Abstract
Importance of the field: Malaria infection during pregnancy is a major public health problem worldwide, with 50 million pregnancies exposed to the infection every year. Approximately 25,000 maternal deaths and between 75,000 and 200,000 infant deaths could be prevented each year by effective malaria control in pregnancy. Antimalarial drug treatment and prevention has been hampered by the appearance of drug resistance, which has been a particular problem in pregnancy due to the inherent safety issues.
Areas covered in this review: New antimalarial drugs and combinations are being studied but there is not yet sufficient information on their efficacy or, more importantly, on their safety in pregnancy. This article provides an overview of the relevance of the topic and reviews the current antimalarial drugs recommended for pregnancy, as well as the guidelines for both treatment and prevention in women living in endemic areas and for travellers.
What the reader will gain: Updated information on the drugs currently used for malaria treatment and prevention in pregnancy, including new drugs under development, is provided. The gaps on efficacy and safety information for use during pregnancy are also discussed.
Take home message: Prevention and case management of malaria during pregnancy is based on risk–benefit criteria and poses one of the greatest challenges to current malaria control.
Keywords::
1. Introduction
1.1 Malaria global distribution
Malaria is the most important protozoan parasitic infection in humans, accounting for nearly one million deaths in 2006 Citation[1]. The World Health Organization (WHO) estimates that half of the world's population is at risk of malaria, sub-Saharan Africa being the region where the infection exacts a major toll.
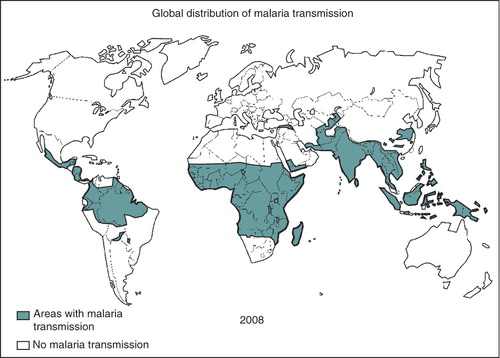
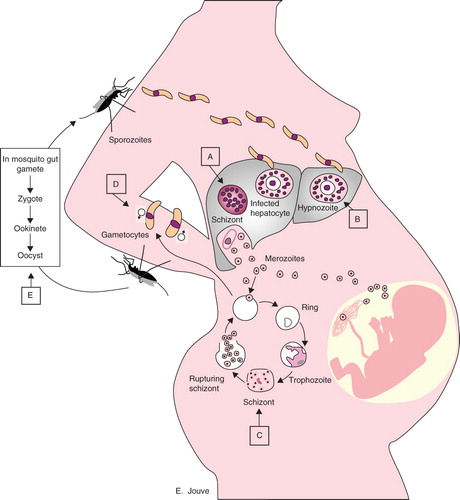
The parasite is transmitted by the bite of an infected female anopheline mosquito and, once in the body of its human host, migrates through the blood to the liver to invade hepatocytes (). Five Plasmodium species can infect humans: P. falciparum, P. vivax, P. ovale, P. malariae and P. knowlesi Citation[2]; P. falciparum is the most virulent. Malaria transmission occurs in sub-Saharan Africa, South East Asia and South America (). Most of the malaria burden in the world is focused on children younger than 5 years of age and on pregnant women.
Figure 1. Malaria life cycle and antimalarial drug Plasmodium stage targets. Plasmodium sporozoites travel from the salivary glands of the anopheline mosquito through the bloodstream of the human host to the liver, where they invade hepatocytes and divide to form multinucleated schizonts. Hypnozoites can be found in P. vivax and P. ovale infections as a quiescent stage in the liver. Liver schizonts rupture and release merozoites into the circulation, where they invade red blood cells. Within the red cells, merozoites mature from ring forms to trophozoites to multinucleated schizonts. Some merozoites differentiate into male or female gametocytes that can then be ingested by the anopheline mosquito. The Plasmodium cycle is completed in the mosquito gut. Capital letters indicate the Plasmodium-stage targets of antimalarial drugs.
1.2 Burden and effects of malaria in pregnancy
About 50 million pregnancies are thought to occur every year in malaria endemic areas Citation[3], and more recent unpublished estimates suggest that this figure may reach up to 100 million pregnancies (ter Kuile, personal communication). Approximately 25 million pregnancies in the sub-Saharan region are exposed to malaria annually, most of them occurring in high-transmission areas where P. falciparum predominates. The rest of the estimated exposed pregnancies occur in regions with low malaria transmission where P. vivax infection co-exists with P. falciparum, or even predominates Citation[1].
Pregnant women have been said to attract twice the number of mosquitoes than their non-pregnant counterparts and are more likely to develop malaria-related complications Citation[4,5]. The deleterious consequences of malaria in pregnancy (MiP) on infant and maternal morbidity and mortality have been extensively described Citation[6-13]. One of the consequences of MiP is the increased risk of low birth weight (< 2500 g), either through intrauterine growth retardation or preterm delivery Citation[14,15]. As low birth weight is strongly associated with infant survival, it has been estimated that between 75,000 and 200,000 infant deaths could be prevented if MiP control was effective Citation[9]. Furthermore, approximately 15.5% of maternal deaths can be attributed to malaria in endemic regions, and therefore an important impact in the reduction of maternal mortality could be achieved by implementing effective malaria control measures Citation[16,17].
1.3 History of malaria treatment
The first reported antimalarial treatment dates back to the seventeenth century and was based on the powder obtained from the bark of the cinchona tree. This tree is found on the slopes of the Andes and Jesuit priests introduced the use of its bark to Europe. However, it took nearly two centuries to isolate the active principle of the cinchona bark powder: the alkaloid quinine (QN) Citation[18]. In the twentieth century, the development of antimalarial drugs ran in parallel with the military history. QN remained the drug of choice for treating malaria until the end of World War II, when chloroquine (CQ) became the mainstream treatment of the disease due to its effectiveness, safety profile and low cost. CQ was first synthesized in Germany in 1934 and was extensively used worldwide until the spread of CQ-resistant P. falciparum parasites in the late 1950s. CQ resistance to P. falciparum was first observed in Thailand and around the Colombian–Venezuelan border Citation[19]. Other antimalarial drugs, such as proguanil (PG), amodiaquine (AQ), primaquine (PQ), sulfadoxine–pyrimethamine (SP), halofantrine and mefloquine (MQ), were developed to counter malaria CQ-resistant parasites. Piperaquine (PIP) was discovered in the 1960s and replaced CQ as first-line treatment in China for P. falciparum and P. vivax malaria in 1978 Citation[20]. CQ resistance was widespread in sub-Saharan Africa by 1989.
Despite the efforts made in the 1950s and 1960s to support the WHO Malaria Eradication Programme, the infection remained neglected by the scientific research community and of the 1393 new chemical entities marketed in the last 25 years of the twentieth century only four were antimalarial drugs Citation[21]. One of the oldest Chinese herbal medicines for treating fevers, the wormwood Artemisia annua, has recently been added to the global antimalarial drugs collection. Its active ingredient, artemisinin (or Qinghaosu), was isolated in 1971 by Chinese scientists. However, widespread availability of the semi-synthetic derivates of this plant was delayed until the last decades of the twentieth century.
1.4 Current recommendations for malaria control in pregnancy in endemic areas
Current WHO recommendations for the control of MiP in areas of stable transmission rely on: i) prompt and effective case management of malaria illness; ii) intermittent preventive treatment (IPT) with at least two treatment doses of SP; and iii) the use of insecticide-treated nets (ITNs) Citation[3]. There are no specific recommendations in areas of low endemicity and treatment depends on each country's guidelines.
The following antimalarial drugs have been recommended for its use in pregnancy: quinine, chloroquine, amodiaquine, mefloquine, sulfadoxine–pyrimethamine, proguanil, artemisinins and clindamycin Citation[22]. The choice of the most suitable drug to be used is based on the severity of the malarial episode, the gestational age of the pregnant woman and the pattern of drug resistance and local availability of antimalarials in the region.
1.5 Review justification and search limits
Pregnant women are systematically excluded from drug trials for ethical, legal and sociological concerns, and for fear of toxicity to the fetus, but issues concerning the liability of pharmaceutical companies dominate the others. This has resulted in a lack of information on safety, efficacy and even correct dosage of most drugs, including antimalarials, in pregnancy. Thus, the administration of antimalarial drugs to pregnant women is frequently based on risk–benefit criteria without the drugs going through adequate clinical reprotoxicity or teratologic evaluation. For MiP, safe and effective antimalarial drugs in a context of widespread CQ resistance of P. falciparum (and, to a lesser extent, of P. vivax parasites) are needed more than ever.
This review examines the available efficacy and safety information on the current antimalarial drugs recommended for treatment and prevention of MiP. We conducted a comprehensive literature search of medical databases (Medline, the Cochrane library, WHO) and non-medical search engines using the following keywords: pregnancy, malaria, treatment, antimalarial, control, prevention, drugs.
2. Clinical manifestations of malaria in pregnancy
The frequency and severity of the effects of MiP depend on the pre-pregnancy level of acquired malaria immunity, which mainly depend on the intensity of malaria transmission. In areas of stable malaria transmission, where women are considered to be semi-immune with regard to malaria infection, it is assumed that most P. falciparum infections are asymptomatic Citation[23]. However, in high transmission areas, infected women have an increased risk of maternal anaemia, low birth weight and premature delivery Citation[15,24]. In areas of low transmission, pregnant women with malaria parasitaemia frequently present symptoms and signs such as fever, headache, vomiting and malaise. If untreated, the infection may develop into severe complications, such as cerebral malaria and pulmonary oedema, and may be a cause of maternal mortality Citation[22,25,26].
3. Classification of antimalarial drugs
Antimalarial drugs can be classified according to their chemical structure and pharmacologic mechanism of action (). Antimalarial drugs target different stages in the life-cycle of the Plasmodium parasites and the following groups of drugs can be distinguished ():
Table 1. Classification of antimalarial drugs according to the chemical structure.
Tissue schizonticides: act against pre-erythrocitic schizonts (e.g., primaquine, atovaquone–proguanil, pyrimethamine).
Hypnozoiticides: act against quiescent liver stage hyponozoites from P. ovale and P. vivax infections (e.g., primaquine).
Blood schizonticides: suppress infection symptoms by elimination of erythrocytic forms; also called ‘clinically curative’ (e.g., atovaquone–proguanil, sulfadoxine, sulfones, tetracyclines, halofantrine, quinine, mefloquine and chloroquine).
Gametocytocides: eliminate gametocytes forms in the blood, preventing mosquito infection (e.g., primaquine has activity against all Plasmodium spp, chloroquine and quinine against P. vivax and P. malariae).
Sporontocides: prevent the development of oocyst and multiplication of parasites in the mosquito gut when ingested with the blood of the human host (e.g., primaquine, chloroguanide, pyrimethamine).
Malaria treatment should ideally include drugs with tissue and blood schizonticide, as well as gametocytocide activities.
4. Antimalarial drugs recommended or with potential use in pregnancy
Malaria treatment during pregnancy has traditionally followed the Hippocratic principle of prima non nocere (i.e., ‘first do not harm’). The labelling of most of the antimalarial drugs refers to pregnancy risk category C. This means that animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but that potential benefits may warrant use of the drug in pregnant women despite potential risks. The choice of the antimalarial drug to treat pregnant women is based on risk–benefit criteria and individual assessment due to lack of human data. The available antimalarial drugs that are currently recommended in pregnancy are outlined below.
4.1 Aminoquinolines
4.1.1 Chloroquine
Apart from its schizonticidal and gametocytocidal activities, CQ also has antipyretic and anti-inflammatory properties Citation[27]. The drug is effective against, P. ovale, P. malariae, P. vivax and sensitive P. falciparum parasites. CQ alone or in combination with proguanil is recommended for chemoprophylaxis for travellers to endemic areas. A CQ-resistant P. falciparum parasite has been described worldwide and resistance to P. vivax has been reported in areas of south-east Asia and the Pacific Citation[28,29]. However, even today CQ remains effective in some areas of Central America and south-western Asia. CQ efficacy could be improved by its combination with other antimalarial drugs, while recovery of P. falciparum sensitivity to CQ has been reported in an area where previous parasite resistance to the drug was documented Citation[30].
Safety: CQ is usually well tolerated although mild side effects such as nausea, dizziness and headache can occur. CQ-induced pruritus has been described to be particularly frequent in black-skinned populations Citation[31]. Long-term use of high doses of CQ may induce retinopathy and neuromyopathy. Malaria prophylactic recommended doses can difficulty result on retinopathy. The drug is considered to be safe in all trimesters of pregnancy and can be given to breast-feeding mothers Citation[32-34].
Pharmacokinetics: CQ is efficiently absorbed when administered orally, peak plasma concentrations being achieved within 3 h (range 2 – 12 h). Chloroquine is slowly eliminated and detected in blood for up to 56 days, with an elimination half-life of around 10 days, and is predominantly excreted as the parent drug. CQ readily crosses the human placenta Citation[35]. A study among African pregnant women suggested that CQ clearance is increased in the third trimester of pregnancy Citation[36]. On the other hand, blood concentrations of CQ were not significantly affected among pregnant women with P. vivax malaria Citation[37].
4.1.2 Amodiaquine
Amodiaquine (AQ) is another 4-aminoquinoline similar in structure and activity to CQ. It also presents antipyretic and anti-inflammatory effects Citation[38]. It is generally effective against CQ-resistant P. falciparum infections. The role of AQ in the treatment of CQ-resistant P. vivax malaria has not been adequately evaluated.
Safety: Adverse reactions to AQ are generally similar to those of chloroquine, being itching probably less common with AQ. However, in contrast, amodiaquine can induce toxic hepatitis and fatal agranulocytosis following its use for prophylaxis Citation[31,39,40]. AQ is contraindicated for chemoprophylaxis and in persons with haematological and hepatic disorders. Recent studies suggest that AQ is safe during second and third trimester of pregnancy Citation[41].
Pharmacokinetics: Oral AQ is rapidly absorbed, peak plasma concentrations are reached after a mean of 1.5 h in malaria patients. It is extensively metabolized in the liver to desethylamodiaquine, the main antimalarial compound. AQ and its metabolites have more than 90% protein bound and have a mean terminal half-life of 5.2 (± 1.7) Citation[42]. There are no data of AQ pharmacokinetics in pregnancy Citation[43,44].
4.1.3 Piperaquine
The extensive use of piperaquine (PIP) as monotherapy, in mass treatment and in prophylactic campaigns in China, led to the development of resistance to the drug, so that its use was abandoned during the 1980s. Recently, interest in PIP has revived and it is now proposed to use it in combination with artemisinin derivatives. PIP is currently used in Asia combined with dihydroartemisinin (DHA) and is under registration by the European and USA regulatory authorities for its use in children and non-pregnant adults Citation[45]. To date, there have not been studies in pregnant women with this drug combination.
Safety: PIP is usually well tolerated with a safety profile similar to that of CQ.
Pharmacokinetics: PIP is a highly lipid-soluble drug with a large volume of distribution at steady state/bioavailability, long elimination half-life and a clearance that is markedly higher in children than in adults.
4.2 Arylaminoalcohols
4.2.1 Quinine
QN sulphate is administered orally for the treatment of uncomplicated malaria and QN hydrochloride as intravenous infusion for the treatment of severe or complicated malaria. In areas of multi-drug-resistance in south-east Asia, Western Oceania and South America, QN is combined with tetracyclines or clindamycin. The efficacy of QN monotherapy has been evaluated in pregnant women and ranges from 66 to 90% depending on the drug resistance in the area Citation[46-49]. QN is not indicated for chemoprophylaxis.
Safety: QN is a potentially toxic drug and most of its adverse effects are dose-related. ‘Cinchonism’ is a characteristic syndrome associated with QN treatment that consists of tinnitus, headache, nausea and dizziness Citation[31]; most of these adverse effects are reversible. QN also has arrhythmogenic potential (QT interval prolongation) and can cause hypotension. In addition, it stimulates insulin secretion and in patients with G6PD deficiency may occur haemolytic anaemia Citation[46,50]. Although QN is generally thought to be safe during pregnancy, pregnant women have an increased risk of hypoglycaemia Citation[51]. Glycaemia monitoring and parenteral dextrose supplementation are thus critical recommendations in the case management of pregnant patients with complicated malaria receiving QN Citation[52]. According to the available documented exposures to QN during pregnancy (including 368 in the first trimester) it has recently been concluded that the drug does not increase the number of birth defect outcomes and, thus, can be considered safe for the treatment of malaria in pregnancy Citation[53,54].
Pharmacokinetics: QN is absorbed rapidly when orally administered. Peak plasma concentrations are achieved within 1 – 3 h of an oral dose. QN is extensively metabolized in the liver and its plasma half-life is 10 – 12 h. The pharmacokinetics of QN in non-pregnant adults Citation[55-57] and in pregnant populations Citation[43,58] have been examined. A recent study on pregnant and non-pregnant Sudanese women found no differences between the two groups regarding QN metabolism and concluded that there is no need to adjust QN doses during pregnancy Citation[59].
4.2.2 Mefloquine
MQ is recommended for malaria prophylaxis in travellers and for treatment in areas of drug resistance. Most of the experience of MQ as malaria treatment during pregnancy comes from south-east Asia, where increasing MQ resistance has been reported Citation[60,61]. In these areas of multi-drug-resistance, MQ is combined with AS for malaria treatment. In Thailand, this combination (MQ + AS) has been found to be more effective than QN in clearing parasites and fever in pregnant women with uncomplicated malaria Citation[48,62]. In a study conducted in eastern Sudan, 25 mg/kg dose of MQ was found to be safe and effective for treating CQ-resistant malaria episodes in pregnant women Citation[63].
Safety: MQ can cause mild gastrointestinal and neurological adverse effects. Severe CNS effects occur in 1:10,000 patients taking MQ as chemoprophylaxis Citation[31,64]. Rare cardiac (bradycardia, hypotension, arial flutter) and pulmonary (alveolar damage, pneumonia) toxic effects have also been described after its use Citation[65-69]. The incidence of adverse events is higher when administered as treatment, indicating a dose-related pattern. Most of the safety data for the use of MQ in pregnant women come from the post-marketing surveillance system of the manufacturer and from retrospective studies Citation[54,70,71]. A retrospective study evaluating antimalarial exposure during pregnancy in Thailand showed an increased risk of stillbirths in the group of pregnant women treated with MQ Citation[72]. However, in a study conducted in Malawi, no differences were found in abortion and stillbirth rates between the group of pregnant women treated with CQ and the group treated with MQ Citation[33]. From the clinical data available, MQ can be considered a safe drug to be administered during pregnancy, even in the first trimester Citation[43,73].
Pharmacokinetics: MQ is highly protein bound (98% in plasma) and has a long elimination half-life, varying between 10 and 40 days in adults but tending to be shorter in pregnant women. The pharmacokinetic parameters of MQ are changed in acute P. falciparum malaria, reaching a higher Cmax, probably due to a contraction of the apparent distribution volume. MQ clearance was found to be increased during late pregnancy in a study conducted in 20 women from an area of multi-drug-resistance in south-east Asia. MQ is considered to be one of the best characterized antimalarial drugs from a pharmacokinetic point of view in pregnancy Citation[59].
4.2.3 Lumefantrine (or benflumetol)
Lumefantrine is used in association with artemisinins derivatives in patients with uncomplicated P. falciparum malaria. Lumefantrine is only available in combination with artemether (AL). The drug clears parasites rapidly and results in fewer gametocyte carriers Citation[74]. AL is not indicated for chemoprophylaxis.
Safety: Lumefantrine is usually well tolerated, with minor and reversible events such as nausea, abdominal pain, diarrhoea, pruritus and skin rashes. It has been found to prolong the QTc interval at the standard recommended dose but no adverse events attributable to QTs prolongation have been reported in patients treated with AL Citation[75,76]. In comparison to halofantrine, the incidence and degree of QTc prolongation are significantly lower in the combination of lumefantrine with artemether. In animal studies, no evidence of mutagenicity was detected but teratogenicity and embryotoxicity occurred with the combination of both drugs. AL was well tolerated and safe in a study conducted in Thailand among 125 pregnant women Citation[77,78].
Pharmacokinetics: Lumefantrine pharmacokinetic data show a considerable variation of all parameters, except for the terminal half-life. This is also mainly ascribed to the variability of absorption. The estimated mean absorption half-life was 3.2 h (range 2 – 7 days), showing little variation between ethnic groups and fat food intake. The terminal half-life of lumefantrine seems to differ between healthy subjects (2 – 3 days) and malaria patients (3 – 6 days). Reduced concentrations of the drug have been described in late pregnancy suggesting that drug-adjustment may be needed Citation[79]. Elimination of lumefantrine is via liver and faeces, and the drug is eliminated very slowly.
4.2.4 Pyronaridine
Pyronaridine is an acridine derivative, a synthetic drug widely used in China that may have utility for multi-resistant P. falciparum malaria Citation[80]. It seems likely that drug resistance would emerge rapidly if pyronaridine is used as monotherapy; therefore, it has been studied in combination with other antimalarials as artesunate Citation[81]. No data on its use in pregnancy are available.
4.3 Antifolates
4.3.1 Sulfadoxine and pyrimethamine
Sulfadoxine and pyrimethamine (SP) are used in fixed combination only in the treatment of malaria. This combination is a highly active blood schizontocide against P. falciparum but is less effective against other species. There is no cross-resistance with the 4-aminoquinolines, mefloquine, quinine, or the artesiminin derivatives. The drug does not have gametocytocidal activity but has been shown to be sporontocidal in animal models. There is extensive experience with the use of SP in pregnancy, as it is the recommended drug for malaria prevention in pregnant women in stable transmission areas and has also been used for treatment in combination with other antimalarial drugs Citation[22].
Safety: The SP combination is generally well tolerated when used at the recommended doses for malaria therapy. The most serious events are associated with hypersensitivity to the sulfa component, involving the skin and mucous membranes and include life-threatening erythema multiforme (Stevens–Johnson syndrome) and toxical epidermal necrolysis Citation[40,82]. Cutaneous drug reactions are more common in patients who are HIV infected Citation[83]. There have been isolated reports of a transient increase in liver enzymes as well as hepatitis occurring after administration of SP; haematological changes, including thrombocytopenia, megaloblastic anaemia and leucopoenia, have also been observed. In very rare cases, agranulocytosis and purpura have occurred. As a rule, these changes regress after withdrawal of the drug. It has a good safety profile in pregnant women. There is a theoretical risk of kernicterus if sulfadoxine is given in the third trimester of pregnancy as sulfonamides compete with bilirrubin for plasma proteins (IPTP) Citation[87]. However, it was reported from a study conducted in western Kenya, that maternal SP exposure or detectable sulfa compounds in maternal urine at delivery were not associated with neonatal kernicterus Citation[85].
Pharmacokinetics: SP pharmacokinetic properties in pregnancy have been studied extensively. Both drugs are highly protein bound, with relatively long mean elimination half-lives of around 180 h for sulfadoxine and 95 h for pyrimethamine. Pyrimethamine is extensively metabolized whereas only a small proportion of sulfadoxine is metabolized to acetyl and glucoronide derivatives. Excretion is mainly in the urine. The two drugs cross the placental barrier and are detected in breast milk Citation[42,86]. Inconsistency of changes in pharmacokinetic parameters between sulfadoxine and pyrimethamine was observed in a recent study where SP was used for intermittent preventive treatment in pregnancy (IPTP) Citation[87]. However, HIV status has little influence on pharmacokinetic parameters of SP in pregnant women Citation[88].
4.3.2 Chloroguanide and proguanil
Recent evidence suggests that mechanisms of action other than antifolate activity may also be involved in the antimalarial activity of chloroguanide and proguanil Citation[38,40]. Unfortunately, effectiveness has been compromised by development of drug-resistant strains of P. falciparum. Proguanil is currently used only for profilaxis and in combination with CQ in areas with low prevalence of CQ-resistant P. falciparum. The combination of proguanil and CQ can be recommended as prophylaxis in non-immune pregnant women such as travellers to malaria P. falciparum-endemic areas Citation[89].
Safety: Proguanil can be given orally, is easily administered and causes few side effects. There are reports indicating that mouth ulcers, hair loss and gastrointestinal discomfort may occur following prophylactic use. The drug should not be used in persons with liver or kidney dysfunction. Gross over dosage gives rise to abdominal pain, vomiting, diarrhoea and haematuria Citation[40]. There is no evidence that proguanil is harmful at prophylactic doses during pregnancy Citation[32].
Pharmacokinetics: Chloroguanide and proguanil are pro-drugs that are metabolized to the active compound, cycloguanil, which was the first antifolate developed. Pregnancy reduces the conversion of proguanil to the active metabolite Citation[90]. Pharmacokinetic studies on proguanil are limited. Absorption is rapid, peak plasma concentrations of both proguanil and its active metabolite, cycloguanil, are achieved within 4 h of administration. The elimination half-life is approximately 16 h Citation[40].
4.4 Peroxides: artemisinin derivatives
Artemisinin derivatives are the fastest active antimalarial drugs with a potent and rapidly acting schizontocide, eliciting shorter parasite clearance times than CQ or QN and rapid symptomatic responses. Different compounds have been used; the derivatives are actually more active than artemisinin itself and all of them are readily metabolized to the biologically active metabolite, DHA. Artemisinin is active at nanomolar concentrations in vitro on both CQ-sensitive and resistant P. falciparum strains. These compounds prevent gametocyte development and, therefore, can reduce transmission Citation[91].
Safety: The artemisinin derivatives are well tolerated when used for acute malaria, despite the fact that neurotoxicity was observed in animals with higher doses than used clinically Citation[91]. Most adverse events are mild and include nausea, vomiting, itching and drug fever. In addition, abnormal bleeding and dark urine have occasionally been documented and minor cardiac changes, mainly non-specific ST changes and first degree A-V block, have been noted during clinical trials. These returned to normal after improvement of malaria symptoms. Preclinical studies have consistently shown that artemisinin and its derivatives exhibit mutagenic or teratogenic activity, and could cause fetal resorption in rodents at relatively low doses when given after the sixth day of gestation Citation[92]. These drugs are also embryolethal in cynomologus monkeys at doses close to therapeutic range Citation[93]. Safety data in human pregnancy are still limited and more information is urgently needed, especially for the first trimester.
Pharmacokinetics: Oral artesunate is rapidly absorbed; peak levels of both the parent compound and DHA are reached by about 60 min. Levels of both can be detected for 4 – 8 h. Similar pharmacokinetics have been reported following the oral administration of artemether; mean peak plasma concentrations and mean plasma half-lives being 3 h, 3.1 h and 10.6 h for the parent compound and DHA respectively. The plasma concentrations of both artemether and DHA were similar in both healthy subjects and those with acute uncomplicated malaria Citation[91]. The kinetics of DHA are modified by pregnancy. The plasma levels of the active antimalarial metabolite DHA are lower than reported previously in non-pregnant adults Citation[94].
4.5 Other drugs with antimalarial activity
Several antibiotic drugs present antimalarial properties and have been used in combination with antimalarials for malaria treatment Citation[95].
Clindamycin is a semi-synthetic antibiotic derived from lincomycin. It is an efficient blood schizontocide with a relatively slow action Citation[96]. It is usually given in combination with QN for the treatment of P. falciparum malaria when decreased susceptibility to quinine has been reported Citation[97]. It has been mainly used in endemic areas of Latin America. Clindamycin use has not been related to adverse events in pregnancy, although it does pass through the placenta and may be accumulated in the fetal liver. The experience of its use during pregnancy is limited.
Azithromycin is a macrolide antibiotic, semi-synthetic derivative of erythromycin with activity against P. falciparum Citation[98]. Azithromycin is synergistic with quinine against P. falciparum strains. The azithromycin-quinine combination appears safe, well tolerated, and effective in drug-resistant P. falciparum malaria Citation[99]. The drug has a slow onset of action and has been proposed to be used in combination with fast-acting substances, such as artemisinin derivatives Citation[98]. Recent studies have demonstrated the safety and efficacy of combinations of azithromycin and SP or artesunate for the treatment of P. falciparum malaria in pregnant women Citation[62,100]. The azithromycin–chloroquine combination is active against P. falciparum and P. vivax infections Citation[101]. Azythromicin combinations are still under clinical evaluation for their use in the prevention or treatment of malaria in pregnant and non-pregnant patients. The interest in using azithromycin for IPTp is not only because of its favourable safety profile in pregnancy but also because of its antibiotic effect on other infections, mainly sexually transmitted infections, which are very prevalent in many malaria-endemic areas Citation[102].
4.6 Combination therapies
The combination of different antimalarial drugs has been suggested because of its potential to rapidly decrease parasitaemia and also because the synergistic effect of the combination could improve the clinical cure, shorten the duration of therapy so as to minimize the risk of recrudescence, provide a way in which resistance can be delayed and might also reduce malaria transmission Citation[103,104]. Sulfadoxine and pyrimethamine is the most widely used combination.
4.6.1 Chlorproguanil-dapsone
Chlorproguanil–dapsone (CD) is a fixed-dose antifolate combination similar to SP but with a shorter half life; thus, it could reduce the probability of selecting resistant parasites. Dapsone is effective in combination with other antifolates in preventing P. falciparum malaria; it is also effective in clearing P. falciparum parasitaemia in children younger than 5 years of age Citation[83]. However, toxicity is a serious concern with this combination Citation[40]. In a recent study for treatment of malaria in pregnancy in Tanzania, CD showed high parasitological failure rates at day 28 (18%) compared with other combinations Citation[105]. Adverse effects reported include hemolytic anaemia, particularly in patients with G6PD deficiency Citation[106]. Due to this unacceptable haematological toxicity, the drug has been withdrawn from clinical use Citation[107].
4.6.2 Atovaquone–proguanil
Atovaquone–proguanil is another antimalarial in fixed-dose combination. The efficacy of atovaquone in prevention and treatment of P. falciparum malaria is maximized in combination with proguanil. Atovaquone is also effective in treating and preventing P. vivax, P. malariae and P. ovale infections, although more evidence is desirable for this indication. As atovaquone is structurally unrelated to the 4-aminoquinolines, quinoline–methanols or other arylaminoalcohols, it is also effective against P. falciparum strains resistant to these drugs Citation[86]. This combination is well tolerated and more effective than CQ alone, CQ-SP, or MQ, against acute uncomplicated multi-drug-resistant P. falciparum Citation[108]. Pregnancy is currently listed as a contraindication to the use of the atovaquone–proguanil combination but studies are in place to evaluate its safety profile Citation[109].
4.6.3 Sulfadoxine–pyrimethamine and amodiaquine
Co-administered sulfadoxine–pyrimethamine and amodiaquine (SP + AQ) was the first antimalarial combination to replace CQ as first-line treatment in many African malaria-endemic countries due to its availability and low cost. The SP + AQ combination has been found to be significantly more effective than CQ in parasite cleaning on day 7 in P. falciparum-uncomplicated malaria Citation[110]. Recent studies in pregnant women have shown that this combination presents low parasitological failure rates at day 28 (1%) than other combinations Citation[105].
4.6.4 Artemisinin-based combination therapies
The artemisinin derivatives are strongly recommended to be used in combination with other antimalarial drugs Citation[22]. Several combinations have been used, including artemether–lumefantrine (AL), artesunate + amodiaquine (AS + AQ), artesunate + SP (AS + SP), artesunate + mefloquine (AS + MQ), dihydroartemisinin + piperaquine (DHA + PIP) and artesunate + pyronaridine (AS + PD).
The combination AS + SP was found to be safe and effective for malaria treatment in pregnant women in The Gambia Citation[111]. A recent study in Tanzania has also shown that AS + AQ was safe and efficacious in pregnancy Citation[105]. In Thailand, the combination AS + MQ was more effective than QN in the treatment of acute uncomplicated P. falciparum malaria in the second or third trimesters of pregnancy Citation[48].
Studies in Vietnam have assessed the efficacy of DHA–PIP for the treatment of uncomplicated malaria in children and adults Citation[112,113]. However, there is very little information on the use of this drug in pregnancy. A report from Thailand, where 50 pregnant women with recurrent P. falciparum infections were treated with DHA–PIP, indicates that the drug was effective and well tolerated Citation[114]. Studies are planned to evaluate the use of this combination for the treatment and prevention of malaria in pregnancy.
Artesunate–pyronaridine was shown to have a good tolerability and safety profile in uncomplicated P. falciparum malaria in children Citation[81]. There is no information on the safety and efficacy of this combination in pregnancy.
Fixed-dose combinations for the existing drugs in the market, in particular with artemisinin derivatives, include AS–SP, AS–AQ, DHA–PIP and AS–MQ, and have completed clinical trials in Africa and south-east Asia. AS–MQ and AS–AQ were evaluated in Brazil, Malaysia, Thailand and France and are now available Citation[115].
4.7 Antimalarial drugs contraindicated in pregnancy
Tetracycline and doxycycline are used in combination with quinine or artesunate in areas of multi-drug resistance. In human pregnancy, their use can be associated with disturbances of fetal bone growth and with irreversible teeth coloration in the infant; congenital cataract has also been reported. The hepatotoxicity of tetracycline is increased in pregnancy.
Halofantrine is embryotoxic in animals. No data exist on the use of halofantrine in pregnant women, but the cardiotoxicity of the drug has compromised its role in the treatment of uncomplicated P. falciparum malaria.
Primaquine is also contraindicated in pregnancy because of the risk of intravascular hemolysis in the mother and the fetus. This risk is linked to the dose administered and the degree of G6PD deficiency.
4.8 Upcoming drugs
The beginning of the twenty-first century is witnessing a renewed enthusiasm in the malaria research community, and malaria in pregnancy has been considered a priority research area. Different institutions are involved in drug discovery and development to achieve the goal of malaria eradication. Most of them are focused on developing antimalarials for use in combination therapy.
Artemisone, a metabolically stable, semi-synthetic derivative of artemisinin, is being developed. Several tioxanes obtained by total synthesis are now available and are being assessed for further development Citation[116]. Isoquine is an isomeric derivative of amodiaquine that should not generate the toxic quione-imine metabolites that are thought to have a role in the hepatic and neutrophil toxicities Citation[117]. Short-chain chloroquine analogues with better efficacy on chloroquine resistant isolates are also being studied Citation[118,119]. Fosmidomycin has recently been tested in small number of patients and produced modest cure rates; however, it could be used in combination with other antimalarials, this compound inhibits 1-deoxy-d-xylulose 5-phosphate reductoisomerase, an enzyme of the non-mevalonate pathway of isoprenoid biosynthesis, which is absent in humans but present in many pathogens and plants. The combination of fosmidomycin and clindamycin has also been tested in a study involving 70 patients with acute uncomplicated P. falciparum malaria. It was well tolerated and a cure rate > 95% was found Citation[120].
5. Guidelines for treatment and prophylaxis of malaria in preganancy
Chemotherapy and prophylaxis of different forms of malaria have become progressively more complex and less satisfactory, primarily due to selection of drug-resistant strains of P. falciparum in areas of extensive antimalarial use. Any guideline should be reviewed appropriately, according to the status and habitat of the patient, the geographic origin, species and drug-resistance profile of the likely infecting parasites, and the antimalarials locally available.
5.1 Preventive treatment
Chemoprophylaxis is recommended for travellers to endemic areas, and CQ, CQ + proguanil, MQ, proguanil–atovaquone or doxycycline are examples of drugs that are widely used according to the patient and to the specific endemic area Citation[121].
Various strategies using antimalarial drugs for the prevention of malaria in endemic areas have been recommended in pregnancy. Studies in pregnant women have shown significant effects increasing birth weight and reducing maternal anaemia and parasitaemia in women who received regular chemoprophylaxis Citation[122,123]. Because of concerns on the sustainability and compliance with the regular prophylaxis regimen and the emergence of resistant parasites to CQ, chemoprophylaxis was abandoned in most African countries Citation[122].
More recent studies have shown that intermittent preventive treatment might maintain the benefits of regular chemoprophylaxis regimens without some of their drawbacks Citation[17,124]. This strategy consists on the administration of a therapeutic dose of an antimalarial drug at predetermined time points to pregnant women regardless of the presence of parasitaemia, and coinciding with the women attendance to the routine antinatal clinics. The current recommendation is to administer at least two doses of SP. from the second trimester of gestation Citation[3].
Although the level of resistance at which SP IPTp becomes ineffective is still unknown Citation[125], the increase in SP resistance is a challenge to the sustainability of the implementation of this strategy. A recent study in Tanzania showed that SP IPTp was associated with an increase in the number of parasites carrying the resistance allele at DHPS codon 581 Citation[126]. With the increasing emergence of P. falciparum parasites resistant to SP, other alternative drugs need to be explored for their use as IPTp. In an IPTp study in Ghana, AQ and SP–AQ were comparable to SP in reducing maternal anaemia and low birth weight; but the lower tolerability of the AQ-containing arms was a concern Citation[127]. Combinations of azithromycin with other antimalarials, such as CQ or PIP, have been proposed for IPTp; however, studies are still needed to evaluate this option through regulatory trials for their use in pregnancy Citation[101]. MQ has also been found to be more efficacious than SP for IPTp in Benin but, again, tolerability was lower in the MQ arm Citation[128]. The pharmacokinetic characteristics and ease of administration of MQ make this drug the most promising available candidate to replace SP. Studies are on-going to explore the full potential of MQ as an alternative to SP for IPTp (C Menéndez, personal communication).
5.2 Treatment
Prompt and effective treatment of confirmed or suspected malaria cases continues to be the cornerstone of malaria control in pregnancy Citation[22].
CQ is the safest antimalarial drug to treat malaria in pregnant women. With the emergence of CQ-resistant P. falciparum parasites, the treatment of malaria in pregnancy has become a very complex issue. There is little information on the efficacy and safety profile of the alternative drugs for pregnancy. The decision on the selection of the appropriate antimalarial drug is based on a risk–benefit assessment taking into account: (1) the severity of the clinical manifestations; (2) the gestational age; (3) the type of Plasmodium spp. causing the infection; and (4) the pattern of the parasite drug resistance in the area.
Artemisinin-based combination therapies (ACTs) are the first-line treatment recommended by the WHO in P. falciparum endemic areas including in pregnancy Citation[22]. For uncomplicated malaria, the WHO recommends ACTs in the second and third trimesters; the ACT to be used should be the one used as first-line treatment in the area. Based on the safety concerns from preclinical studies, artemisinins are not recommended in the first trimester of pregnancy and can therefore only be considered in the absence of effective alternatives after a risk–benefit assessment has been made Citation[22,129]. Thus, oral QN is still the recommended drug for the treatment of uncomplicated malaria in the first trimester of gestation (clindamycin is rarely available and not used in Africa due to its high cost). Oral QN for uncomplicated malaria has inherent problems of compliance due to the duration (7 days) and low tolerability of this therapeutic regimen. With regard to complicated malaria in the second and third trimesters of pregnancy, the WHO guidelines recommend parenteral AS as the first choice, and parenteral artemether (AM) as the second option, depending on the local availability of these drugs Citation[22]. This recommendation of replacing QN for the treatment of complicated malaria is based on a study showing lower mortality among non-pregnant Asian patients treated with parenteral AS than in those treated with QN Citation[130]. Although these are very important results, they should be confirmed among pregnant African women with complicated malaria, since resistance to QN is still negligible in the African region.
Since 2006, new studies have evaluated the efficacy of antimalarial drugs as monotherapy or in combination for treatment of uncomplicated malaria in pregnancy. Citation[78,100,105,131]. summarizes the currently WHO recommendations for the treatment of malaria in pregnancy.
Table 2. Currently recommended drugs for treatment and prevention of malaria in pregnancy by WHO region.
5.3 Malaria treatment in HIV-infected women
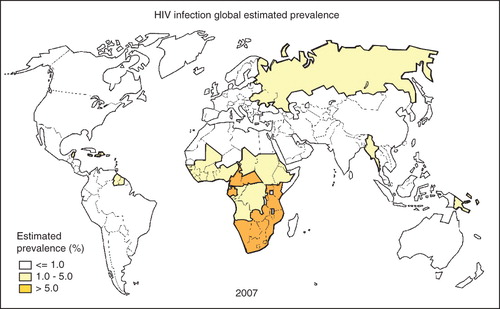
The global spread of the HIV/AIDS epidemic has contributed to increase the burden of MiP, especially in sub-Saharan Africa, where almost 76% of AIDS deaths occurred in 2007 Citation[132]. shows the global HIV/AIDS global distribution. The WHO estimates that 61% of adults living with HIV in sub-Saharan Africa are women. HIV infection increases the risk and severity of malaria in pregnant women and alters the malaria parity-related pattern Citation[133-135]. Antimalarial treatment failure is more common among HIV-infected pregnant women, and more doses of antimalarial preventive treatment are required for an effective prevention Citation[85,136,137].
Co-infection with malaria and HIV is associated with increased maternal anaemia, low birth weight and infant mortality to a greater extent than infection with either disease alone Citation[138]. HIV-infected pregnant women are more likely to be malaria parasitaemic, to have higher parasite densities and to be febrile when parasitaemic than HIV-seronegative pregnant women Citation[139]. Given this increased susceptibility, malaria control among pregnant HIV-infected women is a priority in endemic areas. However, both prevention and treatment of malaria becomes a challenge in this particular group due to potential drug interactions between antimalarial and antiretroviral drugs Citation[140].
WHO guidelines recommend prophylaxis of opportunistic infections with cotrimoxazole (CTX, trimethoprim–sulfamethoxazole) in HIV-infected individuals in developing countries Citation[141]. They also mention that SP should not be given to patients on CTX to avoid the risk of sulfa-related adverse effects. Thus, this implies that IPTp with SP for malaria prevention is not recommended for HIV-infected pregnant women. Although CTX also has antimalarial activity, it is unclear whether CTX prophylaxis would be effective in preventing the harmful effects of malaria in pregnancy in HIV-infected women. A multicentre study involving pregnant African women evaluating CTX is currently ongoing (C Menendez, personal communication).
6. Conclusions
The treatment of malaria in pregnancy has been hampered since the appearance of CQ resistance. New antimalarial drugs and combinations are being studied. However, due to the systematic exclusion of pregnant women from clinical drug trials, decisions are being taken on the basis of limited safety and efficacy data for their use in pregnancy.
The prevention of malaria in pregnancy in endemic areas of Africa is also faced with the problem of drug resistance and alternative antimalarials are urgently needed. To further complicate the issue, in some of the areas with the highest malaria transmission, the incidence of HIV is also highest, making the treatment of both infections a new and yet unresolved challenge especially in pregnancy.
7. Expert opinion
Malaria poses an enormous burden to public health services of endemic countries as well as a challenge to manage it in the context of limited resources. One of the reasons for this challenge is the need to use adequate antimalarial drugs that are not only safe and effective but that are also affordable for the weak economies of endemic countries. Because of changes in the development of drug resistance and of epidemiological situations, like interactions with other infections such as HIV, there is a need to regularly revisit the list of available antimalarial drugs and their recommendation in different populations at risk. This has been possible because in the last years an unprecedented effort from public and private international health agencies has been made. One of these organizations is the Medicines for Malaria Venture (MMV), which has a focus on the development of new antimalarial drugs with specific target product profiles. This new perspective of the research on drugs against malaria is certainly very positive and should be encouraged if the goal is to eradicate malaria in a not too distance future, and because the parasite, with more or less efficiency and speed, will continue developing resistance against the drugs used to destroy it.
New antimalarial drugs also represent new challenges; their efficacy and safety have to be proved also in special populations such as very small babies and pregnant women, none of whom are part of clinical trials for the drug registration process. This issue is especially complex in the case of pregnant women not only because they are systematically excluded from drug trials for ethical, legal, sociological concerns and for fear of toxicity to the fetus, but also – and mainly – for matters concerning the liability of pharmaceutical companies. The pressure of having to resolve the need to treat and prevent malaria in pregnancy has led to the use of antimalarial drugs with very limited information on their safety profile and efficacy in pregnant women. The WHO recommendation of ACTs as first-line treatment for individuals living in endemic areas has improved malaria case management in these areas. However, it has also raised concerns about having to treat pregnant women in the same way as the rest of the population, without any evidence that ACTs are safe in pregnancy. This is especially relevant because artermisinins have been found to be embryotoxic in several animal species. The recommendation of the use of ACTs in pregnancy constitutes an example of how when it comes to treatment in pregnant women the decision is mainly based on a risk-benefit assessment rather than on the balance of safety and efficacy as well.
The choice of antimalarial drug has increased in recent years, although it is still limited and many drugs are still under development. As discussed above, this choice is even more limited in pregnancy. Given that pregnancy is a special physiological situation, pregnant women should be considered specifically and not necessarily included in the recommendations made for the rest of the population with regard to malaria control, both case management and prevention. The decision on which drug should be given has to be based not only on general tolerability and safety but also on reprotoxic safety. It also has to be based on drug resistance in the area; for example, a non-artemisinin combination therapy might be as effective as an ACT in a particular area and may have a better safety profile for pregnancy than the ACT compound, and should thus be preferable. Finally, the decision should also be based on the acquired immunity of the woman, which will depend on the malaria endemicity of the area. Thus, recommendations should not be extrapolated and generalized on the basis from findings from a particular endemic area to another very different one without having carried out the appropriate evaluation.
In conclusion, in recent years malaria in pregnancy has received more attention than ever before from the international malaria community, as exemplified by the Malaria in Pregnancy (MiP) Consortium, among other international initiatives. This interest is clearly very positive and should serve to contribute to establish rational policies for the use of antimalarial drugs in pregnancy, as well as to find more efficacious and safer alternatives for this vulnerable group at risk of malaria. In the renewed effort for malaria eradication pregnant women should not be neglected but being one of the main focuses.
Article highlights.
The systematic exclusion of pregnant women from clinical trials has resulted in a lack of information on the safety and efficacy of most antimalarial drugs.
The choice of antimalarial drugs for malaria treatment and prevention in pregnancy is based on risk–benefit criteria.
Artemisinin-based combinations are the recommended drugs for treatment in the second and third trimesters of pregnancy; the recommendation for the first trimester has not yet been adequately resolved.
The increasing emergence of parasite resistance to sulfadoxine–pyrimethamine requires the urgent evaluation of alternative drugs to be used as intermittent preventive treatment for malaria prevention in pregnancy in stable endemic areas.
To solve the problem of malaria in pregnancy worldwide, research should be focused on the priorities specific for each endemic region. The recent effort from public and private health-funding agencies is going in this direction.
Declaration of interest
This paper did not receive specific funds as all the authors are supported by academic institutions. R González is supported by a grant from the Spanish Ministry of Health (Contrato post-Formación Sanitaria Especializada ‘Rio Hortega’, Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III, ref. CM07/0015).
Acknowledgments
The authors would like to thank E Jouve for drawing the figures of the manuscript and for her dedicated help.
Notes
This box summarizes key points contained in the article.
Bibliography
- WHO. World malaria report 2008. World Health Organization; 2008. WHO/HTM/GMP/2008.1
- Singh B, Kim Sung L, Matuso PA, A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 2004;363:1017-24
- WHO. A strategic framework for malaria prevention and control during pregnancy in the African region. World Health Organization, Geneva 2004, 2004: AFR/MAL
- Lindsay S, Ansell J, Selman C, Effect of pregnancy on exposure to malaria mosquitoes. Lancet 2000;355:1972
- Ansell J, Hamilton KA, Pinder M, Short-range attractiveness of pregnant women to Anopheles gambiae mosquitoes. Trans R Soc Trop Med Hyg 2002;96:113-116
- Desai M, ter Kuile FO, Nosten F, Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007;7:93-104
- Menendez C, D'Alessandro U, ter Kuile FO. Reducing the burden of malaria in pregnancy by preventive strategies. Lancet Infect Dis 2007;7:126-35
- Schwarz NG, Adegnika AA, Breitling LP, Placental malaria increases malaria risk in the first 30 months of life. Clin Infect Dis 2008;47:1017-25
- van Geertruyden JP, Thomas F, Erhart A, D'Alessandro U. The contribution of malaria in pregnancy to perinatal mortality. Am J Trop Med Hyg 2004;71:35-40
- Steketee RW, Nahlen BL, Parise ME, The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg 2001;64:28-35
- Le Hesran JY, Fievet N, Thioulouse J, Development of cellular immune responses to Plasmodium falciparum blood stage antigens from birth to 36 months of age in Cameroon. Acta Trop 2006;98:261-9
- Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull World Health Organ 1983;61:1005-16
- Shulman CE Dorman EK, Bulmer JN. Malaria as a cause of severe anaemia in pregnancy. Lancet 2002;360:494
- Greenwood BM, Greenwood AM, Snow RW, The effects of malaria chemoprophylaxis given by traditional birth attendantes on the course and outcome of pregnancy. Trans R Soc Trop Med Hyg 1989;83:589-94
- Menendez C, Ordi J, Ismail MR, The impact of placental malaria on gestational age and birth weight. J Infect Dis 2000;181:1740-5
- Granja AC, Machungo F, Gomes A, Malaria-related maternal mortality in urban Mozambique. Ann Trop Med Parasitol 1998;92:257-63
- Menendez C, Bardaji A, Sigauque B, A randomized placebo-controlled trial of intermittent preventive treatment in pregnant women in the context of insecticide treated nets delivered through the antenatal clinic. PLoS One 2008;3:e1934
- Greenwood D. The quinine connection. J Antimicrob Chemother 1992;30:417-27
- Payne D. Did medicated salt hasten the spread of chloroquine resistance in Plasmodium falciparum? Parasitol Today 1988;4:112-5
- Myint HY, Ashley EA, Day NP, Efficacy and safety of dihydroartemisinin-piperaquine. Trans R Soc Trop Med Hyg 2007;101:858-66
- Trouiller P, Olliaro P, Torreele E, Drug development for neglected diseases: a deficient market and a public-health policy failure. Lancet 2002;359:2188-94
- WHO. WHO guidelines for the treatment of malaria; 2006. WHO/HTM/MAL/2006.1108
- Nosten F, Rogerson SJ, Beeson JG, Malaria in pregnancy and the endemicity spectrum: what can we learn? Trends Parasitol 2004;20:425-32
- Guyatt HL, Snow RW. The epidemiology and burden of Plasmodium falciparum-related anemia among pregnant women in sub-Saharan Africa. Am J Trop Med Hyg 2001;64:36-44
- Bardaji A, Sigauque B, Bruni L, Clinical malaria in African pregnant women. Malar J 2008;7:27
- Maitra N, Joshi M, Hazra M. Maternal manifestations of malaria in pregnancy: a review. Indian J Matern Child Health 1993;4:98-101
- Kalia S, Dutz JP. New concepts in antimalarial use and mode of action in dermatology. Dermatol Ther 2007;20:160-74
- Khatoon L, Baliraine FN, Bonizzoni M, Prevalence of antimalarial drug resistance mutations in Plasmodium vivax and P. falciparum from a malaria-endemic area of Pakistan. Am J Trop Med Hyg 2009;81:525-8
- Lee SW, Lee M, Lee DD, Biological resistance of hydroxychloroquine for Plasmodium vivax malaria in the Republic of Korea. Am J Trop Med Hyg 2009;81:600-604
- Laufer MK, Thesing PC, Eddington ND, Return of chloroquine antimalarial efficacy in Malawi. N Engl J Med 2006;355:1959-66
- Alkadi HO. Antimalarial drug toxicity: a review. Chemotherapy 2007;53:385-91
- Phillips-Howard PA Wood D. The safety of anti-malarial drugs in pregnancy. Drug Saf 1996;14:131-45
- Steketee RW, Wirima JJ, Slutsker L, Malaria treatment and prevention in pregnancy: indications for use and adverse events associated with use of chloroquine or mefloquine. Am J Trop Med Hyg 1996;55:50-6
- Motta M, Tincani A, Faden D, Antimalarial agents in pregnancy. Lancet 2002;359:524-5
- Akintonwa A, Gbajumo SA, Mabadeje AF. Placental and milk transfer of chloroquine in humans. Ther Drug Monit 1988;10:147-9
- Chukwuani MC, Bolaji OO, Onyeji CO, Evidence for increased metabolism of chloroquine during the early third trimester of human pregnancy. Trop Med Int Health 2004;9:601-5
- Lee SJ, McGready R, Fernandez C, Chloroquine pharmacokinetics in pregnant and nonpregnant women with vivax malaria. Eur J Clin Pharmacol 2008;64:987-92
- Dipiro JT, Talbert RL, Yee GC, Pharmacotherapy: a pathophysiologic approach. Appleton & Lange, Stanford-Connecticut, USA; 1997
- Hatton CS, Peto TE, Bunch C, Frequency of severe neutropenia associated with amodiaquine prophylaxis against malaria. Lancet 1986;1:411-14
- Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf 2004;27:25-61
- Thomas F, Erhart A, D'Alessandro U. Can amodiaquine be used safely during pregnancy? Lancet Infect Dis 2004;4:235-9
- White NJ. Clinical pharmacokinetics of antimalarial drugs. Clin Pharmacokinet 1985;10:187-215
- Ward SA, Sevene EJ, Hastings IM, Antimalarial drugs and pregnancy: safety, pharmacokinetics, and pharmacovigilance. Lancet Infect Dis 2007;7:136-44
- Tagbor HK, Chandramohan D, Greenwood B. The safety of amodiaquine use in pregnant women. Expert Opin Drug Saf 2007;6:631-5
- Ashley EA, Krudsood S, Phaiphun L, Randomized, controlled dose-optimization studies of dihydroartemisinin-piperaquine for the treatment of uncomplicated multidrug-resistant falciparum malaria in Thailand. J Infect Dis 2004;190:1773-82
- Schlitzer M. Antimalarial drugs – what is in use and what is in the pipeline. Arch Pharm (Weinheim) 2008;341:149-63
- Adam I, Idris HM, Elbashir MI. Quinine for chloroquine-resistant falciparum malaria in pregnant Sudanese women in the first trimester. East Mediterr Health J 2004;10:560-5
- McGready R, Brockman A, Cho T, Randomized comparison of mefloquine-artesunate versus quinine in the treatment of multidrug-resistant falciparum malaria in pregnancy. Trans R Soc Trop Med Hyg 2000;94:689-93
- Bounyasong S. Randomized trial of artesunate and mefloquine in comparison with quinine sulfate to treat P. falciparum malaria pregnant women. J Med Assoc Thai 2001;84:1289-99
- Bateman DN, Dyson EH. Quinine toxicity. Adverse Drug React Acute Poisoning Rev 1986;5:215-33
- Kochar DK, Kumawat BL, Kochar SK, Sanwal V. Hypoglycemia after oral quinine administration. J Assoc Physicians India 1995;43:654, 657
- Rogerson SJ, Menendez C. Treatment and prevention of malaria in pregnancy: opportunities and challenges. Expert Rev Anti Infect Ther 2006;4:687-702
- White NJ, Looareesuwan S, Warrell DA, Quinine pharmacokinetics and toxicity in cerebral and uncomplicated falciparum malaria. Am J Med 1982;73:564-72
- Nosten F, McGready R, d'Alessandro U, Antimalarial drugs in pregnancy: a review. Curr Drug Saf 2006;1:1-15
- Babalola CP, Bolaji OO, Ogunbona FA, Pharmacokinetics of quinine in African patients with acute falciparum malaria. Pharm World Sci 1998;20:118-22
- Sowunmi A. Disposition of oral quinine in African patients suffering from acute uncomplicated falciparum malaria. East Afr Med J 1996;73:519-23
- Supanaranond W, Davis TM, Pukrittayakamee S, Disposition of oral quinine in acute falciparum malaria. Eur J Clin Pharmacol 1991;40:49-52
- Phillips RE, Looareesuwan S, White NJ, Quinine pharmacokinetics and toxicity in pregnant and lactating women with falciparum malaria. Br J Clin Pharmacol 1986;21:677-83
- Abdelrahim, II, Adam I, Elghazali G, Pharmacokinetics of quinine and its metabolites in pregnant Sudanese women with uncomplicated Plasmodium falciparum malaria. J Clin Pharm Ther 2007;32:15-19
- Carrara VI, Zwang J, Ashley EA, Changes in the treatment responses to artesunate-mefloquine on the northwestern border of Thailand during 13 years of continuous deployment. PLoS One 2009;4:e4551
- Nosten F, van Vugt M, Price R, Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet 2000;356:297-302
- Orton LC, Omari AA. Drugs for treating uncomplicated malaria in pregnant women. Cochrane Database Syst Rev 2008;(4):CD004912
- Adam I, Ali DA, Alwaseila A, Mefloquine in the treatment of falciparum malaria during pregnancy in Eastern Sudan. Saudi Med J 2004;25:1400-2
- Weinke T, Trautmann M, Held T, Neuropsychiatric side effects after the use of mefloquine. Am J Trop Med Hyg 1991;45:86-91
- Udry E, Bailly F, Dusmet M, Pulmonary toxicity with mefloquine. Eur Respir J 2001;18:890-2
- Katsenos S, Psathakis K, Nikolopoulou MI, Mefloquine-induced eosinophilic pneumonia. Pharmacotherapy 2007;27:1767-71
- Inoue T, Tanaka E, Sakuramoto M, Case of drug-induced pneumonia possibly due to mefloquine (antimalarial drug). Nihon Kokyuki Gakkai Zasshi 2005;43:103-7
- White NJ. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis 2007;7:549-58
- Fonteyne W, Bauwens A, Jordaens L. Atrial flutter with 1:1 conduction after administration of the antimalarial drug mefloquine. Clin Cardiol 1996;19:967-8
- Vanhauwere B, Maradit H, Kerr L. Post-marketing surveillance of prophylactic mefloquine (Lariam) use in pregnancy. Am J Trop Med Hyg 1998;58:17-21
- Newman RD, Parise ME, Slutsker L, Safety, efficacy and determinants of effectiveness of antimalarial drugs during pregnancy: implications for prevention programmes in Plasmodium falciparum-endemic sub-Saharan Africa. Trop Med Int Health 2003;8:488-506
- Nosten F, Vincenti M, Simpson J, The effects of mefloquine treatment in pregnancy. Clin Infect Dis 1999;28:808-15
- Phillips-Howard PA, Steffen R, Kerr L, Safety of mefloquine and other antimalarial agents in the first trimester of pregnancy. J Travel Med 1998;5:121-6
- Omari AA, Preston C, Garner P. Artemether-lumefantrine for treating uncomplicated falciparum malaria. Cochrane Database Syst Rev 2003;3:CD003125
- van Vugt M, Ezzet F, Nosten F, No evidence of cardiotoxicity during antimalarial treatment with artemether-lumefantrine. Am J Trop Med Hyg 1999;61:964-7
- Falade C, Manyando C. Safety profile of Coartem: the evidence base. Malar J 2009;8(Suppl 1):S6
- Tarning J, McGready R, Lindegardh N, Population pharmacokinetics of lumefantrine in pregnant women treated with artemether-lumefantrine for uncomplicated Plasmodium falciparum malaria. Antimicrob Agents Chemother 2009;53:3837-46
- McGready R, Tan SO, Ashley EA, A randomised controlled trial of artemether-lumefantrine versus artesunate for uncomplicated Plasmodium falciparum treatment in pregnancy. PLoS Med 2008;5:e253
- McGready R, Stepniewska K, Lindegardh N, The pharmacokinetics of artemether and lumefantrine in pregnant women with uncomplicated falciparum malaria. Eur J Clin Pharmacol 2006;62:1021-31
- Chavalitshewinkoon-Petmitr P, Pongvilairat G, Auparakkitanon S, Gametocytocidal activity of pyronaridine and DNA topoisomerase II inhibitors against multidrug-resistant Plasmodium falciparum in vitro. Parasitol Int 2000;48:275-80
- Ramharter M, Kurth F, Schreier AC, Fixed-dose pyronaridine-artesunate combination for treatment of uncomplicated falciparum malaria in pediatric patients in Gabon. J Infect Dis 2008;198:911-919
- Steffen R, Somaini B. Severe cutaneous adverse reactions to sulfadoxine-pyrimethamine in Switzerland. Lancet 1986;1:610
- Alloueche A, Bailey W, Barton S, Comparison of chlorproguanil-dapsone with sulfadoxine-pyrimethamine for the treatment of uncomplicated falciparum malaria in young African children: double-blind randomised controlled trial. Lancet 2004;363:1843-8
- White NJ. The treatment of malaria. N Engl J Med 1996;335:800-6
- Parise ME, Ayisi JG, Nahlen BL, Efficacy of sulfadoxine-pyrimethamine for prevention of placental malaria in an area of Kenya with a high prevalence of malaria and human immunodeficiency virus infection. Am J Trop Med Hyg 1998;59:813-22
- Karbwang J, Harinasuta T. Overview: clinical pharmacology of antimalarials. Southeast Asian J Trop Med Public Health 1992;23(Suppl 4):95-109
- Nyunt MM, Adam I, Kayentao K, Pharmacokinetics of sulfadoxine and pyrimethamine in intermittent preventive treatment of malaria in pregnancy. Clin Pharmacol Ther 2010;87(2):226-34
- Green MD, van Eijk AM, van Ter Kuile FO, Pharmacokinetics of sulfadoxine-pyrimethamine in HIV-infected and uninfected pregnant women in Western Kenya. J Infect Dis 2007;196:1403-8
- Schlagenhauf P, Petersen E. Malaria chemoprophylaxis: strategies for risk groups. Clin Microbiol Rev 2008;21:466-72
- Wangboonskul J, White NJ, Nosten F, Single dose pharmacokinetics of proguanil and its metabolites in pregnancy. Eur J Clin Pharmacol 1993;44:247-51
- Meshnick SR. Artemisinin: mechanisms of action, resistance and toxicity. Int J Parasitol 2002;32:1655-60
- White TE, Bushdid PB, Ritter S, Artesunate-induced depletion of embryonic erythroblasts precedes embryolethality and teratogenicity in vivo. Birth Defects Res B Dev Reprod Toxicol 2006;77:413-29
- Clark RL KM, Makori N, Nakata Y, Artesunate: embryolethal effects in cynomolgus monkeys. Am J Trop Med Hyg 2006;75:54
- McGready R, Stepniewska K, Ward SA, Pharmacokinetics of dihydroartemisinin following oral artesunate treatment of pregnant women with acute uncomplicated falciparum malaria. Eur J Clin Pharmacol 2006;62:367-71
- Goodman CD, Su V, McFadden GI. The effects of anti-bacterials on the malaria parasite Plasmodium falciparum. Mol Biochem Parasitol 2007;152:181-91
- Lell B, Kremsner PG. Clindamycin as an antimalarial drug: review of clinical trials. Antimicrob Agents Chemother 2002;46:2315-20
- McGready R, Cho T, Samuel, Randomized comparison of quinine-clindamycin versus artesunate in the treatment of falciparum malaria in pregnancy. Trans R Soc Trop Med Hyg 2001;95:651-6
- Noedl H, Krudsood S, Chalermratana K, Azithromycin combination therapy with artesunate or quinine for the treatment of uncomplicated Plasmodium falciparum malaria in adults: a randomized, phase 2 clinical trial in Thailand. Clin Infect Dis 2006;43:1264-71
- Miller RS, Wongsrichanalai C, Buathong N, Effective treatment of uncomplicated Plasmodium falciparum malaria with azithromycin-quinine combinations: a randomized, dose-ranging study. Am J Trop Med Hyg 2006;74:401-6
- Kalilani L MI, Chaponda M, Rogerson SJ, A randomized controlled pilot trial of azithromycin or artesunate added to sulfadoxine-pyrimethamine as treatment for malaria in pregnant women. PLoS One 2007;2:e1166
- Chico RM, Pittrof R, Greenwood B, Azithromycin-chloroquine and the intermittent preventive treatment of malaria in pregnancy. Malar J 2008;7:255
- Van Vranken M. Prevention and treatment of sexually transmitted diseases: an update. Am Fam Physician 2007;76:1827-32
- Kremsner PG, Krishna S. Antimalarial combinations. Lancet 2004;364:285-94
- WHO. Antimalarial drug combination therapy: report of a WHO Technical Consultation. World Health Organization, Geneva; 2001. WHO/CDS/RBM/2001.35
- Mutabingwa TK, Muze K, Ord R, Randomized trial of artesunate+amodiaquine, sulfadoxine-pyrimethamine+amodiaquine, chlorproguanal-dapsone and SP. for malaria in pregnancy in Tanzania. PLoS One 2009;4:e5138
- Fanello CI, Karema C, Avellino P, High risk of severe anaemia after chlorproguanil-dapsone+artesunate antimalarial treatment in patients with G6PD (A-) deficiency. PLoS One 2008;3:e4031
- Miller AK, Bandyopadhyay N, Wootton DG, Pharmacokinetics of chlorproguanil, dapsone, artesunate and their major metabolites in patients during treatment of acute uncomplicated Plasmodium falciparum malaria. Eur J Clin Pharmacol 2009;65:977-87
- Osei-Akoto A, Orton L, Owusu-Ofori SP. Atovaquone-proguanil for treating uncomplicated malaria. Cochrane Database Syst Rev 2005;(4):CD004529
- McGready R AE, Moo E, Cho T, A randomized comparison of artesunate-atovaquone-proguanil versus quinine in treatment for uncomplicated falciparum malaria during pregnancy. J Infect Dis 2005;192:846-53
- McIntosh HM GB. Chloroquine or amodiaquine combined with sulphadoxine-pyrimethamine as a treatment for uncomplicated malaria: a systematic review. Ann Trop Med Parasitol 1998;92:265-70
- Deen JL, von Seidlein L, Pinder M, The safety of the combination artesunate and pyrimethamine-sulfadoxine given during pregnancy. Trans R Soc Trop Med Hyg 2001;95:424-8
- Tran TH, Dolecek C, Pham PM, Dihydroartemisinin-piperaquine against multidrug-resistant Plasmodium falciparum malaria in Vietnam: randomised clinical trial. Lancet 2004;363:18-22
- Thanh NX, Trung TN, Phong NC, Open label randomized comparison of dihydroartemisinin-piperaquine and artesunate-amodiaquine for the treatment of uncomplicated Plasmodium falciparum malaria in central Vietnam. Trop Med Int Health 2009;14:504-11
- Rijken MJ, McGready R, Boel ME, Dihydroartemisinin-piperaquine rescue treatment of multidrug-resistant Plasmodium falciparum malaria in pregnancy: a preliminary report. Am J Trop Med Hyg 2008;78:543-5
- Sagara I, Diallo A, Kone M, A randomized trial of artesunate-mefloquine versus artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Mali. Am J Trop Med Hyg 2008;79:655-61
- Nagelschmitz J, Voith B, Wensing G, First assessment in humans of the safety, tolerability, pharmacokinetics, and ex vivo pharmacodynamic antimalarial activity of the new artemisinin derivative artemisone. Antimicrob Agents Chemother 2008;52:3085-91
- Casagrande M, Basilico N, Parapini S, Novel amodiaquine congeners as potent antimalarial agents. Bioorg Med Chem 2008;16:6813-23
- Solomon VR, Haq W, Smilkstein M, Synthesis and antimalarial activity of novel side chain modified antimalarial agents derived from 4-aminoquinoline. Med Chem 2008;4:446-56
- Stocks PA, Raynes KJ, Bray PG, Novel short chain chloroquine analogues retain activity against chloroquine resistant K1 Plasmodium falciparum. J Med Chem 2002;45:4975-83
- Ruangweerayut R, Looareesuwan S, Hutchinson D, Assessment of the pharmacokinetics and dynamics of two combination regimens of fosmidomycin-clindamycin in patients with acute uncomplicated falciparum malaria. Malar J 2008;7:225
- CDC. Centers for Disease Control Recommendations for the prevention of malaria among travellers. MMWR 1990;39:1-10
- Greenwood B. The use of antimalarial drugs to prevent malaria in the population of malaria endemic areas. Am J Trop Med Hyg 2004;70:1-7
- Garner P, Gülmezoglu AM. Prevention versus treatment for malaria in pregnant women. Cochrane Database Syst Rev 2000;(2):CD000169
- Brentlinger PE, Dgedge M, Correia MA, Intermittent preventive treatment of malaria during pregnancy in central Mozambique. Bull World Health Organ 2007;85:873-9
- Mockenhaupt FP, Bedu-Addo G, Eggelte TA, Rapid increase in the prevalence of sulfadoxine-pyrimethamine resistance among Plasmodium falciparum isolated from pregnant women in Ghana. J Infect Dis 2008;198:1545-9
- Harrington WE, Mutabingwa TK, Muehlenbachs A, Competitive facilitation of drug-resistant Plasmodium falciparum malaria parasites in pregnant women who receive preventive treatment. Proc Natl Acad Sci USA 2009;106:9027-32
- Clerk CA, Bruce J, Affipunguh PK, A randomized, controlled trial of intermittent preventive treatment with sulfadoxine-pyrimethamine, amodiaquine, or the combination in pregnant women in Ghana. J Infect Dis 2008;198:1202-11
- Briand V, Bottero J, Noel H, Intermittent treatment for the prevention of malaria during pregnancy in Benin: a randomized, open-label equivalence trial comparing sulfadoxine-pyrimethamine with mefloquine. J Infect Dis 2009;200:991-1001
- WHO. Assessment of the safety of artemisinin compounds in pregnancy. Report of two joint informal consultations convened in 2006 by TDR and the Global Malaria Programme of WHO for research and training in tropical diseases. World Health Organization, Geneva; 2006
- Dondorp A, Nosten F, Stepniewska K, Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 2005;366:717-25
- Tagbor H, Bruce J, Browne E, Efficacy, safety, and tolerability of amodiaquine plus sulphadoxine-pyrimethamine used alone or in combination for malaria treatment in pregnancy: a randomised trial. Lancet 2006;368:1349-56
- UNAIDS. WHO. AIDS epidemic update; 2007. UNAIDS/07.27E/JC1322E 2007
- Mount AM, Mwapasa V, Elliott SR, Impairment of humoral immunity to Plasmodium falciparum malaria in pregnancy by HIV infection. Lancet 2004;363:1860-7
- Verhoeff FH, Brabin BJ, Hart CA, Increased prevalence of malaria in HIV-infected pregnant women and its implications for malaria control. Trop Med Int Health 1999;4:5-12
- ter Kuile FO, Parise ME, Verhoeff FH, The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-saharan Africa. Am J Trop Med Hyg 2004;71:41-54
- Filler SJ, Kazembe P, Thigpen M, Randomized trial of 2-dose versus monthly sulfadoxine-pyrimethamine intermittent preventive treatment for malaria in HIV-positive and HIV-negative pregnant women in Malawi. J Infect Dis 2006;194:286-93
- Steketee RW, Wirima JJ, Bloland PB, Impairment of a pregnant woman's acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1. Am J Trop Med Hyg 1996;55:42-9
- Idemyor V. Human immunodeficiency virus (HIV) and malaria interaction in sub-Saharan Africa: the collision of two Titans. HIV Clin Trials 2007;8:246-53
- van Eijk AM, Ayisi JG, ter Kuile FO, HIV increases the risk of malaria in women of all gravidities in Kisumu, Kenya. AIDS 2003;17:595-603
- Skinner-Adams TS, McCarthy JS, Gardiner DL, HIV and malaria co-infection: interactions and consequences of chemotherapy. Trends Parasitol 2008;24:264-71
- WHO. Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults: recommendations for a public health approach; 2006