Abstract
Introduction: The global prevalence of type 2 diabetes mellitus (T2DM) is rapidly increasing and is associated with a high risk of microvascular and macrovascular complications. Although some glucose-lowering therapies are associated with hypoglycemia and weight gain, glucagon-like peptide-1 (GLP-1) receptor agonists represent a significant advance in the treatment of T2DM, as they provide effective glycemic control with a low incidence of hypoglycemia and a beneficial effect on body weight, as well as potential improvements in cardiovascular outcomes.
Areas covered: This article evaluates the pharmacological and clinical profile of the once-daily prandial GLP-1 receptor agonist lixisenatide for the treatment of T2DM.
Expert opinion: Once-daily prandial lixisenatide has been evaluated in an extensive clinical trials program, in which it was shown to have a favorable safety and tolerability profile, and to effectively improve metabolic control. The unique pharmacological properties of lixisenatide clearly differentiate it from other GLP-1 receptor agonists. As a once-daily agonist with a high affinity for the GLP-1 receptor, lixisenatide improves overall glycemic control, with particularly strong effects on postprandial plasma glucose levels. These attributes encourage the application of lixisenatide in those patients with extensive postprandial glucose excursions, or in combination with other antidiabetic drugs that have prevailing effects on fasting glucose levels.
1. Introduction
Inadequate glycemic control in patients with type 2 diabetes mellitus (T2DM) is associated with a risk for developing macrovascular and microvascular complications Citation[1]. Cardiovascular disease (CVD) is the primary cause of death in patients with T2DM Citation[2] and may reduce life expectancy by up to 10 years Citation[3]. Furthermore, CVD accounts for a substantial component of healthcare expenditure associated with T2DM Citation[4,5]. Although improved glycemic control goes some way to curb the risk of developing CVD, there are a number of other contributing factors Citation[6]. Therefore, glucose-lowering agents that confer additional cardiovascular protection are of particular clinical relevance.
Problems associated with standard glucose-lowering therapies include weight gain (sulfonylureas, insulin, meglitinides, thiazolidinediones), hypoglycemia (sulfonylureas, insulin, meglitinides) and a need for multiple injections (insulin) Citation[7,8], all of which highlight the need for alternative treatments. Incretin therapies represent a substantial advance in antidiabetic treatment, as they are rarely associated with severe hypoglycemia Citation[9]. They can be broadly categorized into dipeptidyl peptidase-IV (DPP-IV) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists. Although DPP-IV inhibitors appear to be weight-neutral, GLP-1 receptor agonists have been demonstrated to have a beneficial effect on body weight Citation[10-12].
Native GLP-1 is secreted in anticipation of a meal and in response to food ingestion, and has been demonstrated to enhance insulin secretion and suppress glucagon release. GLP-1 has been shown to improve pancreatic beta-cell function, delay gastric emptying, promote satiety and confer a protective effect on the cardiovascular system Citation[13-17]. In addition to these beneficial effects on the cardiovascular risk profile, there are preliminary reports indicating beneficial effects on diabetic nephropathy and psoriasis Citation[18,19]. Native GLP-1, however, is rapidly degraded by the protease DPP-IV and would therefore require continual infusion to provide a therapeutic benefit for T2DM.
GLP-1 receptor agonists mimic many of the biological actions of endogenous GLP-1, but benefit from extended half-lives Citation[10,20]. They can be characterized as being short-acting or long-acting agents, based on their pharmacokinetic profile. Short-acting GLP-1 receptor agonists predominantly have an effect on postprandial plasma glucose (PPG), whereas long-acting agents primarily affect fasting plasma glucose (FPG). Historically, FPG has always been an important target in achieving normoglycemia, yet PPG is increasingly recognized for its significant contribution to overall glycemic control and to the development of diabetes-related complications Citation[21,22]. As a result, PPG is now accepted to be a crucial therapeutic target for improving glycemic control in patients with T2DM.
The first marketed GLP-1 receptor agonist was the short-acting agent exenatide immediate release (IR), which has a mean half-life of 2.4 h and is given as a twice-daily (BID) subcutaneous injection Citation[23]. Exenatide is a synthetic version of exendin-4, a natural GLP-1 receptor agonist that is derived from the salivary glands of the Gila monster and that shares 53% homology with human GLP-1 Citation[24]. Currently marketed long-acting agents include liraglutide and exenatide long-acting release (LAR). Liraglutide, which shares 97% homology with human GLP-1, has a half-life of 11 – 13 h and is suitable for once-daily (QD) subcutaneous injection Citation[25]. Exenatide-LAR is composed of microspheres encapsulating the exenatide peptide, and is suitable for once-weekly subcutaneous injection Citation[26]. This article summarizes the pharmacological and clinical profile of the once-daily prandial GLP-1 receptor agonist lixisenatide () Citation[27] for the treatment of T2DM.
Box 1. Drug summary.
2. Profile of lixisenatide
2.1 Introduction to the compound
Lixisenatide is a once-daily prandial GLP-1 receptor agonist that is administered 20 µg QD by subcutaneous injection. It has a distinct structure and pharmacokinetic profile Citation[27] that confers a unique duration of action, efficacy and safety profile compared with the other GLP-1 receptor agonists Citation[20].
2.2 Chemical structure
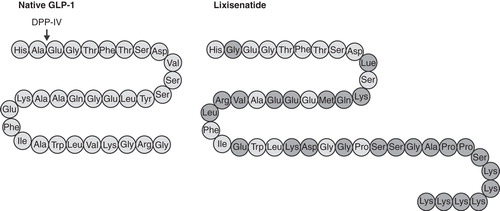
Lixisenatide is a 44-amino-acid peptide based on exendin-4, which differs in structure through the deletion of a proline residue and the addition of six lysine residues at the C terminus. The amino-acid sequence for lixisenatide is shown in .
Figure 1. Structure of lixisenatide. The amino-acid sequence for lixisenatide and native human GLP-1. The amino acids highlighted in dark grey represent differences in the structure of lixisenatide compared with native GLP-1. Dipeptidyl peptidase-IV (DPP-IV) rapidly cleaves and inactivates native GLP-1 at the indicated site.
2.3 Pharmacokinetic and pharmacodynamic profile
Lixisenatide is recommended for once-daily dosing, despite its relatively short half-life, owing to its high affinity for the GLP-1 receptor. Preclinical studies in Chinese hamster ovary (CHO) cells overexpressing human GLP-1 receptor have demonstrated that lixisenatide has a median inhibitory concentration (IC50) for the GLP-1 receptor of 1.43 nM, which is approximately four-fold greater than that of native GLP-1 Citation[28]. Radiolabelling studies suggest that the binding affinity of lixisenatide is higher than that of exenatide or liraglutide, although no direct comparison has been made Citation[20,29].
Lixisenatide is thought to undergo renal filtration and proteolytic degradation. A Phase I, open-label study demonstrated that lixisenatide clearance was not significantly altered in patients with mild or moderate renal function (creatinine clearance 50 – 80 ml/min) compared with patients with normal function. In contrast, clearance was reduced by ∼30% in patients with severe renal impairment (creatinine clearance < 30 ml/min) Citation[30].
A Phase II, 28-day, dose-escalation study assessed the effects of 5 – 20 µg lixisenatide QD or BID, in combination with metformin and/or sulfonylurea, in 64 patients with T2DM Citation[31]. Mean apparent clearance (CL/F) was 21 – 29 L/h. Mean area under the curve (AUC) and peak plasma concentration (Cmax) were demonstrated to increase in a dose-dependent manner (at 20 µg QD, AUC was 847.8 h·pg/ml and Cmax was 187.2 pg/ml). At the 20 µg dose, the median time to maximal concentration (tmax) was 1.25 h for both the once-daily and twice-daily regimens. In contrast, the half-life (t1/2) was slightly affected by the frequency of dosing, with a median t1/2 of 2.8 and 3.2 h with once-daily and twice-daily administration, respectively, at the 20 µg dose.
2.4 Clinical trials program
The efficacy, safety and tolerability of lixisenatide once-daily was examined in the Phase III ‘GetGoal' program, which comprised 11 randomized controlled studies and involved > 5000 adults with T2DM. So far, nine of these trials have been published (), including the effects of lixisenatide once-daily in monotherapy Citation[32], with oral antidiabetic agents (OADs; metformin, sulfonylureas or thiazolidinediones) Citation[33,34], or basal insulin glargine Citation[35-37], as well as in direct comparison with exenatide Citation[38]. In addition, lixisenatide was directly compared with liraglutide in a randomized, Phase II study Citation[39]. The evidence from the GetGoal program demonstrates that lixisenatide once-daily effectively lowers blood glucose levels, predominantly by reducing PPG. Lixisenatide's postprandial effect is predominantly pronounced for the first meal following injection. However, a recent study has shown that once-daily lixisenatide in the morning reduces PPG levels throughout the day. Further, the reduction in PPG at least after breakfast was attributable to a delay in gastric emptying Citation[40].
Table 1. Summary of the Phase III GetGoal clinical trials.
2.4.1 Lixisenatide monotherapy
The effect of lixisenatide once-daily administered as monotherapy was evaluated in a 12-week, randomized, double-blind study (GetGoal-Mono) Citation[32]. A total of 361 patients with T2DM were assigned to receive lixisenatide or placebo in a one-step or two-step dose-increase regimen. Lixisenatide treatment significantly reduced glycated hemoglobin (HbA1c) levels (least squares (LS) mean difference vs placebo: –0.5% (2-step) and –0.7% (1-step); p < 0.0001) and enabled a significantly higher proportion of patients to achieve HbA1c ≤ 6.5 (31.9% (2-step) and 25.4% (1-step) vs 12.5% with placebo; p < 0.01) and HbA1c < 7% (52.2% (2-step) and 46.5% (1-step) vs 26.8% with placebo; p < 0.01). There was a highly statistically significant reduction in PPG levels with lixisenatide (–4.5 (2-step) and –5.5 (1-step) vs –0.7 mmol/l with placebo; p < 0.0001). FPG levels were significantly decreased in lixisenatide-treated patients (LS mean change vs placebo: –0.9 mmol/l (2-step); p < 0.001 and –1.1 mmol/l (1-step); p < 0.0001).There was a reduction in body weight of ∼2 kg in all groups, with no significant difference between lixisenatide and placebo. Lixisenatide was generally well tolerated, with no significant differences between the 2-step and 1-step dose regimens (adverse events (AEs): 52.5% 2-step, 54.6% 1-step vs 45.1% placebo; serious AEs: 0.8% 2-step, 0.0% 1-step vs 4.1% placebo). In total, eight patients in the lixisenatide group and one patient in the placebo group discontinued treatment during the study due to a treatment-emergent AE (TEAE; 4.2% 2-step, 2.5% 1-step vs 0.8% placebo), mostly due to gastrointestinal (GI) events. The most common AEs in the lixisenatide group were GI in nature, with nausea being the most frequent AE (lixisenatide combined 22.1% vs placebo 4.1%). Nausea events were generally mild-to-moderate in intensity and were reported more frequently during the first 3 weeks of treatment, with a reduced occurrence from Week 7 to the end of treatment. There was also a higher incidence of vomiting in the lixisenatide group compared with the placebo group (7.1% lixisenatide combined vs 0.0% placebo). Symptomatic hypoglycemia occurred in 1.7% of patients in the lixisenatide group compared with 1.6% in the placebo group, with no severe episodes. There were no reports of suspected pancreatitis during the study. Injection-site reactions were reported by 4.6% of patients in the lixisenatide group and none in the placebo group, with no serious or severe events reported.
2.4.2 Lixisenatide in combination with metformin
Lixisenatide treatment in combination solely with metformin was assessed in three studies: GetGoal-M, GetGoal-F1 and GetGoal-X. GetGoal-M was a randomized, double-blind, 24-week study in 680 patients comparing morning (AM) and evening (PM) doses of lixisenatide versus placebo Citation[33]. Both the lixisenatide morning and evening doses significantly reduced HbA1c levels compared with placebo (LS mean change: –0.9% AM, –0.8% PM vs –0.4% with placebo), and enabled almost twice as many patients to achieve an HbA1c level of < 7% (43% AM or 41% PM vs 22% with placebo). Lixisenatide significantly reduced FPG compared with placebo (p < 0.005). The lixisenatide AM group also significantly decreased PPG versus the placebo AM regimen (LS mean difference: –4.5 mmol/l; p < 0.0001). Body weight was reduced in all groups, with no significant difference between lixisenatide and placebo (–2 vs –1.6 kg, respectively). Lixisenatide was well tolerated, with a comparable number of AEs and serious AEs between the groups (AEs: 69.4% AM, 69.4% PM vs 60.0% with placebo; serious AEs: 2.0% AM, 3.1% PM vs 1.2% with placebo). Discontinuations due to AEs were reported in 7.1% of patients in the lixisenatide AM group and in 5.5% of patients in the lixisenatide PM group, compared with 1.2% in the placebo group. The most common TEAE in the lixisenatide group was nausea (22.7% AM, 21.2% PM vs 7.6% with placebo), which generally occurred more frequently during the first 5 – 6 weeks. The majority of nausea events were mild-to-moderate in intensity, with no serious TEAEs reported. Vomiting occurred in 9.4% (AM) and 13.3% (PM) of patients in the lixisenatide group versus 2.9% of patients in the placebo group. Diarrhea was reported for 10.6% of both lixisenatide regimens compared with 8.8% of patients in the placebo group. Symptomatic hypoglycemia occurred in 2.4% (AM) and 5.1% (PM) of patients in the lixisenatide group, compared with 0.6% in the placebo group. There were no severe episodes.
GetGoal-F1 was a randomized, double-blind, placebo-controlled, 24-week study that compared lixisenatide administered in a 1-step or 2-step dose-increase regimen in 482 patients with T2DM Citation[41]. Lixisenatide was demonstrated to significantly reduce HbA1c levels at Week 24 (p < 0.0001) and enabled a significantly higher proportion of patients to achieve HbA1c ≤ 6.5% and < 7% (p < 0.001). Significant improvements in FPG and body weight were associated with lixisenatide treatment compared with placebo. The overall incidence of AEs was 70.8% (2-step), 67.7% (1-step) versus 65.6% (placebo), with serious AEs reported in 4.3% (2-step), 3.1% (1-step) versus 2.5% (placebo) of patients. There was one AE leading to death, which occurred in the lixisenatide 1-step group. Discontinuations due to AEs occurred in 8.1% of patients in the 2-step group, and 5.6% of patients in the 1-step group, compared with 2.5% of patients in the placebo group. The most frequent AE reported with lixisenatide was nausea (35.4% (2-step) and 26.1% (1-step) vs 4.4% with placebo) and vomiting (15.5% (2-step) and 11.8% (1-step) vs 0% with placebo). There was no significant difference in the incidence of symptomatic hypoglycemia in the lixisenatide groups compared with placebo (2.5% (2-step) and 1.9% (1-step) vs 0.6%, respectively), and no cases of severe hypoglycemia.
The GetGoal-X randomized, open-label study directly compared lixisenatide once-daily treatment with exenatide twice-daily treatment, in patients with T2DM inadequately controlled on metformin Citation[38]. A total of 634 patients were randomized to receive lixisenatide 20 μg QD (n = 318) or exenatide 10 μg BID (n = 316). Lixisenatide achieved its primary outcome of noninferiority in HbA1c reduction from baseline at Week 24. Both the proportion of patients achieving HbA1c < 7.0% and the level of FPG reduction were comparable between the two groups. Mean body weight decreased from baseline in both groups (94.5 to 91.7 kg with lixisenatide vs 96.7 to 92.9 kg with exenatide). The incidence of AEs and serious AEs were similar between lixisenatide and exenatide groups (AEs: 69.5% vs 72.2%; serious AEs: 2.8% vs 2.2%, for lixisenatide and exenatide, respectively). There were two deaths during the study – one in the lixisenatide group and one in the exenatide group. Discontinuations due to AEs were slightly lower in the lixisenatide group (10.4% vs 13.0% with exenatide) and were mainly due to GI events. Overall GI tolerability appeared to be slightly better for lixisenatide compared with exenatide, with fewer patients experiencing nausea (24.5% vs 35.1%, respectively). Vomiting occurred in 10.1% of patients in the lixisenatide group compared with 13.3% of patients in the exenatide group. Lixisenatide treatment was associated with a significantly lower incidence of symptomatic hypoglycemia, with six-fold fewer hypoglycemic events compared with exenatide. There were no cases of severe hypoglycemia. A higher proportion of patients in the lixisenatide group tolerated the targeted maintenance dose of 20 μg/day (93% vs 83% with exenatide). A total of 0.9% of patients in the lixisenatide group had injection-site reactions that led to discontinuation of study treatment, none of which were serious or considered severe by the investigator. No allergic reactions adjudicated as possibly related to treatment were reported in either of the treatment groups. A TEAE of blood calcitonin of ≥ 20 ng/l was reported for 0.3% of patients in each treatment group, with none of the events being serious or considered to be severe by the investigator, and no events of calcitonin ≥ 50 ng/l were reported. No clinically relevant changes in creatinine, aspartate aminotransferase and alanine aminotransferase were observed in either treatment group. No cases of pancreatitis were reported.
2.4.3 Lixisenatide in combination with pioglitazone
GetGoal-P assessed lixisenatide treatment in combination with pioglitazone with or without metformin Citation[34]. In this 24-week, double-blind study, 484 patients were randomized to receive either lixisenatide 20 µg QD (n = 323) or placebo (n = 161). Lixisenatide significantly reduced HbA1c compared with placebo (LS mean difference: –0.6%; p < 0.0001) and enabled a significantly higher proportion of patients to achieve HbA1c ≤ 6.5% (28.9% vs 10.1% with placebo) and < 7% (52% vs 26% with placebo). Lixisenatide was associated with a significant improvement in FPG (LS mean difference: –0.8 mmol/l vs placebo; p < 0.0001). There was a small reduction in body weight with lixisenatide (–0.2 kg) and a small increase with placebo (+0.2 kg), with no significant difference between the groups. The incidence of AEs and serious AEs was 72.4% and 2.5% with lixisenatide versus 72.7% and 1.9% with placebo, respectively. There were two deaths in the study, both in the placebo group. The most commonly reported AEs in the lixisenatide group were GI in nature, mostly nausea (23.5% vs 10.6% with placebo). Events of nausea were generally mild-to-moderate in intensity and usually occurred during the first week of treatment and in the week following each increase in dose. Diarrhea occurred in 7.1% of patients in the lixisenatide group versus 10.6% in the placebo group, with vomiting reported for 6.8% versus 3.7%, respectively. The occurrence of symptomatic hypoglycemia was low in both groups (3.4% with lixisenatide vs 1.2% with placebo), with no severe episodes being reported. Events of allergic reaction were reported for nine patients (2.8%) in the lixisenatide group versus three patients (1.9%) in the placebo group. Of these, three patients (0.9%) in the lixisenatide group reported five events that were adjudicated as possibly related to treatment. Injection-site reactions were reported for 6.8% of patients in the lixisenatide group and 5.0% in the placebo group, none of which were serious or severe in intensity, or led to permanent treatment discontinuation. No confirmed diagnoses of pancreatitis were reported in either treatment group.
2.4.4 Lixisenatide in combination with sulfonylurea
The efficacy and safety of lixisenatide in combination with sulfonylurea with or without metformin was assessed in a 24-week, randomized, double-blind, placebo-controlled study (GetGoal-S) Citation[42]. A total of 859 patients with T2DM were assigned to receive either lixisenatide 20 μg QD (n = 573) or placebo (n = 286) in a 2-step dose-increase regimen. Lixisenatide treatment was associated with a significant reduction in HbA1c (–0.9% vs –0.1% with placebo; p < 0.0001) and enabled a significantly higher proportion of patients to reach HbA1c < 7% (36.4% vs 13.5%; p < 0.0001). Patients treated with lixisenatide had significant decreases in PPG and FPG levels (p < 0.0001 for both), and experienced a significant reduction in body weight (–1.8 vs –0.9 kg with placebo; p < 0.0001). The overall incidence of AEs was 68.3% with lixisenatide versus 61.1% with placebo, with serious AEs reported for 3.5% versus 5.6% of patients, respectively. AEs leading to discontinuation of treatment occurred in 9.8% of patients in the lixisenatide group compared with 4.9% of patients in the placebo group. There was a higher incidence of AEs relating to GI events in the lixisenatide group, mainly nausea (25.3% vs 7.0% with placebo) and vomiting (8.7% vs 3.5% with placebo). The incidence of symptomatic hypoglycemia was 15.3% with lixisenatide compared with 12.3% with placebo. There was one case (0.2%) of severe hypoglycemia in the lixisenatide group and none in the placebo group.
2.4.5 Lixisenatide treatment in combination with basal insulin
The effects of lixisenatide treatment in combination with basal insulin glargine were evaluated in three separate studies: GetGoal-L-Asia, GetGoal-L and GetGoal-Duo1. GetGoal-L-Asia was a 24-week, randomized, double-blind, placebo-controlled study in Asian patients with T2DM inadequately controlled on basal insulin with or without sulfonylurea Citation[35]. A total of 311 patients from Japan, the Republic of Korea, Taiwan and the Philippines were randomized to receive either lixisenatide (n = 154) or placebo (n = 157). Lixisenatide treatment significantly reduced HbA1c levels compared with placebo (LS mean difference vs placebo: –0.9%; p < 0.0001) and enabled a significantly higher proportion of patients to achieve HbA1c ≤ 6.5% (17.8% vs 1.3%) and < 7% (35.6% vs 5.2%). Notable reductions in PPG levels were reported with lixisenatide treatment versus placebo (LS mean difference: –7.8 mmol/L, p < 0.0001). A possible explanation for the marked improvements in HbA1c and PPG levels observed in this study may be the difference in the pathophysiology of T2DM in Asian people, which is characterized by reduced insulin secretion rather than insulin resistance Citation[43], as well as a GLP-1 deficiency Citation[44]. FPG and glucose excursions were also significantly improved with lixisenatide versus placebo. Mean changes in body weight were small, with no significant differences between the two groups (LS mean change: –0.4 kg with lixisenatide vs +0.1 kg with placebo, p = 0.0857). AEs were reported for 89.0% of patients in the lixisenatide group versus 70.1% of patients in the placebo group. Serious AEs occurred in 6.5% of patients treated with lixisenatide and 5.7% of patients receiving placebo. A higher proportion of patients discontinued treatment due to AEs in the lixisenatide group (9.1% vs 3.2% with placebo), which were mainly GI in nature. There was a higher incidence of nausea and vomiting in the lixisenatide group compared with the placebo group (nausea: 39.6% vs 4.5%; vomiting: 18.2% vs 1.9%). The incidence of symptomatic hypoglycemia was higher in the lixisenatide group compared with placebo (42.9% vs 23.6%), but was similar between groups in patients not receiving sulfonylureas (32.6% vs 28.3%). There were no episodes of severe hypoglycemia reported. No pancreatitis was reported during this study. Injection-site reactions were reported for two patients (1.3%) in each of the treatment groups, with none of these reactions considered serious or severe or leading to treatment discontinuation. Seven possible allergic reactions were reported during the study (five events in the lixisenatide group and two events in the placebo group), with one of these events (urticaria, in the lixisenatide group) adjudicated as an allergic reaction possibly related to study medication.
In GetGoal-L, 495 patients (predominantly Caucasian (78%)) with T2DM inadequately controlled on basal insulin with or without metformin, were randomized 2:1 to receive either lixisenatide or placebo for 24 weeks Citation[37]. Lixisenatide treatment significantly improved HbA1c levels at Week 24 (–0.4% vs placebo) and enabled a significantly higher proportion of patients to reach target HbA1c levels of < 7% (28% vs 12% with placebo; p < 0.0001). PPG levels were significantly lower in the lixisenatide group compared with the placebo group at Week 24 (–3.8 mmol/l vs placebo; p < 0.0001). Reductions in body weight (–1.3 kg vs placebo; p < 0.0001) and insulin dosage (–3.7 units/day vs placebo; p = 0.012) were more marked in patients treated with lixisenatide. TEAEs occurred in 73.5% of patients in the lixisenatide group compared with 68.3% of patients in the placebo group, with serious TEAEs reported for 3.7% versus 4.2% of patients, respectively. There was one TEAE leading to death in the lixisenatide group. A total of 7.6% of lixisenatide-treatment patients and 4.8% of placebo-treated patients had a TEAE leading to discontinuation. The most frequent AEs with lixisenatide were GI in nature (40.2% vs 20.4% with placebo). Nausea occurred in 26.2% versus 8.4% of patients in the lixisenatide and placebo groups, respectively. The incidence of hypoglycemia was 28% with lixisenatide compared with 22% with placebo. Four patients (1.2%) in the lixisenatide group, and no patients in the placebo group, experienced severe hypoglycemia. Injection-site reactions were reported for four patients (1.2%) in the lixisenatide group compared with one patient (0.6%) in the placebo group. None of these events were considered severe by the investigator and no participant discontinued as a result. The occurrence of events adjudicated as allergic reactions was 1.5% in the lixisenatide group and 1.8% in the placebo group, three of which were considered to be possibly related to treatment (two events in the lixisenatide group vs one event in the placebo group). No confirmed cases of acute pancreatitis were reported.
GetGoal-Duo1 evaluated the effects of lixisenatide in combination with titrated basal insulin glargine in patients with T2DM inadequately controlled on metformin with or without sulfonylureas/thiazolidinediones Citation[36]. Sulfonylurea treatment was discontinued during the screening phase, after which insulin glargine was titrated during a 12-week run-in phase. Patients with a FPG of 80 – 100 mg/dl who had an HbA1c ≥ 7 and ≤ 9% were then randomized to lixisenatide (n = 223) or placebo (n = 223) for 24 weeks, during which time insulin glargine titration continued. Lixisenatide significantly reduced HbA1c compared with placebo (LS mean difference: –0.3%; p < 0.0001), and enabled more patients to achieve HbA1c < 7.0% (56% vs 39% with placebo). PPG levels were significantly improved in the lixisenatide group compared with the placebo group. There was a beneficial effect on body weight with lixisenatide treatment (LS mean difference vs placebo: –0.9 kg; p = 0.0012), despite insulin glargine titration. TEAEs occurred in 79.8% of patients in the lixisenatide group compared with 68.2% of patients in the placebo group, with serious TEAEs reported for 7.6% versus 4.5%, respectively. There were two TEAEs leading to death during the study, both in the placebo group. The most common AEs in the lixisenatide group were GI events, with a higher incidence of nausea and vomiting versus placebo (nausea: 27.4% vs 4.9%; vomiting: 9.4% vs 1.3%, respectively). Two participants in the lixisenatide group (0.9%) and one in the placebo group (0.4%) had an allergic reaction adjudicated as possibly related to study treatment. Fifteen patients (6.7%) in the lixisenatide group and five (2.2%) in the placebo group had injection-site reactions; two patients treated with lixisenatide discontinued for this reason. One patient in the placebo group had a TEAE of pancreatitis, compared with none in the lixisenatide group.
2.4.6 Lixisenatide versus liraglutide (Phase II study)
Lixisenatide once-daily (n = 77) was directly compared with liraglutide once-daily (n= 71) in a 28-day, randomized, open-label, Phase II study in patients with T2DM insufficiently controlled on metformin Citation[39]. The primary endpoint was change in PPG from baseline to Day 28. Lixisenatide significantly reduced PPG compared with liraglutide (mean change in ΔAUC0:30–4:30h: –12.6 vs –4.0 h·mmol/l, respectively; mean difference vs liraglutide: –8.6 h.mmol/l; p < 0.0001). Maximum PPG excursion also significantly decreased in the lixisenatide group (mean difference vs liraglutide: –2.5 mmol/l; p < 0.0001). FPG and HbA1c were reduced in both treatment groups, but with a significantly greater effect with liraglutide (FPG: –0.3 vs –1.3 mmol/l, p < 0.0001; HbA1c: –0.3 vs –0.5%, p < 0.01 with lixisenatide and liraglutide, respectively). Glucagon levels were lower with lixisenatide compared with liraglutide (mean difference: –21.4 h·ng/l; p < 0.05). The frequency of AEs was 58% for lixisenatide versus 73% for liraglutide, with no serious AEs reported during the study. AEs leading to treatment discontinuation occurred in 2.6% of patients in the lixisenatide group versus 2.8% in the liraglutide group. There was a lower incidence of GI disorders in the lixisenatide group (36.4% vs 46.5% with liraglutide). The frequency of nausea was similar between the two groups (22.1% with lixisenatide versus 22.5% with liraglutide), with a lower incidence of diarrhea in patients taking lixisenatide (2.6% vs 15.5% with liraglutide). There were no events of symptomatic hypoglycemia in either group.
2.4.7 Lixisenatide treatment in elderly patients
The effect of once-daily lixisenatide treatment in elderly (aged ≥ 65 years; n = 612) and very elderly (aged ≥ 75 years; n = 59) patients was evaluated in a pooled analysis of six Phase III studies (GetGoal-Mono, -M, -F1, -S, -L and -L-Asia) Citation[45]. Lixisenatide was effective and well tolerated in elderly and very elderly patients. HbA1c reductions were comparable in patients aged ≥ 65 years compared with patients aged < 65 years, with significantly greater reductions versus placebo in both age categories. The lixisenatide safety profile was comparable regardless of age (overall AEs: 72% (≥ 65 years) vs 69% (< 65 years) and 73% (≥ 75 years) and 69% (< 75%); GI events: 43% (≥ 65 years) vs 41% (< 65 years) vs 46% (≥ 75 years) vs 41% (< 75%)). There was no relevant difference in the incidence of symptomatic hypoglycemia between the different age groups. These results are particularly relevant as, owing to improved life expectancy, the number of elderly people with T2DM is increasing. Furthermore, owing to the increased number of comorbidities typically observed in elderly patients with T2DM, the efficacy and tolerability profile of lixisenatide may be particularly suitable for this patient group.
2.5 Beyond glycemic control
In addition to its role in lowering blood glucose levels, lixisenatide has a number of interesting properties that may be beneficial in the treatment of T2DM (). The data in this section is a combination of in vivo, in vitro, animal and human data.
Table 2. Biological actions of native GLP-1 and lixisenatide.
2.5.1 Cardioprotective effect
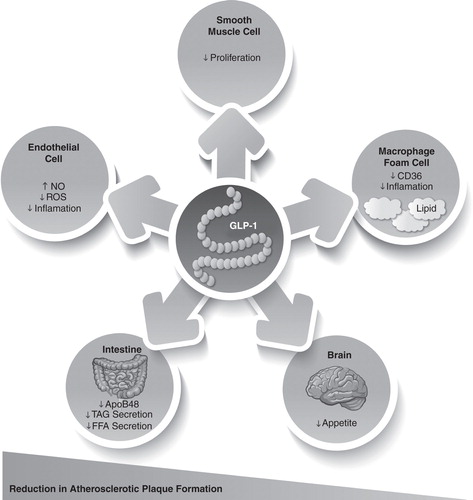
GLP-1 acts through a distinct heptahelical G-protein-coupled receptor, which has been located in beta-cells and the GI tract, as well as in the nervous system, heart, vascular smooth muscle cells, endothelial cells and macrophages Citation[46-49]. GLP-1 appears to modulate a wide range of physiological effects, not only regulating blood glucose and metabolic control, but also directly affecting several cardiovascular pathways that are involved in atherogenesis (). The binding of GLP-1 to the GLP-1 receptor in the myocardium leads to an increase in the production of cyclic adenosine monophosphate (cAMP) and the activation of protein kinase A (PKA), which results in an increase in glucose uptake and the inotropic effects in myocardial tissue. GLP-1 knockout mice exhibit reduced resting heart rate, elevated left ventricular and diastolic pressure and increased left ventricular thickness compared with normal controls Citation[46]. In agreement with these findings, treatment with GLP-1 or GLP-1 receptor agonists was found to improve left ventricular function Citation[15,50] and to reduce circulating levels of brain natriuretic peptide BNP; a biomarker for heart failure Citation[51,52].
Figure 2. GLP-1-mediated regulation of atherogenesis. GLP-1 directly targets endothelial cells, smooth muscle cells and macrophages to potentially reduce atherosclerotic plaque development.
Lixisenatide has been shown to protect against myocardial ischemia-reperfusion (I/R)-induced injury ex vivo, in Langendorff-perfused isolated rat hearts. In this study, I/R-induced injury was reduced by almost 40% with lixisenatide treatment compared with placebo Citation[53]. In an in vivo study performed in male Wistar rats, the effects of chronic lixisenatide treatment on cardiovascular outcomes were investigated, following I/R-induced injury of the left coronary artery Citation[54]. Lixisenatide significantly reduced I/R-induced injury by improving diastolic function, reducing myocardial relaxation and decreasing lung weight (a measure of congestion) compared with control. Lixisenatide treatment also significantly decreased ventricular wall thickness and lowered plasma BNP levels.
Compared with liraglutide, lixisenatide may differentiate with some effects on myocardial physiology Citation[39]. Supine heart rate decreased with lixisenatide by 3.6 beats per minute (bpm) and increased with liraglutide by 5.3 bmp over the 28-day treatment period (mean difference (95% CI): 8.9 bpm (–12.2, –5.6)), which is consistent with previous reports of an increase in heart rate with liraglutide Citation[55]. The mechanism underlying the differential effects on heart rate of these two agents remains elusive and requires further investigation.
There is increasing evidence of the direct effects of GLP-1 on endothelium and vascular smooth muscle cells () Citation[46,56-58]. In human vascular endothelial cells, GLP-1 receptor agonist treatment has been shown to cause endothelial nitric oxide synthase (eNOS) phosphorylation and potentiated eNOS activity, with increased nitric oxide (NO) production Citation[59]. In healthy nondiabetic subjects, GLP-1 enhanced the endothelium-mediated increase in forearm blood flow Citation[56]. In patients with T2DM and coronary artery disease, infusion of GLP-1 increased flow-mediated vasodilatation in the brachial artery, affirming a NO-dependent mechanism of GLP-1 in the vascular system Citation[60]. Treatment with GLP-1 receptor agonists has been consistently demonstrated to reduce blood pressure Citation[61-65]. Interestingly, the reduction of blood pressure in most studies occurred before weight reduction could be achieved. The mechanisms beyond the blood pressure-lowering effects of GLP-1 are still unclear, but in addition to changes in the release of adipokines from the adipose tissue, these may be attributed to direct vasodilatory effects Citation[46] and renal natriuretic effects Citation[66]. A recent metaanalysis assessing the cardiovascular outcomes of patients with T2DM treated with GLP-1 receptor agonists in clinical trials up to November 2010 revealed a significantly lower rate of major cardiovascular events in GLP-1 receptor agonist-treated patients compared with placebo-treated patients Citation[67]. It remains unclear, however, whether this reduction in cardiovascular morbidity was due to direct myocardial effects or to improvements in glycemic control.
Taken together, these studies indicate that both GLP-1 and lixisenatide may have additional cardiovascular benefits beyond their effects on glycemic control. Clinical studies investigating these CV effects are currently ongoing and include the Phase III ELIXA trial, which is due for completion in May 2014 Citation[68]. This randomized, double-blind, placebo-controlled study aims to assess whether lixisenatide treatment can improve cardiovascular outcomes in up to 6000 patients with T2DM, who have recently experienced an acute coronary syndrome (ACS) event.
2.5.2 Slowing of gastric emptying
Several studies have demonstrated that lixisenatide delays gastric emptying, which is a key mechanism in PPG reduction. In normoglycemic dogs, lixisenatide was shown to decrease plasma glucose excursions by up to 73% compared with control treatment. Paracetamol absorption was also significantly reduced with lixisenatide treatment, corresponding to a delay in gastric emptying Citation[69].
Lixisenatide has also been shown to delay gastric emptying within a clinical setting Citation[40]. In a 28-day double-blind study, patients with T2DM were randomized to lixisenatide (n = 19) or placebo (n = 22) in combination with up to two OADs. Lixisenatide was shown to significantly delay gastric emptying compared with placebo, which was accompanied by a significant reduction in PPG. In contrast to long-acting agents, the effects of delayed gastric emptying with short-acting agonists do not appear to be subject to tachyphylaxis Citation[70].
2.5.3 Protective effect on pancreatic beta-cells
T2DM is a progressive disease associated with deteriorating beta-cell function. An early laboratory marker for beta-cell dysfunction is an increase in circulating proinsulin levels. Although a disproportionate increase in the release of insulin and proinsulin indicates beta-cell dysfunction, elevated absolute proinsulin levels are associated with an increased risk for cardiovascular events in diabetic and nondiabetic subjects Citation[71-76]. Beta-cell workload increases after the ingestion of carbohydrate-containing meals, and recent studies suggest that an increase in proinsulin levels after beta-cell stimulation with an oral glucose load might be a more sensitive method to characterize beta-cell function Citation[77,78]. Several clinical studies have shown an improvement in the proinsulin/insulin ratio during treatment with GLP-1 receptor agonists Citation[79,80] and DPP-IV inhibitors Citation[81-83]. Recently, treatment with a DPP-IV inhibitor was shown to improve the proinsulin/insulin ratio and to lower the postprandial release of intact proinsulin in patients with T2DM, whereas treatment with a sulfonylurea worsened beta-cell function and increased the postprandial secretion of intact proinsulin Citation[84].
In vitro studies in the pancreatic beta-cell line INS-1 have demonstrated that lixisenatide is able to reduce cytokine- or fatty-acid-induced apoptotic activity by 50 – 60% Citation[85]. The protective effect of lixisenatide was further enhanced when used in combination with insulin analogs (in particular, insulin glargine), with apoptotic activity reduced by approximately 80%.
Lixisenatide was demonstrated to improve first-phase insulin secretion in a rodent model of diabetes Citation[86], as well as in healthy and diabetic subjects Citation[87]. In addition, lixisenatide was shown to reduce proinsulin levels compared with placebo treatment, in patients with T2DM inadequately controlled on sulfonylurea in the GetGoal-S study Citation[88].
2.5.4 Increased insulin mRNA expression and hormone secretion
A 90-day in vivo study in diabetic db/db mice has demonstrated that lixisenatide treatment is associated with increased pancreatic insulin mRNA expression compared with vehicle-control-treated mice Citation[28]. In a study performed in the isolated pancreas of normoglycemic Wistar rats Citation[86], lixisenatide treatment was shown to significantly augment glucose-stimulated insulin secretion compared with saline control, whereas insulin secretion was unaffected at low glucose levels.
3. Conclusions
Lixisenatide is a once-daily prandial GLP-1 receptor agonist for the treatment of T2DM. Lixisenatide has a unique pharmacokinetic and pharmacodynamic profile compared with the other GLP-1 receptor agonists. Despite its relatively short half-life, lixisenatide is suitable for once-daily dosing, partly due to its high affinity for the GLP-1 receptor as well as its ability to delay gastric emptying.
Clinical studies evaluating the effects of once-daily lixisenatide in monotherapy or in combination with OADs (metformin, sulfonylureas, thiazolidinediones) or basal insulin glargine demonstrate that lixisenatide improves glycemic control, is generally well tolerated and has a fairly low incidence of hypoglycemia. In addition, lixisenatide appears to have a beneficial effect on body weight, as it is either associated with no weight gain or weight loss.
Lixisenatide potentially has a number of additional benefits beyond glycemic control for the treatment of T2DM, including delayed gastric emptying, which is a key mechanism in PPG reduction. In contrast to long-acting agents, lixisenatide-mediated slowing of gastric emptying is not subject to tachyphylaxis, which occurs due to sustained activation of the GLP-1 receptor. Lixisenatide may also provide a protective effect to the cardiovascular system. As CVD is the main cause of death among patients with T2DM, the results of the ELIXA cardiovascular trial investigating lixisenatide treatment in patients with T2DM after ACS (due to be completed in 2014) will be of particular interest Citation[68]. Furthermore, preclinical and preliminary clinical data suggest that lixisenatide may protect pancreatic beta-cells and improve insulin secretory response patterns, resulting in the restoration of first- and second-phase insulin secretion, as well as reduced proinsulin levels.
In summary, lixisenatide is a promising addition to the treatment armamentarium for patients with T2DM. Lixisenatide has proven efficacy in a variety of patient subpopulations, as well as an acceptable tolerability profile. It offers the potential for a range of unique additional health benefits, as well as convenient administration owing to its once-daily dosing regimen.
4. Expert opinion
Incretin-based therapies such as DPP-IV inhibitors and GLP-1 receptor agonists have become an essential part of the pharmacological treatment for T2DM. In addition to providing effective metabolic control without increasing hypoglycemic events, treatment with GLP-1 receptor agonists has been shown to reduce body weight and to improve the overall cardiovascular risk profile in patients with T2DM.
GLP-1 acts through a distinct heptahelical G-protein-coupled receptor, which has been located not only in beta-cells and the GI tract, but also in several other cell systems that are not involved in metabolic regulation Citation[46-49]. GLP-1 appears to have distinct, physiological effects on endothelium and vascular smooth muscle cells. In both healthy nondiabetic subjects and patients with T2DM, GLP-1 was shown to improve the endothelium-mediated increase in forearm blood flow, affirming a NO-dependent mechanism of GLP-1 Citation[56]. In addition, treatment with GLP-1 or GLP-1 receptor agonists seems to improve left ventricular function and protect myocardial tissue in ischemia reperfusion models Citation[15,50].
T2DM is a progressive disease that is characterized by a steady decline in beta-cell function. A loss of first-phase insulin release following the ingestion of a meal and an increase in the release of intact proinsulin are early markers of deteriorating beta-cell function, preceding an overall breakdown in the synthetic capacity of the beta-cell with the development of relative insulin deficiency. Several clinical studies have shown an improvement in the release of insulin and a decline in the proinsulin/insulin ratio following treatment with a GLP-1 receptor agonist Citation[79,80] or DPP-IV inhibitor Citation[81-83]. Overall, these studies indicate an improvement in the functional capacity of the beta-cell during GLP-1-based treatments. Whether GLP-1 also improves beta-cell survival by increasing the differentiation of new beta-cells or decreasing their rate of apoptosis in humans is still a matter of debate. In addition to its effects on beta-cell function, GLP-1 appears to interact with alpha cells in the pancreatic islet, by suppressing the postprandial release of glucagon without affecting the glucagon response during hypoglycemia. As shown in , GLP-1-based treatments seem to restore overall islet-cell function by improving the regulation of glucagon and insulin secretion from alpha- and beta-cells.
Figure 3. Restoration of islet-cell function by glucagon-like peptide-1 (GLP-1). GLP-1 reduces islet-cell dysfunction by a number of mechanisms, including increasing insulin secretion from pancreatic beta-cells and restoring first-phase insulin secretory patterns. GLP-1 has also been shown to improve the proinsulin/insulin ratio, which is an early marker of beta-cell dysfunction.

Lixisenatide is a once-daily GLP-1 receptor agonist, which has been evaluated in a broad clinical study program. In these studies, lixisenatide was shown to have a favorable safety and tolerability profile and to improve metabolic control in monotherapy, as well as in combination with a number of different antidiabetic drugs. Treatment with lixisenatide improved blood glucose control without increasing the risk of hypoglycemia. In addition, lixisenatide had a beneficial effect on body weight in the majority of patients.
The unique pharmacological properties of lixisenatide clearly differentiate the molecule from other GLP-1 receptor agonists. As a once-daily agent with a high affinity to the GLP-1 receptor, it improves overall glycemic control, with especially strong effects on PPG excursions. Lixisenatide's postprandial effect is predominantly pronounced for the first meal following injection, but continues to be sustained throughout the day Citation[40]. These attributes encourage the application of lixisenatide in those patients with extensive postprandial glucose excursions, or in combination with other antidiabetic drugs that have prevailing effects on fasting glucose levels, such as long-acting insulin analogs. Ongoing trials, such as the ELIXA study, will provide further information on the overall effects of lixisenatide on the cardiovascular risk profile and mortality.
Declaration of interest
T Forst has received advisory and speaker honoraria from Sanofi. A Pfützner has received research grants, travel support, consultancy fees and/or speaker fees during the last five years from the following pharmaceutical companies with products related to the content of this article: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Novartis, NovoNordisk and Sanofi. This paper was supported by Sanofi. Editorial support was provided by H Brereton from Medicus International (London, UK).
Notes
Bibliography
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405-12
- Morrish NJ, Wang SL, Stevens LK, et al. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001;44(Suppl 2):S14-21
- Cubbon R, Kahn M, Kearney MT. Secondary prevention of cardiovascular disease in type 2 diabetes and prediabetes: a cardiologist's perspective. Int J Clin Pract 2008;62:287-99
- White JR. Economic considerations in treating patients with type 2 diabetes mellitus. Am J Health Syst Pharm 2002;59(Suppl 9):S14-17
- Shamseddeen H, Getty JZ, Hamdallah IN, Ali MR. Epidemiology and economic impact of obesity and type 2 diabetes. Surg Clin North Am 2011;91:1163-72, vii
- Fergusson LD, Sattar N. Reducing cardiovascular disease risk in type 2 diabetes: is the focus on glycaemia warranted? Diabetes Obes Metab 2012;15:387-91
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577-96
- van Dieren S, Czernichow S, Chalmers J, et al. Weight changes and their predictors amongst 11 140 patients with type 2 diabetes in the ADVANCE trial. Diabetes Obes Metab 2012;14:464-9
- Nauck MA, Vilsboll T, Gallwitz B, et al. Incretin-based therapies: viewpoints on the way to consensus. Diabetes Care 2009;32(Suppl 2):S223-31
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696-705
- Vilsboll T, Christensen M, Junker AE, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012;344:d7771
- Nauck MA. Incretin-based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. Am J Med 2011;124:S3-18
- Tourrel C, Bailbe D, Meile MJ, et al. Glucagon-like peptide-1 and exendin-4 stimulate beta-cell neogenesis in streptozotocin-treated newborn rats resulting in persistently improved glucose homeostasis at adult age. Diabetes 2001;50:1562-70
- Farilla L, Hui H, Bertolotto C, et al. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology 2002;143:4397-408
- Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 2004;109:962-5
- Gutzwiller JP, Goke B, Drewe J, et al. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut 1999;44:81-6
- Forst T, Michelson G, Ratter F, et al. Addition of liraglutide in patients with type 2 diabetes well controlled on metformin monotherapy improves several markers of vascular function. Diabet Med 2012;29:1115-18
- Kodera R, Shikata K, Kataoka HU, et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 2011;54:965-78
- Drucker DJ, Rosen CF. Glucagon-like peptide-1 (GLP-1) receptor agonists, obesity and psoriasis: diabetes meets dermatology. Diabetologia 2011;54:2741-4
- Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012;8:728-42
- Ceriello A, Colagiuri S, Gerich J, Tuomilehto J; Guideline Development Group. Guideline for management of postmeal glucose. Nutr Metab Cardiovasc Dis 2008;18:S17-33
- Schrot RJ. Targeting plasma glucose: preprandial versus postprandial. Clin Diabetes 2004;22:169-72
- Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2003;88:3082-9
- Eng J, Kleinman WA, Singh L, et al. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem 1992;267:7402-5
- Knudsen LB, Nielsen PF, Huusfeldt PO, et al. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem 2000;43:1664-9
- Malone J, Trautmann M, Wilhelm K, et al. Exenatide once weekly for the treatment of type 2 diabetes. Expert Opin Investig Drugs 2009;18:359-67
- Barnett AH. Lixisenatide: evidence for its potential use in the treatment of type 2 diabetes. Core Evid 2011;6:67-79
- Thorkildsen C, Neve S, Larsen BD, et al. Glucagon-like peptide 1 receptor agonist ZP10A increases insulin mRNA expression and prevents diabetic progression in db/db mice. J Pharmacol Exp Ther 2003;307:490-6
- Werner U, Haschke G, Herling AW, Kramer W. Pharmacological profile of lixisenatide: a new GLP-1 receptor agonist for the treatment of type 2 diabetes. Regul Pept 2010;164:58-64
- Liu Y-H, Ruus P. Pharmacokinetics and safety of the GLP-1 agonist AVE0010 in patient with renal impairment. Ann Meet Am Diabetes Assoc 2009;69:abstract 557-P
- Distiller L, Ruus PE. Pharmacokinetics and pharmacodynamics of GLP-1 agonist AVE0010 in type 2 diabetes patients. Ann Meet Am Diabetes Assoc 2008;68:abstract 520-P
- Fonseca VA, Alvarado-Ruiz R, Raccah D, et al. Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono). Diabetes Care 2012;35:1225-31
- Ahrén B, Dimas A, Miossec P, et al. Efficacy and safety of lixisenatide once daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M). Diabetes Care 2013;36:2543-50
- Pinget M, Goldenberg R, Niemoeller E, et al. Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P). Diabetes Obes Metab 2013; Epub ahead of print
- Seino Y, Min KW, Niemoeller E, Takami A; Investigators EG-LAS. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab 2012;14:910-17
- Riddle MC, Forst T, Aronson R, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). Diabetes Care 2013;36:2497-503
- Riddle MC, Aronson R, Home P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care 2013;36:2489-96
- Rosenstock J, Raccah D, Korányi L, et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care 2013; Epub ahead of print
- Kapitza C, Forst T, Coester HV, et al. Pharmacodynamic characteristics of lixisenatide once daily versus liraglutide once daily in patients with type 2 diabetes insufficiently controlled on metformin. Diabetes Obes Metab 2013;15:642-9
- Lorenz M, Pfeiffer C, Steinsträßer A, et al. Effects of lixisenatide once daily on gastric emptying in type 2 diabetes - Relationship to postprandial glycemia. Regul Pept 2013;185C:1-8
- Bolli G, Munteanu M, Dotsenko S, et al. Efficacy and safety of lixisenatide once-daily versus placebo in patients with type 2 diabetes insufficiently controlled on metformin (GetGoal-F1). Diabetic Medicine 2013; In press
- Ratner R, Hanefeld M, Shamanna P, et al. Efficacy and safety of lixisenatide once daily versus placebo in patients with T2DM insufficiently controlled on sulfonylurea + metformin (GetGoal-S) [abstract 785]. Diabetologia 2011;54:1-542
- Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract 2004;66(Suppl 1):S37-43
- Yabe D, Kuroe A, Lee S, et al. Little enhancement of meal-induced glucagon-like peptide 1 secretion in Japanese: comparison of type 2 diabetes patients and healthy controls. J Diabetes Invest 2010;1:56-9
- Raccah D, Miossec P, Esposito V, et al. Efficacy and safety of lixisenatide in elderly (≥65 yr) and very elderly (≥75 yr) patients with type 2 diabetes: an analysis from the GetGoal phase 3 program. Poster 972-P, presented at 72nd Scientific Sessions of the American Diabetes Association; 8 – 12 June 2012; Philadelphia, PA; 2012
- Ban K, Noyan-Ashraf MH, Hoefer J, et al. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 2008;117:2340-50
- Gardiner SM, March JE, Kemp PA, et al. Possible involvement of GLP-1(9-36) in the regional haemodynamic effects of GLP-1(7-36) in conscious rats. Br J Pharmacol 2010;161:92-102
- Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev 1999;20:876-913
- Tomas E, Habener JF. Insulin-like actions of glucagon-like peptide-1: a dual receptor hypothesis. Trends Endocrinol Metab 2010;21:59-67
- Sokos GG, Nikolaidis LA, Mankad S, et al. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail 2006;12:694-9
- Bergenstal RM, Wysham C, Macconell L, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 2010;376:431-9
- Courreges JP, Vilsboll T, Zdravkovic M, et al. Beneficial effects of once-daily liraglutide, a human glucagon-like peptide-1 analogue, on cardiovascular risk biomarkers in patients with Type 2 diabetes. Diabet Med 2008;25:1129-31
- Huber J, Janecek E, Meister S, et al. Cardioprotective effect of the GLP-1 receptor agonist lixisenatide on ischemia-reperfusion-induced injury in the isolated rat heart. Diabetes 2011;60:abstract A265
- Wohlfart P, Linz W, Linz D, et al. Cardioprotective effect of chronic treatment with lixisenatide in an in vivo rat model of myocardial ischaemia/reperfusion-induced injury. Presented at the 48th European Association for the Study of Diabetes Annual Meeting; 1 – 5 October 2012; Berlin, Germany; 2012
- Robinson LE, Holt TA, Rees K, et al. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open 2013;3; e001986
- Basu A, Charkoudian N, Schrage W, et al. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab 2007;293:E1289-95
- Golpon HA, Puechner A, Welte T, et al. Vasorelaxant effect of glucagon-like peptide-(7-36)amide and amylin on the pulmonary circulation of the rat. Regul Pept 2001;102:81-6
- Richter G, Feddersen O, Wagner U, et al. GLP-1 stimulates secretion of macromolecules from airways and relaxes pulmonary artery. Am J Physiol 1993;265:L374-81
- Hattori Y, Jojima T, Tomizawa A, et al. A glucagon-like peptide-1 (GLP-1) analogue, liraglutide, upregulates nitric oxide production and exerts anti-inflammatory action in endothelial cells. Diabetologia 2010;53:2256-63
- Nystrom T, Gutniak MK, Zhang Q, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 2004;287:E1209-15
- Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;374:39-47
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008;24:275-86
- Mafong DD, Henry RR. The role of incretins in cardiovascular control. Curr Hypertens Rep 2009;11:18-22
- Marre M, Shaw J, Brandle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet Med 2009;26:268-78
- Okerson T, Yan P, Stonehouse A, Brodows R. Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. Am J Hypertens 2010;23:334-9
- Gutzwiller JP, Tschopp S, Bock A, et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab 2004;89:3055-61
- Monami M, Cremasco F, Lamanna C, et al. Glucagon-like peptide-1 receptor agonists and cardiovascular events: a meta-analysis of randomized clinical trials. Exp Diabetes Res 2011;2011:215764
- ClinicalTrials Evaluation of cardiovascular outcomes in patients with type 2 diabetes after acute coronary syndrome during treatment with AVE0010 (Lixisenatide) (ELIXA). Available from: http://clinicaltrials.gov/show/NCT01147250 [Accessed 28 February 2013]
- Werner U, Gerlach M, Hofmann M, Herling AW. The GLP-1 receptor agonist AVE0010 abolishes OGTT-induced blood glucose excursion in healthy, normoglycemic dog without risk of hypoglycaemia [abstract 0486-P]. Poster presented at the 67th Scientific Session of the American Diabetes Association; 22 – 26 June 2007; Chicago, IL
- Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 2011;60:1561-5
- Alssema M, Dekker JM, Nijpels G, et al. Proinsulin concentration is an independent predictor of all-cause and cardiovascular mortality: an 11-year follow-up of the Hoorn Study. Diabetes Care 2005;28:860-5
- Zethelius B, Lithell H, Hales CN, Berne C. Insulin sensitivity, proinsulin and insulin as predictors of coronary heart disease. A population-based 10-year, follow-up study in 70-year old men using the euglycaemic insulin clamp. Diabetologia 2005;48:862-7
- Oh JY, Barrett-Connor E, Wedick NM. Sex differences in the association between proinsulin and intact insulin with coronary heart disease in nondiabetic older adults: the Rancho Bernardo Study. Circulation 2002;105:1311-16
- Wiberg B, Sundstrom J, Zethelius B, Lind L. Insulin sensitivity measured by the euglycaemic insulin clamp and proinsulin levels as predictors of stroke in elderly men. Diabetologia 2009;52:90-6
- Wohlin M, Sundstrom J, Arnlov J, et al. Impaired insulin sensitivity is an independent predictor of common carotid intima-media thickness in a population sample of elderly men. Atherosclerosis 2003;170:181-5
- Zethelius B, Byberg L, Hales CN, et al. Proinsulin is an independent predictor of coronary heart disease: report from a 27-year follow-up study. Circulation 2002;105:2153-8
- Forst T, Pfutzner A, Lubben G, et al. Effect of simvastatin and/or pioglitazone on insulin resistance, insulin secretion, adiponectin, and proinsulin levels in nondiabetic patients at cardiovascular risk–the PIOSTAT Study. Metabolism 2007;56:491-6
- Fritsche A, Madaus A, Stefan N, et al. Relationships among age, proinsulin conversion, and beta-cell function in nondiabetic humans. Diabetes 2002;51(Suppl 1):S234-9
- DeFronzo RA, Ratner RE, Han J, et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005;28:1092-100
- Vilsboll T, Brock B, Perrild H, et al. Liraglutide, a once-daily human GLP-1 analogue, improves pancreatic B-cell function and arginine-stimulated insulin secretion during hyperglycaemia in patients with type 2 diabetes mellitus. Diabet Med 2008;25:152-6
- Del Prato S, Barnett AH, Huisman H, et al. Effect of linagliptin monotherapy on glycaemic control and markers of beta-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab 2011;13:258-67
- Pratley RE, Schweizer A, Rosenstock J, et al. Robust improvements in fasting and prandial measures of beta-cell function with vildagliptin in drug-naive patients: analysis of pooled vildagliptin monotherapy database. Diabetes Obes Metab 2008;10:931-8
- Raz I, Hanefeld M, Xu L, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006;49:2564-71
- Forst T, Dworak M, Berndt-Zipfel C, et al. Effect of vildagliptin compared to glimepiride on postprandial proinsulin processing in the β cell of patients with type 2 diabetes mellitus. Diabetes Obes Metab 2013;15:576-9
- Tews D, Werner U, Eckel J. Enhanced protection against cytokine- and fatty acid-induced apoptosis in pancreatic beta cells by combined treatment with glucagon-like peptide-1 receptor agonists and insulin analogues. Horm Metab Res 2008;40:172-80
- Haschke G, Haag-Diergarten S, Werner U, et al. The GLP-1 receptor agonist AVE0010 preserves beta cell function and insulin secretion after a 6 week treatment in make obese Zucker diabetic fatty rats – an isolated perfusion pancreas study [abstract]. Diabetologia 2006;49:400-1
- Becker RH, Kapitza C, Stechl J, et al. Restitution of glucose disposition with lixisenatide in healthy subjects and patients with type 2 diabetes [abstract 813]. Diabetologia 2012;55:S1-S538
- Ratner RE, Hanefeld M, Shamanna P, et al. Post-meal pharmacodynamic profile of lixisenatide once daily vs placebo in T2DM insufficiently controlled on sulfonylurea±metformin (GetGoal-S) [abstract D-0743]. Poster presented at the International Diabetes Federation's 21st World Diabetes Congress; 5 – 8 December 2011; Dubai, United Arab Emirates; 2011
- Gutniak M, Orskov C, Holst JJ, et al. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med 1992;326:1316-22
- Nauck MA, Kleine N, Orskov C, et al. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993;36:741-4
- Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 2002;359:824-30
- Hui H, Nourparvar A, Zhao X, Perfetti R. Glucagon-like peptide-1 inhibits apoptosis of insulin-secreting cells via a cyclic 5'-adenosine monophosphate-dependent protein kinase A- and a phosphatidylinositol 3-kinase-dependent pathway. Endocrinology 2003;144:1444-55
- Wang Y, Perfetti R, Greig NH, et al. Glucagon-like peptide-1 can reverse the age-related decline in glucose tolerance in rats. J Clin Invest 1997;99:2883-9
- Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993;91:301-7
- Quddusi S, Vahl TP, Hanson K, et al. Differential effects of acute and extended infusions of glucagon-like peptide-1 on first- and second-phase insulin secretion in diabetic and nondiabetic humans. Diabetes Care 2003;26:791-8
- Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev 2012;33:187-215