathogenesis of HAE, which has, in addition, new clinical trials with new therapeutic agents and strategies. The following editorial covers drugs currently under investigation that have the potential to be promising new therapeutic options. While some compounds show promise for the future, there are currently no oral treatments available for the treatment of acute attacks. Furthermore, some of the intravenous therapies currently available require numerous injections and do not always prevent acute attacks. Attenuated androgens also may have problematic side effects, highlighting the need for new treatment options.
1. Hereditary angioedema
Recurrent angioedema is clinically characterized by episodes of marked edema (‘attacks’) involving the skin, gastrointestinal tract and other organs, including potentially fatal upper airway obstruction Citation[1]. Various forms of acquired and hereditary angioedema (HAE) share this clinical presentation. ‘Classic’ HAE is associated with a quantitative (type I) or qualitative (type II) deficiency of C1 esterase inhibitor (C1-INH) caused by mutations of the SERPING1 gene (HAE-C1-INH). Since 2000, a novel type of HAE is well known: HAE with normal C1-INH (HAEnCI) Citation[2,3]. HAE-C1-INH is a rare disease: the prevalence is estimated at 1 individual per 50,000. The number of attacks varies considerably in patients. The burden of disease is high, especially in patients with frequent attacks.
2. Pathophysiology of HAE-C1-INH
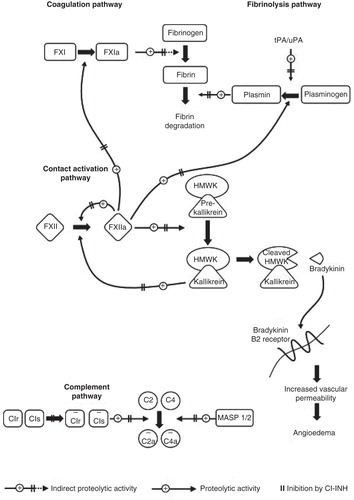
C1-INH is a serine protease inhibitor and inhibits not only C1r and C1s in the complement system but also FXIIa, kallikrein and FXIa in the kallikrein-kinin system (KKS) and the coagulation system (). In patients with HAE-C1-INH, the KKS (‘contact system’) is activated in an acute attack leading to the release of bradykinin followed by vasodilation, increased vascular permeability and localized fluid extravasation. In individuals with C1-INH deficiency, there is an increased tendency for phases of activation of the KKS, leading to the clinical phases of edema. These phases, however, do not occur in all individuals with a genetic C1-INH deficiency. In a series of 514 individuals with C1-INH deficiency, 45 permanently had no clinical symptoms of HAE-C1-INH Citation[1]. This and the alternation between symptomatic and asymptomatic phases show that besides the C1-INH deficiency, further factors seem to play a role in eliciting an attack. These factors are currently unknown. Acute attacks may occur ‘spontaneously’, for example, without any detectable triggers, others may be elicited by one of the numerous trigger factors well known for eliciting HAE attacks, including treatment with angiotensin-converting enzyme inhibitors and estrogens. The molecular mechanisms, however, for triggering the attacks are unknown.
3. Currently available treatment options for HAE-C1-INH
There are two treatment strategies in HAE-C1-INH. The first is to treat an acute swelling attack (on-demand) and the second is to prevent swelling attacks (short-term or long-term prophylaxis).
Drugs targeted specifically to the disease have become available for treatment of acute angioedema attacks (). They are highly effective and include C1-inhibitors, either plasma-derived or recombinant, the kallikrein-inhibitor, ecallantide and the bradykinin B2-receptor antagonist, icatibant Citation[4-8]. Treatment for long-term prophylaxis include C1-INH (highly effective), attenuated androgens (highly effective), and tranexamic acid (less effective) Citation[9-11].
Table 1. Currently available treatments and future treatment options for hereditary angioedema-C1 esterase inhibitor.
4. New potential treatment options for HAE-C1-INH
The continuing search for new therapies in HAE-C1-INH has resulted in a number of drug candidates, which are at various stages of clinical development (). The goal is to develop new HAE-C1-INH prophylactic treatments. Hopefully these new preventive treatments can potentially further reduce the burden of disease with less disruption of daily activities. Currently there are some new options in different stages of development.
In 2006, a series of small molecule compounds were reported that potently inhibited kallikrein activity in vitro Citation[12]. BCX4161 is an oral small molecule kallikrein inhibitor that is being developed by BioCryst Pharmaceuticals Ltd. (Durham, NC, USA). Preclinical studies showed that it is a potent and specific inhibitor of plasma kallikrein Citation[13]. A Phase IIa proof-of-concept clinical trial (OPuS-1 [Oral ProphylaxiS-1]) of BCX4161 in patients with HAE-C1-INH has been recently initiated Citation[14]. The main goals for the OPuS-1 trial are to estimate efficacy in reducing the frequency of angioedema attacks and to evaluate the safety and tolerability of BCX4161 in patients with HAE-C1-INH. The OPuS-1 trial will test 400 mg of BCX4161 administered three times daily for 28 days in up to 25 HAE patients who have a high frequency of attacks (≥ 1 per week), in a randomized, placebo-controlled, two-period crossover design.
Using a phage display technology, a long-acting inhibitor of plasma kallikrein has been developed by Dyax Corp. (Burlington, MA, USA). DX-2930 is a recombinant fully human monoclonal antibody inhibitor of plasma kallikrein for the treatment of HAE-C1-INH Citation[15]. Preclinical studies revealed that subcutaneous dosing of DX-2930 effectively reduced carrageenan-induced paw edema in vivo in rats when injected 24 h prior to challenge Citation[16]. DX-2930 is developed to be a long-acting, prophylactic agent that prevents HAE attacks. Development plans include a dosage formulation that will permit infrequent self administration by a small volume, subcutaneous injection. DX-2930 is currently being studied in a randomized, placebo-controlled, dose-escalation Phase I trial in normal individuals. The purpose of this study is to assess the safety and tolerability of DX-2930. Results from this study are expected in the first quarter of 2014.
A recent study has shown that a subcutaneous injection of 1,000 international units of Berinert® is clinically feasible. The absolute bioavailability of the subcutaneously administered C1-INH concentrate was about 40% Citation[17]. Currently, an international Phase III study of a volume-reduced formulation of Berinert® for subcutaneous injection in patients with HAE-C1-INH has started enrollment Citation[18]. It is a double-blind, randomized, placebo-controlled, crossover study called Clinical Study for Optimal Management in Preventing Angioedema with Low-Volume Subcutaneous C1-inhibitor Replacement Therapy (COMPACT). The aim of this study is to assess the efficacy and safety of subcutaneously administered C1-INH in preventing HAE attacks. The study will also evaluate the pharmacokinetics and pharmacodynamics of two different doses of subcutaneous C1-INH concentrate.
ViroPharma, Inc. completed a clinical Phase II study to evaluate the safety, tolerability, and efficacy of subcutaneously administered Cinryze with recombinant human hyaluronidase (rHuPH20) to prevent attacks in patients with HAE due to C1-INH deficiency. It is a randomized, double-blind, crossover study Citation[19]. In the study, a recently developed low-volume subcutaneous formulation of Cinryze® is used. The primary objectives of the ongoing study are to evaluate the safety, tolerability and efficacy of two doses of CINRYZE with recombinant human hyaluronidase (rHuPH20) administered by subcutaneous injection. At present, the FDA is evaluating the potential risk of long-term effect of anti-rHuPH20 non-neutralizing antibodies associated with the use of the recombinant human hyaluronidase enzyme (rHuPH20) that were detected in a separate development program not involving Cinryze. Therefore, studies of the combination of Cinryze and rHuPH20 were being placed on temporary clinical hold.
Recently, a factor XIIa inhibitory antibody has been developed. It is a fully human function-neutralizing antibody that binds into the FXIIa enzymatic pocket Citation[20]. CSL Behring recently announced that a fully humanized monoclonal antibody against coagulation factor XIIa has been added to the company’s research pipeline Citation[21]. Preclinical proof-of-concept (inhibition of edema) has been demonstrated in a relevant animal model of HAE. Preliminary pharmacokinetic data indicate that less frequent dosing intervals than current therapeutic options may be feasible in humans.
In conclusion, there are currently five new treatment options for HAE-C1-INH in development. All of them target the kallikrein-kinin pathway with the aim to inhibit bradykinin formation as the primary mediator of an HAE-C1-INH attack.
5. Expert opinion
Treatment goals in HAE-C1-INH comprise the avoidance of death cases and an improved quality of life. Besides the medication treatment, numerous other measures are necessary to reach these goals, including the provision of an HAE alert card for patients with clear instructions on how to manage an acute upper airway obstruction caused by an HAE attack Citation[22]. Current treatment options include several effective medications. This is true for the treatment of acute attacks as well as for avoiding attacks in long-term prophylaxis. For treatment of acute attacks there is no oral treatment available, only intravenously and subcutaneously applicable medication. An early and appropriate medication treatment is essential and contributes to reaching the treatment goals. Concerning long-term prophylaxis, there are intravenous (i.v.) medications (C1-INH) requiring a high number of i.v. injections for many years or even decades. Breakthrough attacks may occur despite long-term prophylaxis. Attenuated androgens can be administrated orally; however, possible side effects may limit their use. Thus there is still a medical need for new therapies that can be used alone or in combination with other therapies. Promising new investigational drugs are being developed for long-term prophylaxis. All four mentioned agents are in a different stage of development. All of them focus on the stabilization of the KKS/contact system. Two of the new investigational drugs are specific kallikrein inhibitors. The small molecule BCX4161 would allow a novel oral treatment. The long-acting antibody inhibitor to kallikrein, DX-2930, can be injected subcutaneously. First results on safety and efficacy in patients with HAE-C1-INH can already be expected in this year. For long-term prophylaxis with subcutaneous injections of C1-INH twice a week, the aim is to maintain a constant and functionally relevant C1-INH level in plasma. The rationale for this approach has been established with i.v. administration of C1-INH concentrate as an effective treatment for long-term prophylaxis.
In summary, a number of new promising treatment options are currently in development that may further help to reduce the burden of disease for patients with HAE-C1-INH.
Declaration of interest
K Bork is a consultant for CSL Behring and Shire Pharmaceuticals.
Notes
Bibliography
- Bork K, Hardt J, Wtzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1-INH deficiency. J Allergy Clin Immunol 2012;130:692-7
- Bork K, Barnstedt SE, Koch P, et al. Hereditary angioedema with normal C1-inhibitor activity in women. Lancet 2000;356:213-17
- Bork K. Hereditary angioedema with normal C1 inhibitor. Immunol Allergy Clin North Am 2013;33:457-70
- Craig TJ, Levy RJ, Wasserman RL, et al. Efficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacks. J Allergy Clin Immunol 2009;124:801-8
- Zuraw B, Cicardi M, Levy RJ, et al. Recombinant human C1-inhibitor for the treatment of acute angioedema attacks in patients with hereditary angioedema. J Allergy Clin Immunol 2010;26:821-7. e814
- Cicardi M, Levy RJ, McNeil DL. Ecallantide for the treatment of acute attacks in hereditary angioedema. N Engl J Med 2010;363:523-31
- Levy RJ, Lumry WR, McNeil DL, et al. EDEMA4: a phase 3, double-blind study of subcutaneous ecallantide treatment for acute attacks of hereditary angioedema. Ann Allergy Asthma Immunol 2010;104:523-9
- Cicardi M, Banerji A, Bracho F, et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med 2010;363:532-41
- Zuraw BL, Busse PJ, White M, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med 2010;363:513-22
- Bork K, Bygum A, Hardt J. Benefits and risks of danazol in hereditary angioedema: a long-term survey of 118 patients. Ann Allergy Asthma Immunol 2008;100:153-61
- Sheffer AL, Austen KF, Rosen FS. Tranexamic acid therapy in hereditary angioneurotic edema. N Engl J Med 1972;287:452-4
- Zhang J, Krishnan R, Arnold CS, et al. Discovery of highly potent small molecule kallikrein inhibitors. Med Chem 2006;2:545-53
- Bantia S, Zhang J, Wilson R, et al. BCX4161, a small molecule orally bioavailable plasma kallikrein inhibitor for the treatment of hereditary angioedema [P756]. Proceedings of the Annual Meeting of the American Academy of Allergy, Asthma and Immunology; San Antonio, TX, USA; 2013
- BioCryst Initiates OPuS-1: a phase 2a clinical trial of BCX4161 in patients with hereditary angioedema. http://investor.shareholder.com/biocryst/releasedetail.cfm?ReleaseID=805452 [Accessed March 2014]
- Sexton D, Faucette R, Viswanathan M, et al. Discovery and characterization of a fully human monoclonal antibody inhibitor of plasma kallikrein for the treatment of plasma kallikrein-mediated edema. J Allergy Clin. Immunol 2013;131(Suppl):AB32-116
- Kenniston JA, Sexton DJ, Martik D, et al. Discovery and characterization of a highly specific antibody inhibitor of plasma kallikrein [P1067]. Annual Meeting of the American Society of Hematology; New Orleans, LA, USA; 2013
- Martinez-Saguer I, Cicardi M, Suffritti C, et al. Pharmacokinetics of plasma-derived C1-esterase inhibitor after subcutaneous versus intravenous administration in subjects with mild or moderate hereditary angioedema: the PASSION study. Transfusion 2013. [Epub ahead of print]
- A study to evaluate the clinical efficacy and safety of subcutaneously administered C1-esterase inhibitor in the prevention of hereditary angioedema. Available from: http://clinicaltrials.gov/show/NCT01912456 [Accessed March 2014]
- Subcutaneous CINRYZE with recombinant human hyaluronidase for prevention of angioedema attacks. Available from: http://clinicaltrials.gov/ct2/show/NCT01756157?term=viropharma&rank=4 [Accessed March 2014]
- Larsson M, Rayzman V, Nolte MW, et al. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med 2014;6:222ra17
- R&D Briefing December 2013. Available from: http://www.csl.com.au/investors/briefings-presentations/operational-briefing.htm [Accessed March 2014]
- German Guidelines for Hereditary Angioedema due to C1-INH deficiency. Available from: www.angioedema.de/englisch/infos.htm [Accessed March 2014]