Abstract
Ketolides are erythromycin A derivatives with a keto group replacing the cladinose sugar and an aryl-alkyl group attached to the lactone macrocycle. The aryl-alkyl extension broadens its antibacterial spectrum to include all pathogens responsible for community-acquired pneumonia (CAP): Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis as well as atypical pathogens (Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella pneumophila). Ketolides have extensive tissue distribution, favorable pharmacokinetics (oral, once-a-day) and useful anti-inflammatory/immunomodulatory properties. Hence, they were considered attractive additions to established oral antibacterials (quinolones, β-lactams, second-generation macrolides) for mild-to-moderate CAP. The first ketolide to be approved, Sanofi-Aventis’ telithromycin (RU 66647, HMR 3647, Ketek®), had tainted clinical development, controversial FDA approval and subsequent restrictions due to rare, irreversible hepatotoxicity that included deaths. Three additional ketolides progressed to non-inferiority clinical trials vis-à-vis clarithromycin for CAP. Abbott’s cethromycin (ABT-773), acquired by Polymedix and subsequently by Advanced Life Sciences, completed Phase III trials, but its New Drug Application was denied by the FDA in 2009. Enanta’s modithromycin (EDP-420), originally codeveloped with Shionogi (S-013420) and subsequently by Shionogi alone, is currently in Phase II in Japan. Optimer’s solithromycin (OP-1068), acquired by Cempra (CEM-101), is currently in Phase III. Until this hepatotoxicity issue is resolved, ketolides are unlikely to replace established antibacterials for CAP, or lipoglycopeptides and oxazolidinones for gram-positive infections.
1. Introduction and overview
Bacterial resistance to antibacterial drugs has been increasing relentlessly over the past two decades driving the need for new compounds Citation[1,2]. While nosocomial infections have received most of the publicity Citation[3], common, community-acquired infections, pneumonia in particular, can be almost as deadly. There are currently three antibacterial classes for community-acquired pneumonia (CAP): quinolones (levofloxacin, moxifloxacin, gemifloxacin), β-lactams (amoxicillin–clavulanic acid combination) and second-generation macrolides (clarithromycin, azithromycin) Citation[4].
Ketolides are third-generation macrolides designed specifically to act against macrolide-resistant, respiratory pathogens. They are semisynthetic derivatives of erythromycin A with the cladinose sugar at C-3 on the 14-membered lactone macrocycle removed and the resulting hydroxyl oxidized to a keto group (hence the name, ketolides). In addition, they usually have a cyclic carbamate group attached to C-11 and C-12 of the macrocycle and an aryl-alkyl chain, usually attached to the cyclic carbamate group () Citation[5]. The aryl-alkyl extension broadens the antibacterial spectrum and improves activity against most macrolide-resistant respiratory pathogens Citation[5]. In addition, ketolides have improved pharmacodynamics, pharmacokinetics (oral, once-a-day) and tissue distribution Citation[6]. These properties established ketolides as potential contenders for the large but competitive market of mild-to-moderate CAP.
Figure 1. Structures of the 14-membered macrolide erythromycin and its 6-O-methyl derivative clarithromycin, the 15-membered azilide azithromycin and the ketolides telithromycin, solithromycin, cethromycin and modithromycin.
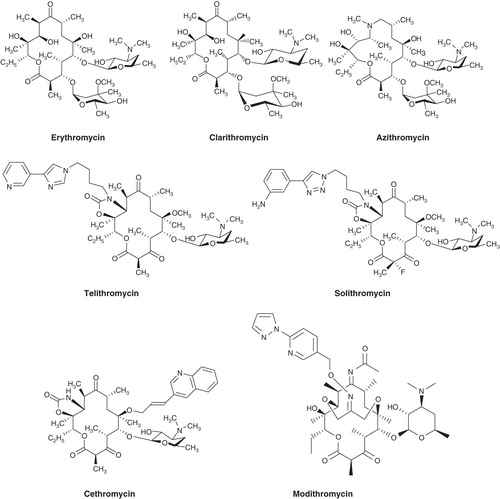
In the mid-90s, ketolides became the focus of intense research activity at several companies (Aventis, Johnson and Johnson, Abbott, Merck, Shionogi, Taisho, Pliva, Rib-x, Chiron, Enanta, Optimer) and academic groups Citation[7]. Ketolides were even attached to quinolones, the latter serving as a ‘silent partner’ in the hybrid molecule, affecting pharmacokinetic properties rather than antibacterial activity Citation[8]. Fueling the research activity was a renaissance involving the bacterial ribosome thanks to the advent of oxazolidinones (linezolid), glycylcyclines (tigecycline) and streptogramins (quinupristin-dalfopristin) Citation[9-12].
Research activity slowed to a trickle after the tainted clinical development and controversial FDA approval of Aventis’ telithromycin (RU 66647, HMR 3647, Ketek®) Citation[13-16]. Nevertheless, three additional ketolides progressed to non-inferiority clinical trials for mild-to-moderate CAP:
Cethromycin (ABT-773, A-195773, Abbott/Taisho), licensed to Polymedix and subsequently to Advanced Life Sciences Citation[17,18]. It completed Phase III non-inferiority clinical trials, and a New Drug Application was submitted to FDA (US) in 2008. It was denied approval in 2009 and has an orphan drug designation for tularemia, plague and anthrax prophylaxis. With only 2 years left in its patent life, its prospects are uncertain.
Modithromycin (EDP-420, EP-013420, Enanta); a 6,11-bridged bicyclic ketolide, originally codeveloped with Shionogi (S-013420) and subsequently by Shionogi alone Citation[19]. It is currently in Phase II clinical trials in Japan.
Solithromycin (OP-1068, Optimer), licensed to Cempra (CEM-101). It is currently in Phase III clinical trials Citation[20].
2. Mechanism of action, antibacterial spectrum, resistance
Like macrolide antibiotics, ketolides affect bacterial protein synthesis. The macrocycle of both macrolides and ketolides interacts with the peptidyltransferase center (PTC) on the 50S ribosomal subunit (proteins L4 and L22), partially blocking the polypeptide exit tunnel of the ribosome Citation[21-24]. The sugar substituent in both subclasses interacts with A2058 of 23S rRNA, while the alkyl-aryl arm of ketolides interacts with A752 on domain II and U2609 on domain V of 23S rRNA. As would be expected from the identical orientation of the alkyl-aryl arm, telithromycin and solithromycin bind to PTC in an identical fashion, while cethromycin binds somewhat differently. The two-site binding of ketolides in the bacterial ribosome preserves binding even when A2058 is methylated by erythromycin-resistant methyltransferases (Erms). Hence ketolides maintain activity against most erythromycin- (and penicillin-) resistant isolates of Streptococcus pneumoniae.
Ketolides (telithromycin, cethromycin, modithromycin, solithromycin) have excellent activity against gram-positive aerobes (MIC90s, < 0.05 mcg/ml) including macrolide-resistant (mefA and ermB) strains of S. pneumoniae Citation[25-31]. They also have good-to-moderate activity against some gram-negative aerobes such as Haemophilus influenzae and Moraxella catarrhalis (MIC90s < 8 mcg/ml), and excellent activity (MIC90s, < 0.5 mcg/ml) against atypical/intracellular CAP pathogens C. pneumoniae, Legionella pneumophila, Mycoplasma pneumoniae Citation[32,33].
While rare, ketolide (telithromycin)-resistant strains have been isolated worldwide Citation[34,35]. Inducible resistance to both ketolides and macrolides is by energy-dependent drug efflux and through rRNA methylation Citation[36-38]. Efflux is particularly important in gram-negative resistance Citation[39,40]. Interestingly, the molecular details of resistance induction by ketolides differ from those by macrolides; ketolides stimulate a novel ribosomal frameshift Citation[41].
Unlike macrolides, which are considered time-dependent killers, ketolides show concentration-dependent killing Citation[41]. Due to their cidality, they have a long (3 – 6 h) post-antibiotic effect, both properties stemming from their mechanism of action: derailing, rather than stalling protein synthesis.
Like macrolides, ketolides also inhibit the synthesis of proinflammatory cytokines (TNF, IL-1β, IFNγ) and have anti-inflammatory/immunomodulatory properties that may be beneficial to CAP patients Citation[42-46].
3. Pharmacokinetics, metabolism, clinical trials
Ketolides have extensive tissue distribution relative to serum and excellent pharmacokinetics allowing once daily dose administration Citation[47-49]. Similarly to the parent compound erythromycin, they are primarily metabolized in the liver by the cytochrome P450 enzyme system, specifically the CYP 3A4 superfamily. They are eliminated by a combination of biliary, hepatic and urinary excretion. CYP3A enzymes metabolize many common drugs, such as atorvastatin (Lipitor®), and can be induced or inhibited by certain drugs, such as dexamethasone (Decadron®). Thus, ketolides have a high potential for clinically significant drug−drug interactions. In addition, they (telithromycin) decrease protein levels, and thereby activity, of CYP1A2 and CYP3A2, the result being decreased metabolic clearance of the common asthma drug theophylline Citation[50].
Clinical trials of ketolides have focused on respiratory infections, primarily CAP. Telithromycin was found to be non-inferior to trovafloxacin and clarithromycin Citation[51,52], cethromycin to clarithromycin Citation[53] and solithromycin to levofloxacin Citation[54]. There is no clinical information on modithromycin, and its development status is uncertain.
4. Adverse effects, future prospects
Like first- and second-generation macrolides, ketolides produce mild gastrointestinal effects: diarrhea, nausea, abdominal pain, vomiting. Some (telithromycin, cethromycin) also cause QT interval prolongation that could lead to irregular heart rhythm in patients with preexisting conditions. The cardiac effects, while serious, are predictable, and at-risk patients can be identified and given alternative treatments. Some (telithromycin) reportedly inhibit nicotinic acetylcholine receptors thereby blocking neuromuscular transmission and exacerbating myasthenia gravis Citation[55]. There is also a single report of an anaphylactoid reaction in a patient, not allergic to erythromycin or azithromycin, after treatment with a single dose of telithromycin Citation[56]. Other potential adverse effects, alluded earlier, stem from drug−drug interactions with common drugs due to ketolide metabolism by the cytochrome P450 enzyme system (CYP 3A4).
The most serious adverse effect of ketolides is rare, but irreversible hepatotoxicity. It has been extensively documented for the first ketolide to be approved, Sanofi-Aventis’ telithromycin (RU 66647, HMR 3647, Ketek) Citation[57-59]. Telithromycin had tumultuous clinical development, controversial FDA approval in 2004 and subsequent restrictions (in 2007 and 2010) due to rare but severe, irreversible hepatotoxicity that included deaths Citation[14-16]. This very serious adverse effect of telithromycin, discovered after FDA approval, is rare, idiosyncratic, but acute and potentially fatal. It appears to have a distinct clinical signature: rapid onset with no early signs. It thus contrasts to hepatotoxicity from other drugs, acetaminophen (Tylenol®) for example, which is predictable, dose-related and reversible, hence manageable. Hepatotoxicity has not been reported for cethromycin, solithromycin or modithromycin, but safety data are very limited.
5. Expert opinion
The issue of hepatotoxicity continues to cast a shadow over ketolide prospects. Its etiology, or whether it is a class effect, is unclear. It could be due to the imidazole in telithromycin’s structure, in which case it would be less of an issue in solithromycin, which has a triazole instead. Replacing imidazole in the antifungal ketoconazole with a triazole in itraconazole reduced – but did not eliminate – its hepatotoxicity Citation[60]. Specific ketolide metabolites (the alkyl-aryl arm?) may also be responsible, but studies are needed to explore this possibility. Since hepatotoxicity is a rare event, genetic variability in liver sensitivity and/or ketolide metabolism may be key predisposing factors. Until the issue of hepatotoxicity is resolved, ketolides are unlikely to replace established, mostly generic, antibacterials in the treatment of CAP (). They are also unlikely to replace lipoglycopeptides and oxazolidinones for gram-positive infections.
Table 1. Key properties, issues and prospects of ketolides.
In conclusion, the potential of ketolides, particularly solithromycin that is structurally very similar to telithromycin, hinges on the hepatotoxicity issue. To move forward, this issue needs to be investigated and resolved: risk factors identified and biomarkers for vulnerable patients developed. Once at-risk patients are identified and excluded, ketolides can be added to existing, safe and effective treatments for CAP Citation[61]. Otherwise, in the circumspect post-telithromycin regulatory climate Citation[62], this once promising macrolide subclass will be reserved for serious, problematic gram-positive infections for which there are few therapeutic alternatives.
Declaration of interest
NH Georgopapadakou is an independent consultant with NHG Preclinical Research Consulting, LLC and has declared that she has no conflict of interest and received no payment in preparation of the manuscript.
Bibliography
- Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century – a clinical super-challenge. N Engl J Med 2009;360:439–43
- Georgopapadakou NH. Prospects for new antibacterials: can we do better? Expert Opin Investig Drugs 2014;23:145-8
- Watkins RR, Bonomo RA. Increasing prevalence of carbapenem-resistant Enterobacteriaceae and strategies to avert a looming crisis. Expert Rev Anti Infect Ther 2013;11:543-5
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44:S27-72
- Liang J-H, Han X. Structure-activity relationships and mechanism of action of macrolides derived from erythromycin as antibacterial agents. Curr Top Med Chem 2013;13:3131-64
- Zhanel GG, Walters M, Noreddin A, et al. The ketolides: a critical review. Drugs 2002;62:1771-804
- Kirst HA. New macrolide, lincosaminide and streptogramin B antibiotics. Expert Opin Ther Pat 2010;20:1343-57
- Pavlović D, Fajdetić A, Mutak S. Novel hybrids of 15-membered 8a- and 9a-azahomoerythromycin A ketolides and quinolones as potent antibacterials. Bioorg Med Chem 2010;18:8566-82
- Hansen LH, Mauvais P, Douthwaite S. The macrolide-ketolide antibiotic binding site is formed by structures in domains II and V of 23S ribosomal RNA. Mol Microbiol 1999;31:623-31
- Wilson DN, Harms JM, Nierhaus KH, et al. Species-specific antibiotic-ribosome interactions: implications for drug development. Biol Chem 2005;386:1239-52
- Zuckerman JM, Qamar F, Bono BR. Macrolides, ketolides, and glycylcyclines: azithromycin, clarithromycin, telithromycin, tigecycline. Infect Dis Clin North Am 2009;23:997-1026
- McCuster KP, Fujimori DG. The chemistry of peptidyltransferase center-targeted antibiotics: enzymatic resistance and approaches to countering resistance. ACS Chem Biol 2012;7:64-72
- Tellier G, Niederman MS, Nusrat R, et al. Clinical and bacteriological efficacy and safety of 5 and 7 day regimens of telithromycin once daily compared with a 10 day regimen of clarithromycin twice daily in patients with mild to moderate community-acquired pneumonia. J Antimicrob Chemother 2004;54:515-23
- Ross DB. The FDA and the case of Ketek. N Engl J Med 2007;356:1601-4
- Soreth J, Cox E, Kweder S, et al. Ketek - the FDA perspective. N Engl J Med 2007;356:1675-6
- Young D. Limit Ketek to pneumonia, experts advise: advisers urge black-box warning. Am J Health Syst Pharm 2007;64:124-5
- Hammerschlag MR, Sharma R. Use of cethromycin, a new ketolide, for treatment of community-acquired respiratory infections. Expert Opin Investig Drugs 2008;17:387-400
- Rafie S, MacDougall C, James CL. Cethromycin: a promising new ketolide antibiotic for respiratory infections. Pharmacotherapy 2010;30:290-303
- Revill P, Bolós J, Serradell N. EDP-420: ketolide antibiotic. Drugs Future 2006;31:479-83
- Fernandes P, Pereira D, Jamieson B, Keedy K. Solithromycin. Macrolide antibiotic. Drugs Future 2011;36:751-8
- Dunkle JA, Xiong L, Mankin AS, Cate JH. Structures of the Escherichia coli ribosome with antibiotics bound near the peptidyl transferase center explain spectra of drug action. Proc Natl Acad Sci USA 2010;107:17152-7
- Kannan K, Vázquez-Laslop N, Mankin AS. Selective protein synthesis by ribosomes with a drug-obstructed exit tunnel. Cell 2012;151:508-20
- Krokidis MG, Márquez V, Wilson DN, et al. Insights into the mode of action of novel fluoroketolides, potent inhibitors of bacterial protein synthesis. Antimicrob Agents Chemother 2014;58:472-80
- Llano-Sotelo B, Dunkle J, Klepacki D, et al. Binding and action of CEM-101, a new fluoroketolide antibiotic that inhibits protein synthesis. Antimicrob Agents Chemother 2010;54:4961-70
- Blasi F, Farrell DJ, Dubreuil L. Antibacterial activity of telithromycin and comparators against pathogens isolated from patients with community-acquired respiratory tract infections: the Prospective Resistant Organism Tracking and Epidemiology for the Ketolide Telithromycin study year 5 (2003-2004). Diagn Microbiol Infect Dis 2009;63:302-8
- Azoulay-Dupuis E, Mohler J, Bédos JP, et al. Efficacy of cethromycin, a new ketolide, against Streptococcus pneumoniae susceptible or resistant to erythromycin in a murine pneumonia model. Antimicrob Agents Chemother 2006;50:3033-8
- Wierzbowski AK, Karlowsky JA, Hoban DJ, Zhanel GG. In vitro activity of the investigational ketolide cethromycin against macrolide- and penicillin-resistant Streptococcus pneumoniae: review of the 1998 to 2006 Canadian Respiratory Organism Susceptibility Study (CROSS). J Antimicrob Chemother 2009;63:620-2
- Sato T, Tateda K, Kimura S, et al. In vitro antibacterial activity of modithromycin, a novel 6,11-bridged bicyclolide, against respiratory pathogens, including macrolide-resistant Gram-positive cocci. Antimicrob Agents Chemother 2011;55:1588-93
- Woosley LN, Castanheira M, Jones RN. CEM-101 activity against gram-positive organisms. Antimicrob Agents Chemother 2010;54:2182-7
- Farrell DJ, Castanheira M, Sader HS, Jones RN. The in vitro evaluation of solithromycin (CEM-101) against pathogens isolated in the United States and Europe (2009). J Infect 2010;61:476-83
- Putnam SD, Castanheira M, Moet GJ, et al. CEM-101, a novel fluoroketolide: antimicrobial activity against a diverse collection of Gram-positive and Gram-negative bacteria. Diagn Microbiol Infect Dis 2010;66:393-401
- Bébéar CM, Renaudin H, Aydin MD, et al. In-vitro activity of ketolides against mycoplasmas. J Antimicrob Chemother 1997;39:669-70
- Hammerschlag MR, Roblin PM, Bébéar CM. Activity of telithromycin, a new ketolide antibacterial, against atypical and intracellular respiratory pathogens. J Antimicrob Chemother 2001;48(Suppl T1):25-31
- Doern GV. Macrolide and ketolide resistance with Streptococcus pneumoniae. Med Clin North Am 2006;90:1109-24
- Felmingham D, Cantón R, Jenkins SG. Regional trends in β-lactam, macrolide, fluoroquinolone and telithromycin resistance among Streptococcus pneumoniae isolates 2001-2004. J Infect 2007;55:111-18
- Hirakata Y, Mizuta Y, Wada A, Kohno S. The first telithromycin-resistant Streptococcus pneumoniae isolate in Japan associated with erm(B) and mutations in 23S rRNA and riboprotein L4. Jpn J Infect Dis 2007;60:48-50
- Reynolds ED, Cove JH. Resistance to telithromycin is conferred by msr(A), msrC and msr(D) in Staphylococcus aureus. J Antimicrob Chemother 2005;56:1179-80
- Wilson DN. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat Rev Microbiol 2014;12:35-48
- Bogdanovich T, Bozdogan B, Appelbaum PC. Effect of efflux on telithromycin and macrolide susceptibility in Haemophilus influenzae. Antimicrob Agents Chemother 2006;50:893-8
- Nikaido H, Pagès JM. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol Rev 2012;36:340-63
- Gupta P, Kannan K, Mankin A, Vázquez-Laslop N. Regulation of gene expression by macrolide-induced ribosomal frameshifting. Mol Cell 2013;52:629-42
- Al-Lahham A, Reinert RR. Time-kill kinetics of Streptococcus pneumoniae with reduced susceptibility to telithromycin. Chemotherapy 2007;53:190-3
- Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev 2010;23:590-615
- Leiva M, Ruiz-Bravo A, Moreno E, Jiménez-Valera M. Telithromycin inhibits the production of proinflammatory mediators and the activation of NF-κB in in vitro-stimulated murine cells. FEMS Immunol Med Microbiol 2008;53:343-50
- Lotter K, Höcherl K, Bucher M, Kees F. In vivo efficacy of telithromycin on cytokine and nitric oxide formation in lipopolysaccharide-induced acute systemic inflammation in mice. J Antimicrob Chemother 2006;58:615-21
- Johnston SL, Blasi F, Black PN, et al. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med 2006;354:1589-600
- Zeitlinger M, Wagner CC, Heinisch B. Ketolides - the modern relatives of macrolides: the pharmacokinetic perspective. Clin Pharmacokinet 2009;48:23-38
- Traunmüller F, Fille M, Thallinger C, Joukhadar C. Multiple-dose pharmacokinetics of telithromycin in peripheral soft tissues. Int J Antimicrob Agents 2009;34:72-5
- Still JG, Schranz J, Degenhardt TP, et al. Pharmacokinetics of solithromycin (CEM-101) after single or multiple oral doses and effects of food on single-dose bioavailability in healthy adult subjects. Antimicrob Agents Chemother 2011;53:1997-2003
- Nosaka H, Nadai M, Kato M, Hasegawa T. Effect of a newly developed ketolide antibiotic, telithromycin, on metabolism of theophylline and expression of cytochrome P450 in rats. Life Sci 2006;79:50-6
- Pullman J, Champlin J, Vrooman PS Jr. Efficacy and tolerability of once-daily oral therapy with telithromycin compared with trovafloxacin for the treatment of community-acquired pneumonia in adults. Int J Clin Pract 2003;57:377-84
- Mathers DL, Hassman J, Tellier G. Efficacy and tolerability of once-daily oral telithromycin compared with clarithromycin for the treatment of community-acquired pneumonia in adults. Clin Ther 2004;26:48-62
- English ML, Fredericks CE, Milanesio NA, Eiznhamer DA. Cethromycin versus clarithromycin for community-acquired pneumonia: comparative efficacy and safety outcomes from two double-blinded, randomized, parallel-group, multicenter, multinational noninferiority studies. Antimicrob Agents Chemother 2012;56:2037-47
- Oldach D, Clark K, Schranz J, et al. Randomized, double-blind, multicenter phase 2 study comparing the efficacy and safety of oral solithromycin (CEM101) to those of oral levofloxacin in the treatment of patients with community-acquired bacterial pneumonia. Antimicrob Agents Chemother 2013;57:2526-34
- Liu C-N, Somps CJ. Telithromycin blocks neuromuscular transmission and inhibits nAChR currents in vitro. Toxicol Lett 2010;194:66-9
- Bottenberg MM, Wall GC, Hicklin GA. Apparent anaphylactoid reaction after treatment with a single dose of telithromycin. Ann Allergy Asthma Immunol 2007;98:89-91
- Bolesta S, Roslund BP. Elevated hepatic transaminases associated with telithromycin therapy: a case report and literature review. Am J Health Syst Pharm 2008;65:37-41
- Clay KD, Hanson JS, Pope SD, et al. Brief communication: severe hepatotoxicity of telithromycin: three case reports and literature review. Ann Intern Med 2006;144:415-20
- Brinker AD, Wassel RT, Lyndly J, et al. Telithromycin-associated hepatotoxicity: clinical spectrum and causality assessment of 42 cases. Hepatology 2009;49:250-7
- Como JA, Dismukes WE. Oral azole drugs as systemic antifungal therapy. N Engl J Med 1994;330:263-72
- Van Bambeke F, Harms JM, Van Laethem Y, Tulkens PM. Ketolides: pharmacological profile and rational positioning in the treatment of respiratory tract infections. Expert Opin Pharmacother 2008;9:267-83
- Echols RM. Understanding the regulatory hurdles for antibacterial drug development in the post-Ketek world. Ann N Y Acad Sci 2011;1241:153-61
