Abstract
Objective: To assess the safety and efficacy of long-term administration of teneligliptin alone and in combination with oral antidiabetic drugs in Japanese type 2 diabetes mellitus (T2DM) patients with insufficient glycemic control.
Methods: This post-hoc pooled analysis used data from two Phase III clinical studies involving 702 Japanese patients. We evaluated teneligliptin as monotherapy and combined with a sulfonylurea, glinide, biguanide, or α-glucosidase inhibitor. Safety measures included adverse events (AEs), adverse reactions and hypoglycemia. The main efficacy measure was the change in glycated hemoglobin (HbA1c) from baseline.
Results: Incidences of AEs and adverse reactions were similar among the teneligliptin monotherapy group and all combination therapy groups except the combination with sulfonylurea. Hypoglycemia was more frequent in the sulfonylurea combination therapy group than in other groups. Teneligliptin administered once daily as monotherapy or combination therapy resulted in a decrease in HbA1c, which was maintained for 52 weeks. Bodyweight showed no change or a slight increase at the end of 52 weeks in all groups.
Conclusions: This pooled analysis provides evidence for the safety and efficacy of long-term use of teneligliptin as monotherapy or combination therapy in Japanese T2DM patients.
1. Introduction
The incretin hormones, released by the small intestine in response to a meal, have been shown to improve glycemic control. One such incretin, glucagon-like peptide-1 (GLP-1), is released from intestinal L cells and plays a critical role in the regulation of postprandial glucose levels by stimulating insulin secretion and inhibiting glucagon secretion in a glucose-dependent manner. However, GLP-1 is rapidly degraded by dipeptidyl peptidase-4 (DPP-4). Therefore, DPP-4 inhibitors were developed to increase endogenous intact GLP-1 levels, and hence, lower blood glucose levels Citation[1-4]. In Japan, seven DPP-4 inhibitors are approved Citation[5] and can be used in a broad range of patients with type 2 diabetes mellitus (T2DM). The DPP-4 inhibitor teneligliptin was approved for the treatment of T2DM in Japan in 2012 and in Korea in 2014 Citation[6,7].
There are some differences in the structural and pharmacokinetic properties of DPP-4 inhibitors. An X-ray crystallography study Citation[8] revealed that DPP-4 inhibitors can be categorized into three classes on the basis of their binding subsites of the DPP-4 molecule: i) vildagliptin and saxagliptin (Class 1) form interactions with the core S1 and S2 subsites and a covalent bond with Ser630 in the catalytic triad; ii) alogliptin and linagliptin (Class 2) form interactions with the S1’ and/or S2’ subsites in addition to the S1 and S2 subsites; and iii) sitagliptin and teneligliptin (Class 3) form interactions with the S1, S2, and S2 extensive subsites. Teneligliptin binds to the S2 extensive subsites via an ‘anchor lock domain’, and this interaction may be related to the potency of inhibition, the residence time for binding to DPP-4, and the long duration of action in vivo Citation[8]. Regarding the pharmacokinetic properties, the elimination route differs among DPP-4 inhibitors, and these differences are related to the need for dose adjustments in patients with renal impairment in particular. For example, dose adjustments in patients with renal impairment are not necessary for linagliptin and teneligliptin, but are needed for other DPP-4 inhibitors (sitagliptin, vildagliptin, alogliptin, saxagliptin, and anagliptin) Citation[5].
Teneligliptin has a half-life of 24.2 h, with resulting DPP-4 inhibition throughout the day, and suppression of postprandial hyperglycemia after all three daily meals Citation[6,9]. In addition to the effect on postprandial hyperglycemia, a study of Japanese T2DM patients treated for 3 days with teneligliptin showed that it improved the mean, standard deviation (SD), and mean amplitude of glycemic excursions of 24-h glucose levels Citation[10]. These results suggest that teneligliptin improves glucose fluctuations throughout the day. Clinical studies in Japanese patients have examined the efficacy and safety of teneligliptin as monotherapy Citation[11], or as add-on therapy to pioglitazone Citation[12] or glimepiride Citation[13].
Drug administration in patients with diabetes is usually performed over extended periods, so the evaluation of safety and efficacy in long-term use is important. In addition, differences in the efficacy of DPP-4 inhibitors have been observed between Asian and non-Asian or Japanese and non-Japanese patients Citation[14,15]. Because of these observed differences between populations, data from Japanese patients are important to evaluate the utility of DPP-4 inhibitors in the Japanese setting. We therefore performed a post-hoc pooled analysis using data from two Japanese clinical studies to examine the safety and efficacy of teneligliptin in long-term administration (52 weeks) alone and in combination with a sulfonylurea, glinide, biguanide, or α-glucosidase inhibitor in Japanese T2DM patients with inadequately controlled blood glucose levels. In addition, a post-hoc evaluation of safety and efficacy was performed according to patient background factors, which will provide useful information to support treatment strategies in a real-world clinical practice. This is because treatment strategies in diabetes patients may vary depending on patient background factors such as the disease condition, age, extent of metabolic abnormality, and status of diabetic complications Citation[16].
2. Patients and methods
This post-hoc analysis used pooled data from two Phase III clinical trials in Japanese patients (N = 702). These trials were a long-term, open-label study of teneligliptin both as monotherapy and combined with a sulfonylurea (3000-A8) (N = 240), and a long-term, open-label study of teneligliptin both as monotherapy and combined with a glinide, biguanide, or α-glucosidase inhibitor (3000-A14) (N = 462).
Because the two studies shared a similar design, including the teneligliptin dose (20 mg, titrated to 40 mg if no adequate efficacy is obtained), treatment period (52 weeks), inclusion and exclusion criteria, and the method of evaluation of safety and efficacy, it was considered possible to evaluate the pooled data for safety and efficacy, therefore achieving a more comprehensive overview by increasing the number of patients evaluated. Japanese T2DM patients with poorly controlled blood glucose levels were the target group. Endpoints evaluated in the present post-hoc analysis were safety (adverse events [AEs], adverse reactions, and hypoglycemia) and efficacy (glycated hemoglobin [HbA1c] and bodyweight).
The first study included in this pooled analysis was a long-term study of teneligliptin both as monotherapy and in combination with a sulfonylurea (3000-A8). As glimepiride is the most commonly used sulfonylurea in Japan, it was selected as the concomitant drug in this study. The study comprised a 4-week run-in period, during which placebo was administered orally before breakfast, followed by a 52-week period in which 20 mg teneligliptin was administered orally 30 min before breakfast. If HbA1c was ≥ 7.3% at and after week 24 of the treatment period, and there was no safety problem as judged by the investigator, the teneligliptin dose was increased from 20 to 40 mg. If hypoglycemia occurred or if fasting blood glucose was ≤ 70 mg/dl in the glimepiride concomitant therapy group, the dose was decreased by 1 mg/day at a time, at the discretion of the investigator.
The second study was a long-term study of teneligliptin both as monotherapy and in combination with a glinide, biguanide, or α-glucosidase inhibitor (3000-A14). The study comprised a 4-week run-in period, during which placebo was administered orally once daily, followed by a 52-week period in which 20 mg teneligliptin was administered orally once daily. If HbA1c was ≥ 7.4% at and after week 24 of the treatment period, and there was no safety problem as judged by the investigator, the teneligliptin dose was increased from 20 to 40 mg. If hypoglycemia occurred or if fasting blood glucose was ≤ 70 mg/dl in the glinide concomitant therapy group, the dose was decreased at the discretion of the investigator. The biguanide or α-glucosidase inhibitor doses were not adjusted during the study.
In both studies, HbA1c was measured by high-performance liquid chromatography using a reference standard approved by the US National Glycohemoglobin Standardization Program. Both studies were carried out in compliance with Good Clinical Practice. The protocols were approved by institutional review boards at each participating site. Written informed consent was obtained from all patients before enrolment. These trials were registered with ClinicalTrials.gov (nos. NCT02314637 and NCT01301833 for 3000-A8 and 3000-A14, respectively).
2.1 Statistical analysis
Safety analyses were performed on the safety population, which included all enrolled patients who received at least one dose of study medication and had at least one post-dose safety assessment. Efficacy analyses were performed on the full analysis set, which included all enrolled T2DM patients who received at least one dose of study medication, and had at least one post-dose efficacy assessment. Numerical values are indicated as mean ± SD. For efficacy data (HbA1c and bodyweight), missing data at week 52 were imputed using the last observation carried forward (LOCF) method. All the tests were performed by two-sided and paired t-test. The level of significance was set at 5%.
3. Results
3.1 Patient disposition and baseline characteristics
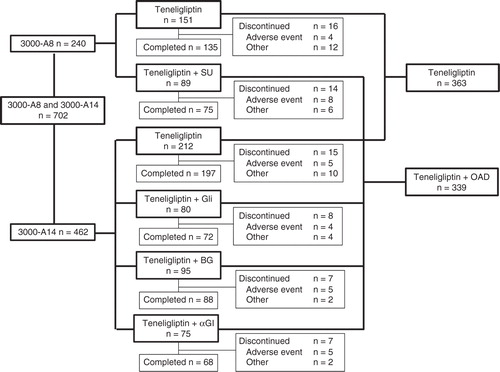
A total of 702 patients were enrolled and all patients were included in both the safety population and full analysis set. The disposition of patients is shown in . In this pooled analysis, there were 702 patients overall, with 363 in the teneligliptin monotherapy group and 339 in all combination therapy groups. The characteristics of patients are described in and were not remarkably different across groups.
Table 1. Patient characteristics at baseline.
3.2 Safety
Of the 702 patients who took at least one dose of teneligliptin during the 52-week treatment period, 619 (88.2%) had AEs, and 69 (9.8%) had adverse drug reactions (ADRs) (). The incidences of AEs and ADRs were similar to those of the teneligliptin monotherapy group (88.2 and 8.5%, respectively) in all combination therapy groups (88.2 and 11.2%, respectively). However, ADRs were more frequent in the sulfonylurea combination therapy group (18.0%), because of the increased incidence of hypoglycemia. The incidence rates of serious AEs were similar between the teneligliptin monotherapy group (5.5%) and all combination therapy groups (6.5%) (). Thyroid cancer was observed in one patient in the teneligliptin monotherapy group, ileus was observed in one patient in the glinide combination therapy group, hemorrhoids were observed in one patient in the biguanide combination therapy group, uterine cancer and Mallory–Weiss syndrome were observed in one patient in the α-glucosidase inhibitor combination therapy group, and testicular neoplasm was observed in one patient in the sulfonylurea combination therapy group. These were classified as serious ADRs.
Table 2. Summary of adverse events in the teneligliptin treatment groups.
Hypoglycemic events were reported infrequently in all groups except the sulfonylurea combination therapy group. All episodes of hypoglycemia were classified as mild in severity. No hypoglycemic events leading to discontinuation occurred in any of the groups during the study. Hypoglycemia occurred in nine patients (2.5%) in the teneligliptin monotherapy group, four patients (5.0%) in the glinide combination therapy group, one patient (1.1%) in the biguanide combination therapy group, one patient (1.3%) in the α-glucosidase inhibitor combination therapy group, and nine patients (10.1%) in the sulfonylurea combination therapy group. Similar to other DPP-4 inhibitors Citation[17], the incidence of hypoglycemia was higher in the sulfonylurea combination therapy group than in the other combination therapy groups. The doses of glimepiride used in study 3000-A8 were 1, 2, 3 and 4 mg. Hypoglycemia was observed in 2/36 (5.6%), 5/25 (20.0%), 1/19 (5.3%), and 1/9 (11.1%) patients in the 1, 2, 3 and 4 mg dosage groups, respectively. The AEs that occurred in ≥ 5% of patients in any group are listed in the Supplementary Table.
3.3 Efficacy
Teneligliptin administered once daily as monotherapy or combination therapy resulted in a decrease in HbA1c, which was maintained for 52 weeks (). The changes in HbA1c (mean ± SD) from baseline to week 52 (LOCF) were −0.63 ± 0.65% in the teneligliptin monotherapy group, −0.76 ± 0.70% in the glinide combination therapy group, −0.78 ± 0.75% in the biguanide combination therapy group, −0.89 ± 0.64% in the α-glucosidase inhibitor combination therapy group, and −0.81 ± 0.76% in the sulfonylurea combination therapy group. HbA1c was significantly lower at week 52 (LOCF) than at baseline in all groups (p < 0.001).
Figure 2. Time-course of HbA1c (A) and bodyweight (B) in Japanese patients receiving teneligliptin monotherapy and combination therapy. Data are expressed as means ± SD.
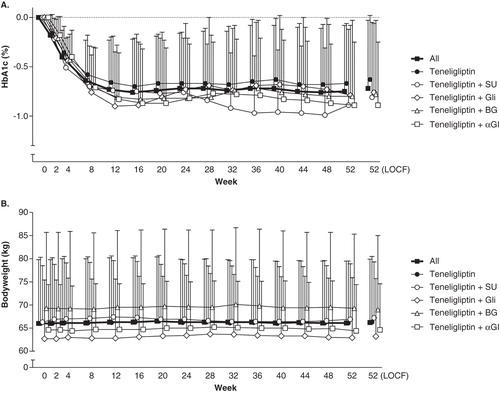
The changes in bodyweight (mean ± SD) from baseline to week 52 (LOCF) were 0.3 ± 2.1 kg in the teneligliptin monotherapy group (p < 0.01), 0.5 ± 2.0 kg in the glinide combination therapy group (p < 0.05), −0.3 ± 2.4 kg in the biguanide combination therapy group, −0.1 ± 2.0 kg in the α-glucosidase inhibitor combination therapy group, and 0.5 ± 1.7 kg in the sulfonylurea combination therapy group (p < 0.01) ().
3.4 Subgroup analyses of safety and efficacy by patient background characteristics
To provide practical information that supports treatment strategies, we performed a post-hoc analysis of the safety and efficacy of teneligliptin in patient subgroups according to various background characteristics. The incidences of overall AEs and ADRs were not markedly different across treatment groups subdivided on the basis of age (< 65 and ≥ 65 years), duration of diabetes (< 5, 5 – < 10 and ≥ 10 years), renal function (creatinine clearance ≥ 80, 50 – < 80, and 30 – < 50 ml/min), and hepatic function (alanine aminotransferase ≤ 30 and ≥ 31 IU/l) ( and ). Because only a few patients with creatinine clearance < 50 ml/min and none with creatinine clearance < 30 ml/min were enrolled, analysis of safety in patients with moderate or severe renal impairment could not be carried out in detail.
Table 3. Summary of adverse events based on patient background factors: age and duration of diabetes.
Table 4. Summary of adverse events based on patient background factors: renal and hepatic function.
HbA1c decreased significantly from baseline to week 52 (LOCF) in all subgroups divided on the basis of background characteristics, and did not markedly differ among age groups, baseline body mass index (BMI), duration of diabetes, baseline low- and high-density lipoprotein cholesterol, triglycerides, systolic and diastolic blood pressure, creatinine clearance, and alanine aminotransferase. Reductions in HbA1c (LOCF) were dependent on the baseline values, with reductions of −0.26 ± 0.30% for HbA1c < 7.0% at baseline, −0.57 ± 0.47% for HbA1c 7.0 – < 8.0% at baseline, and −1.02 ± 0.87% for HbA1c > 8.0% at baseline ().
Table 5. Time-course of HbA1c measurements based on patient background factors.
4. Discussion
The present pooled analysis found that long-term use of teneligliptin as monotherapy or combination therapy was well tolerated and significantly improved hyperglycemia in Japanese patients with T2DM. In the present pooled analysis, the incidence of hypoglycemia was higher in the sulfonylurea-drug combination therapy group than in other combination therapy groups. Similar observations were previously reported when sulfonylureas were used in combination with other DPP-4 inhibitors Citation[17]. After the launch of DPP-4 inhibitors in Japan, cases of severe hypoglycemia were reported. Although the causality was not certain, it appeared that their use in combination with sulfonylureas was a risk factor. In light of this, in 2010, the Committee for appropriate use of incretin-related drugs (GLP-1 receptor agonists and DPP-4 inhibitors) recommended lowering the dose of three major sulfonylureas (glimepiride, glibenclamide, and gliclazide) when adding a DPP-4 inhibitor. Following the release of this recommendation, the rates of severe hypoglycemia with DPP-4 inhibitors in combination with sulfonylureas decreased Citation[18]. The recommendation states that the dose of glimepiride should be < 2 mg when used in combination with DPP-4 inhibitors. The 3000-A8 study was performed before the release of the recommendation, and therefore, not only 1 and 2 mg but also 3 and 4 mg glimepiride doses were used. Although rates of hypoglycemic events may have decreased following the recommendation, it remains important to exercise caution if teneligliptin is concomitantly administered with a sulfonylurea.
Differences in the efficacy of DPP-4 inhibitors have been observed between Asian and non-Asian or Japanese and non-Japanese patients Citation[14,15]. Because of these observed differences between populations, data from Japanese patients are important to evaluate the utility of DPP-4 inhibitors in the Japanese setting. The current pooled analysis revealed that the HbA1c-lowering effect of teneligliptin monotherapy and combinations with other drugs was sustained throughout the 52-week treatment period. HbA1c was significantly lower at week 52 than at baseline in all groups. DPP-4 inhibitors have a neutral or positive effect on bodyweight Citation[14,15]. We observed a tendency toward weight gain of 0.3 – 0.5 kg with monotherapy or combination therapy with a sulfonylurea or glinide at the end of 52 weeks; these differences, albeit small, were statistically significant at the end of 52 weeks. However, no definite tendency for weight gain was observed in the biguanide or α-glucosidase inhibitor combination therapy groups. Overall, there were no clinically significant changes in bodyweight throughout the 52-week period in any groups.
Previous studies have shown that higher baseline HbA1c, lower BMI and shorter duration of diabetes are related to reductions in HbA1c when using DPP-4 inhibitors in T2DM patients Citation[14,19]. Teneligliptin also produced greater HbA1c reductions with higher baseline HbA1c, and slightly improved HbA1c in patients with lower BMI (< 25 kg/m2), although these patients had lower baseline HbA1c compared with patients with BMI ≥ 25 kg/m2 (7.83 ± 0.73 vs 7.94 ± 0.81%, respectively). Meanwhile the reduction in HbA1c tended to be increased in the group with a longer duration of diabetes, but baseline HbA1c levels were also higher in this group. Therefore, there was no clear evidence that the duration of diabetes influences efficacy in these populations. Further studies are needed to explore these findings.
We found no relationships between age, duration of diabetes, renal or hepatic dysfunction, and the onset of AEs or ADRs. However, our study included a limited number of patients with moderate or severe renal dysfunction, and patients with severe renal dysfunction were not examined specifically. Teneligliptin is eliminated via both hepatic metabolism and renal excretion Citation[6,20]. Hepatic impairment was associated with increased exposure to teneligliptin, but within FDA boundaries for requiring dose adjustment Citation[21]. The pharmacokinetics of teneligliptin have also been evaluated in patients with severe renal impairment. Patients with mild, moderate, severe or end-stage renal disease have been shown to tolerate teneligliptin, and dialysis was not expected to affect the drug’s efficacy or safety Citation[22]. Thus, these results indicate that, although caution is required, dose adjustments may not be necessary in patients with impaired renal or hepatic function. Furthermore, teneligliptin at a dose of 20 mg appeared to be well tolerated, and to significantly improve glycemic control in diabetic patients with end-stage renal disease Citation[23]. Because renal disease is a common complication in patients with T2DM, it will be necessary to continuously collect information regarding safety in patients with renal dysfunction.
There are several limitations to this pooled analysis. First, there was no placebo group and the study was not double-blinded. Second, the sample size was relatively small. Several results may have been more significant or clinically useful with a larger sample size. Third, the present studies excluded patients with a high risk of cardiovascular disease (CVD), such as patients who were diagnosed with CVD within 6 months of enrolment, patients with heart failure (New York Heart Association functional classification ≥ III), and patients with severe diabetic complications. Prior studies have examined the long-term safety of saxagliptin (SAVOR-TIM53) Citation[24] and alogliptin (EXAMINE) Citation[25] in patients with a high risk of CVD. However, further studies are needed to examine the cardiovascular safety of teneligliptin in patients with a high risk of CVD. Fourth, in patients receiving teneligliptin combination therapy, this analysis did not address the effects of important antihyperglycemic agents such as insulin, thiazolidinedione and sodium glucose co-transporter 2 inhibitors, although the results of short-term and placebo controlled studies have been previously reported Citation[12,26,27]. These safety and efficacy results will help clinicians determine the optimal use of teneligliptin in Japanese patients with T2DM.
5. Conclusion
This pooled analysis provides evidence of the safety and efficacy of the long-term use of teneligliptin as monotherapy or in combination therapies with a sulfonylurea, glinide, biguanide or α-glucosidase inhibitor in Japanese patients with T2DM. In addition, this pooled analysis showed no marked differences in safety or efficacy according to patient background factors.
IEOP_A_1032249_SM9077.docx
Download MS Word (31.5 KB)Acknowledgement
The authors wish to thank all of the investigators and institutions involved in this study, a list for which is provided in the Appendix.
Author contributions
T Kadowaki supervised the design and protocol of the study, and contributed to the interpretation and discussion of the results. F Marubayashi contributed to the development of the protocol and the design, and prepared the data. S. Yokota contributed to the data processing and statistical analysis. M Katoh and H Iijima contributed to the preparation of the outline of the paper, and the interpretation and discussion of the data. All authors contributed to manuscript preparation and have approved the final draft.
Declaration of interest
T Kadowaki was the independent medical adviser for this study and is on the Advisory Panel of Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Taisho Pharmaceutical Co., Ltd. and Takeda Pharmaceutical Co., Ltd.; has received consulting fees from MSD K.K. and Nippon Boehringer Ingelheim Co., Ltd.; has received research support from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation, MSD K.K., Ono Pharmaceutical Co., Ltd., Sanofi K.K. and Takeda Pharmaceutical Co., Ltd.; and is on speakers bureaus for Astellas Pharma Inc., AstraZeneca K.K., Daiichi Sankyo Co., Ltd., Eli Lilly Japan K.K., Kowa Company, Ltd., Kyowa Hakko Kirin Co., Ltd., Mitsubishi Tanabe Pharma Corporation, MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., Sanofi K.K., Sanwa Kagaku Kenkyusho Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Taisho Pharmaceutical Co., Ltd. and Takeda Pharmaceutical Co., Ltd. All other authors are employees of Mitsubishi Tanabe Pharma Corporation. This study was funded by Mitsubishi Tanabe Pharma Corporation, Tokyo, Japan. Medical writing support was provided by Helen Roberton and Nicholas Smith, PhD from Edanz Group Ltd and was funded by Mitsubishi Tanabe Pharma Corporation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
Bibliography
- Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest 2007;117:24-32
- Russell S. Incretin-based therapies for type 2 diabetes mellitus: a review of direct comparisons of efficacy, safety and patient satisfaction. Int J Clin Pharm 2013;35:159-72
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696-705
- Scheen AJ. A review of gliptins for 2014. Expert Opin Pharmacother 2015;16:43-62
- Filippatos TD, Athyros VG, Elisaf MS, et al. The pharmacokinetic considerations and adverse effects of DPP-4 inhibitors. Expert Opin Drug Metab Toxicol 2014;10:787-812
- Goda M, Kadowaki T. Teneligliptin for the treatment of type 2 diabetes. Drugs Today (Barc) 2013;49:615-29
- Morishita R, Nakagami H. Teneligliptin: expectations for its pleiotropic action. Expert Opin Pharmacother 2015;16:417-26
- Nabeno M, Akahoshi F, Kishida H, et al. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochem Biophys Res Commun 2013;434:191-6
- Eto T, Inoue S, Kadowaki T. Effects of once-daily teneligliptin on 24-h blood glucose control and safety in Japanese patients with type 2 diabetes mellitus: a 4-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab 2012;14:1040-6
- Tsuchimochi W, Ueno H, Yamashita E, et al. Teneligliptin improves glycemic control with the reduction of postprandial insulin requirement in Japanese diabetic patients. Endocr J 2015;62:13-20
- Kadowaki T, Kondo K. Efficacy, safety and dose-response relationship of teneligliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab 2013;15:810-18
- Kadowaki T, Kondo K. Efficacy and safety of teneligliptin in combination with pioglitazone in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig 2013;4:576-84
- Kadowaki T, Kondo K. Efficacy and safety of teneligliptin added to glimepiride in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study with an open-label, long-term extension. Diabetes Obes Metab 2014;16:418-25
- Kim YG, Hahn S, Oh TJ, et al. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia 2013;56:696-708
- Park H, Park C, Kim Y, Rascati KL. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes: meta-analysis. Ann Pharmacother 2012;46:1453-69
- Japan Diabetes Society. Evidence-based Practice Guideline for the Treatment for Diabetes in Japan. 2013. Available from: http://www.jds.or.jp/modules/en/index.php?content_id =44 [Last accessed 11 November 2014]
- Konya H, Yano Y, Matsutani S, et al. Profile of saxagliptin in the treatment of type 2 diabetes: focus on Japanese patients. Ther Clin Risk Manag 2014;10:547-58
- Yabe D, Seino Y. Dipeptidyl peptidase-4 inhibitors and sulfonylureas for type 2 diabetes: friend or foe? J Diabetes Investig 2014;5:475-7
- Maeda H, Kubota A, Tanaka Y, et al. The safety, efficacy and predictors for HbA1c reduction of sitagliptin in the treatment of Japanese type 2 diabetes. Diabetes Res Clin Pract 2012;95:e20-2
- Nakamaru Y, Hayashi Y, Ikegawa R, et al. Metabolism and disposition of the dipeptidyl peptidase IV inhibitor teneligliptin in humans. Xenobiotica 2014;44:242-53
- Halabi A, Maatouk H, Siegler KE, et al. Pharmacokinetics and safety of teneligliptin in subjects with hepatic impairment. Clin Pharmacol Drug Dev 2014;3:290-6
- Halabi A, Maatouk H, Siegler KE, et al. Pharmacokinetics of teneligliptin in subjects with renal impairment. Clin Pharmacol Drug Dev 2013;2:246-54
- Otsuki H, Kosaka T, Nakamura K, et al. Safety and efficacy of teneligliptin: a novel DPP-4 inhibitor for hemodialysis patients with type 2 diabetes. Int Urol Nephrol 2014;46:427-32
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317-26
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327-35
- Tanaka S, Suzuki K, Aoki C, et al. Add-on treatment with teneligliptin ameliorates glucose fluctuations and improves glycemic control index in Japanese patients with type 2 diabetes on insulin therapy. Diabetes Technol Ther 2014;16:1-6
- Kinoshita S, Kondo K. Evaluation of pharmacokinetic and pharmacodynamic interactions of canagliflozin and teneligliptin in Japanese healthy male volunteers. Expert Opin Drug Metab Toxicol 2015;11:7-14
Supplementary material available online
Appendix
Supplementary Table.