Abstract
Nanoparticle albumin-bound (nab)-paclitaxel is paclitaxel linked to albumin nanoparticles, which makes it soluble and is an example of an application of nanotechnology for cancer treatment. The development of nanotechnology as a delivery system for nab-paclitaxel has improved the pharmacokinetics and pharmacodynamics of paclitaxel, in part by decreasing its hydrophobicity. Nab-paclitaxel in combination with gemcitabine has slightly improved survival in pancreatic cancer, compared to gemcitabine alone, as demonstrated in Phase III clinical trials. Cell cycle phase-specific drugs, such as nab-paclitaxel, which target cells in the G2/M phase of the cell cycle, can only have limited efficacy since the vast majority of cells in a tumor are quiescent in G0/G1 phase. Recent advances in our laboratory on how to decoy cancer cells to cycle and then trap them in a sensitive phase of the cell cycle, can, in the hopefully near future, allow drugs such as nab-paclitaxel to have high efficacy, even in a treatment-resistant tumor such as pancreatic cancer.
Pancreatic cancer is the most lethal cancer, with a survival rate of ∼ 5% at 5 years. Pancreatic cancer is the fourth most common cause of cancer-related death. Over half of the pancreatic cancer patients have metastatic disease at diagnosis, with an approximate median survival of 6 months Citation[1]. Gemcitabine is first-line treatment for locally advanced and metastatic pancreatic cancer, with slight or no increase in survival of the treated patients Citation[2,3].
Nanoparticle albumin-bound (nab)-paclitaxel (Abraxane®, Celgene, Summit, NJ) is a microtubule inhibitor, as is paxlitaxel. Nab-paclitaxel was developed to avoid the toxicities of polyoxyethylated castor oil solvent (Cremophor), which is used for paclitaxel because of poor aqueous solubility Citation[4]. Pharmacokinetics of Cremophor may result in hypersensitivity reactions, neutropenia, peripheral neuropathy and liver toxicity Citation[5-7], red blood cells lysis and microvesicle formation Citation[8]. Nab-paclitaxel is now used in the US as first-line combination therapy with gemcitabine for metastatic adenocarcinoma of the pancreas Citation[4].
The use of nab-paclitaxel, in combination with gemcitabine, was based on molecular profiling of pancreatic cancer tumors, which identified overexpression of the secreted protein, acidic and rich in cysteine, an albumin-binding protein Citation[2].
In pancreatic cancer patient-derived xenografts (PDX), the gemcitabine nab-paclitaxel combination resulted in more tumor regression than either drug alone Citation[2]. Pancreatic cancer PDX mice treated with vehicle or gemcitabine had highly desmoplastic stroma. In contrast, nab-paclitaxel treatment depleted the desmoplastic stroma, which contained dilated blood vessels vascularizing the tumor. The depleted stroma and the increased vascularization increased delivery of gemcitabine by 2.8-fold in the gemcitabine – nab-paclitaxel-treated tumors compared with gemcitabine-alone-treated mice Citation[2].
In operable pancreatic cancer, neoadjuvant nab-paclitaxel increased the rate of R0 surgery of the patients Citation[8,9].
In a Phase III clinical trial, pancreatic cancer patients with advanced disease were randomly assigned to nab-paclitaxel – gemcitabine (431 patients) or gemcitabine-alone (430). In the nab-paclitaxel–gemcitabine group, the median overall survival was 8.5 months compared to 6.7 months in the gemcitabine-alone group (hazard ratio for death, 0.72; 95% confidence interval [CI], 0.62 – 0.83; p < 0.001). The median progression-free survival was 5.5 months in the nab-paclitaxel–gemcitabine group, compared to 3.7 months in the gemcitabine-alone group (hazard ratio for disease progression or death, 0.69; 95% CI, 0.58 – 0.82; p < 0.001). Neutropenia and neuropathy were the common toxicities Citation[10]. A case of acute exacerbation of congestive heart failure in gemcitabine – nab-paclitaxel-treated pancreatic cancer patients has been observed Citation[3,11].
Pancreatic ductal adenocarcinomas is characterized by an extensive fibrosis as mentioned above. Provenzano et al. Citation[12] showed that the desmoplastic reaction of pancreatic cancer generates very high interstitial fluid pressures (IFP), inducing vascular collapse, inhibiting perfusion of small molecule therapeutics. These authors showed that systemic administration of the enzyme agent PEGPH20 can decrease stromal hyaluronic acid in mouse models of pancreatic cancer, reducing IFP and re-expanding the microvasculature. In combination with gemcitabine, PEGPH20 remodeled the tumor microenvironment and increased overall survival of mouse models with pancreatic cancer.
Based in part on the preclinical results described above, there is an ongoing clinical trial that compares the efficacy of PEGPH20 combined with nab-paclitaxel and gemcitabine (PAG) to nab-paclitaxel and gemcitabine (AG) in patients with stage IV pancreatic cancer (https://clinicaltrials.gov/ct2/show/study/NCT01839487).
Other current clinical trials include gemcitabine + nab-paclitaxel with LDE-225 (Hedgehog inhibitor) as neoadjuvant therapy for pancreatic adenocarcinoma (https://clinicaltrials.gov/ct2/show/NCT01431794?term=nab-paclitaxel&rank=1).
There is another clinical trial in which patients with metastatic pancreatic cancer are treated with nab-paclitaxel and gemcitabine in combination with necuparanib (M402), derived from heparin, a blood thinner. Blood thinners have been shown in previous animal and human studies to have anticancer effects. Necuparanib is derived from heparin and has lower blood thinning activity. The trial is testing whether necuparanib administered in combination with nab-paclitaxel and gemcitabine is more effective than the combination of nab-paclitaxel and gemcitabine (https://clinicaltrials.gov/ct2/show/NCT01621243?term=nab-paclitaxel&rank=2).
Positive results from such clinical trials as described above should increase the use of nab-paclitaxel in combination chemotherapy in pancreatic cancer. The combination could be used in the adjuvant and neo-adjuvant settings as well as for inoperable stage IV pancreatic cancer. Patient selection will depend in part on performance status since these combinations are potentially toxic.
1. Expert opinion
Nab-paclitaxel is a microtubule-stabilizing agent that enhances microtubule polymerization resulting in arrest in the G2/M phases of the cell cycle. Rapidly dividing cells are the main targets for this drug Citation[8].
1.1 The problem
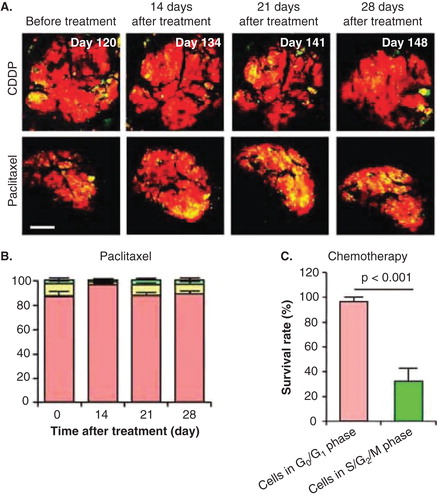
The phase of the cell-cycle can determine whether a cancer cell within a tumor can respond to a given drug. We previously monitored real-time cell-cycle dynamics of cancer cells throughout live tumors growing in mice using a fluorescence ubiquitination-based cell cycle indicator (FUCCI) before, during and after chemotherapy Citation[13]. Approximately 90% of cancer cells in the center and 80% of total cells of an established tumor are in G0/G1 phase (). Longitudinal real-time imaging demonstrated that cytotoxic agents, such as paclitaxel, killed only proliferating cancer cells at the surface and, in contrast, had little effect on quiescent cancer cells, which are the vast majority (). Moreover, resistant quiescent cancer cells restarted cycling after the cessation of chemotherapy. These results suggest why most drugs currently in clinical use, which target cancer cells in S/G2/M phase, including nab-paclitaxel, are mostly ineffective on solid tumors Citation[13]. Nab-paclitaxel is an M-phase drug being used to treat pancreatic cancer where M-phase cells are most probably a small minority in a typical tumor.
Figure 1. Cell-cycle phase distribution of cancer cells at the tumor surface and center. (A) fluorescence ubiquitination-based cell cycle indicator (FUCCI)-expressing MKN45 human stomach cancer cells were implanted directly in the liver of nude mice and imaged at day 35. (B) Histogram shows the cell cycle distribution in the tumor at day 35 after implantation. Histogram shows the distribution of FUCCI-expressing cells at different distances from the center. The vast majority of cancer cells are in G0/G1 below 120 μm from the tumor surface.
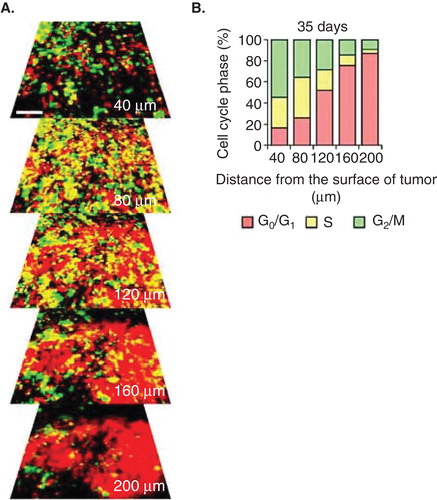
Figure 2. The efficacy of chemotherapy depends on the cell cycle-phase distribution within the tumor. (A) Representative images of a slowly-growing fluorescence ubiquitination-based cell cycle indicator (FUCCI)-expressing tumor in the liver before and after cisplatinum (CDDP) or paclitaxel treatment. (B) Histogram shows the cell cycle phase distribution within the tumor at the indicated time points after paclitaxel. (C) Histogram shows the survival rate of the cancer cells in G0/G1 phase or in S/G2/M phases after chemotherapy. The tumor is resistant to CDDP and paclitaxel because almost all the cells in the tumor are in G0/G1. Scale bars: 500 μm.
1.2 A solution
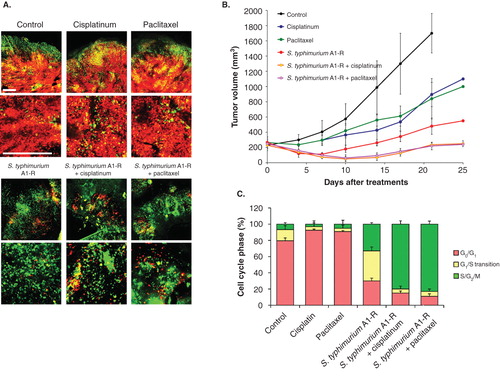
Time-lapse FUCCI imaging demonstrated that tumor-targeting Salmonella typhimurium A1-R decoyed FUCCI-expressing cancer cells in tumors growing in nude mice to cycle from G0/G1 to S/G2/M, thereby making them sensitive to cytotoxic agents. The combination of S. typhimurium A1-R and paclitaxel reduced tumor size compared with A1-R monotherapy or paclitaxel alone (). These results suggest a new paradigm of decoy chemotherapy of cancer Citation[14].
Figure 3. S. typhimurium A1-R-decoyed tumors became sensitive to chemotherapy. Fluorescence ubiquitination-based cell cycle indicator (FUCCI)-expressing MKN45 cells (5 × 106 cells/mouse) were injected subcutaneously into the left flank of nude mouse. When the tumors reached ∼ 8 mm in diameter (tumor volume, 300 mm3), mice were administered iv S. typhimurium A1-R alone, or with CDDP (4 mg/kg ip) or paclitaxel (5 mg/kg ip) for five cycles every 3 days. (A) Representative images of cross-sections of FUCCI-expressing MKN45 subcutaneous tumors, untreated control, S. typhimurium A1-R-treated, CDDP-treated, paclitaxel-treated or treated with the combination of S. typhimurium A1-R and either CDDP or paclitaxel. (B) Growth curves of tumors derived from FUCCI-expressing MKN45 cells after treatment with chemotherapy, S. typhimurium A1-R or the combination of S. typhimurium A1-R and chemotherapy. The difference between control and CDDP-treated, p < 0.01; the difference between control and paclitaxel-treated, p < 0.05; the difference between control and S. typhimurium A1-R-treated, p < 0.05; the difference between control and the combination of S. typhimurium A1-R and either CDDP or paclitaxel-treated, p < 0.01; the difference between S. typhimurium A1-R and the combination of S. typhimurium A1-R and either paclitaxel or CDDP-treated, p < 0.05. (C) Histogram shows cell cycle phase of FUCCI-expressing MKN45 subcutaneous tumors, untreated control, S. typhimurium A1-R-treated, CDDP-treated, paclitaxel-treated, or the combination of S. typhimurium A1-R and either cisplatinum or paclitaxel-treated. These results show that S.typhimurium A1-R decoy of the cancer cells to cycle sensitizes the cancer cells to CDDP and paclitaxel. Scale bars: 500 μm.
Thus far, nab-paclitaxel has shown a limited benefit. If tumors can be cell-cycle decoyed by an agent, it is possible that nab-paclitaxel will have more efficacy against pancreatic cancer. It is possible that different agents can act as cell-cycle decoys on tumors. Currently, in addition to tumor-targeting S. typhimurium A1-R, the tumor-targeting adenovirus OBP-301 has been shown to be a tumor cell-cycle decoy agent Citation[15].
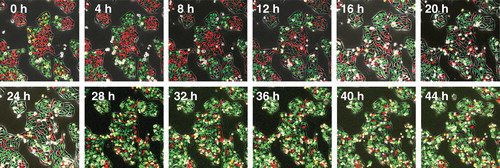
Cancer cells can also be selectively trapped in S/G2 by methionine depletion such as by recombinant methioninase (rMETase) () Citation[16,17]. With this new paradigm of treatment, the decoyed-trapped cancer cells should become highly sensitive to nab-paclitaxel as they synchronously enter M-phase upon release of the trap by repletion of methionine Citation[18].
Figure 4. Time-lapse imaging of FUCCI-expressing HeLa cells treated with rMETase being trapped in S/G2 phase. After seeding on 35 mm glass dishes and culture over night, FUCCI-expressing HeLa cells were treated with rMETase at a dose of 1.0 unit/ml. The cells in G0/G1, S or G2/M phases appear red, yellow or green, respectively. These results show that rMETase treatment traps cancer cells in S/G2, a chemo-sensitive phase of the cell-cycle. Scale bar: 50 μm.
Declaration of interest
The work related to this paper done in the authors' laboratory was supported in part by National Cancer Institute grant CA132791. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
Bibliography
- Tarver T. Cancer Facts & Figures 2012. American Cancer Society (ACS). J Consum Health Internet 2012;16(3):366-7
- Von Hoff D, Ramanathan R, Borad M, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a Phase I/II Trial. J Clin Oncol 2011;29(34):4548-54
- John P, Butler H, Saif MW. Congestive heart failure secondary to gemcitabine nab-paclitaxel in patients with pancreatic cancer. Anticancer Res 2014;34(12):7267-70
- Hoy SM. Albumin-bound paclitaxel: a review of its use for the first-line combination treatment of metastatic pancreatic cancer. Drugs 2014;74(15):1757-68
- Authier N, Gillet JP, Fialip J, et al. Assessment of neurotoxicity following repeated cremophor/ethanol injections in rats. Neurotox Res 2001;3(3):301-6
- Postma TJ, Vermorken JB, Liefting AJ, et al. Paclitaxel-induced neuropathy. Ann Oncol 2004;6(5):484-94
- Brat DJ, Windebank AJ, Brimijoin S. Emulsifier for intravenous cyclosporine inhibits neurite outgrowth, causes deficits in rapid axonal transport and leads to structural abnormalities in differentiating N1E.115 neuroblastoma. J Pharmacol Exp Ther 1992;261(2):803-10
- Viúdez A, Ramírez N, Hernández-García I, et al. Nab-paclitaxel: a flattering facelift. Crit Rev Oncol Hematol 2014;92(3):166-80
- Alvarez R, Musteanu M, Garcia-Garcia E, et al. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br J Cancer 2013;109(4):926-33
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369(18):1691-703
- Senkus E, Jassem J. Cardiovascular effects of systemic cancer treatment. Cancer Treat Rev 2011;37(4):300-11
- Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21(3):418-29
- Yano S, Zhang Y, Miwa S, et al. Spatial-temporal FUCCI imaging of each cell in a tumor demonstrates locational dependence of cell cycle dynamics and chemoresponsiveness. Cell Cycle 2014;13(13):2110-19
- Yano S, Zhang Y, Zhao M, et al. Tumor-targeting Salmonella typhimurium A1-R decoys quiescent cancer cells to cycle as visualized by FUCCI imaging and become sensitive to chemotherapy. Cell Cycle 2014;13(24):3683-8
- Yano S, Tazawa H, Hashimoto Y, et al. A genetically engineered oncolytic adenovirus decoys and lethally traps quiescent cancer stem-like cells into S/G2/M phases. Clin Cancer Res 2013;19(23):6495-505
- Hoffman RM, Jacobsen SJ. Reversible growth arrest in simian virus 40-transformed human fibroblasts. Proc Natl Acad Sci USA 1980;77(12):7306-10
- Yano S, Li S, Han Q, et al. Selective methioninase-induced trap of cancer cells in S/G2 phase visualized by FUCCI imaging confers chemosensitivity. Oncotarget 2014;5(18):8729-36
- Stern PH, Hoffman RM. Enhanced in vitro selective toxicity of chemotherapeutic agents for human cancer cells based on a metabolic defect. J Natl Cancer Inst 1986;76(4):629-34
