Abstract
Objective: This meta-analysis was conducted to analyze and compare the efficacy outcomes associated with the fentanyl iontophoretic transdermal system (ITS) and morphine intravenous (IV) patient-controlled analgesia (PCA) in the management of postoperative pain.
Research design and methods: This meta-analysis assessed the efficacy of the fentanyl ITS versus morphine IV PCA using data from four randomized, active-controlled trials (n = 1271 fentanyl ITS and 1298 morphine IV PCA patients). Main outcome measures were patient global assessment (PGA) of the method of pain control at 24 h.
Results: Fentanyl ITS and morphine IV PCA did not significantly differ regarding ‘good’ and ‘excellent’ ratings on the PGA of the method of pain control at 24 h (odds ratio = 0.95, p = 0.66), however, fentanyl ITS was superior in terms of ‘excellent’ PGA ratings at that time point (odds ratio = 1.53, p < 0.0001). No significant differences were found in weighted mean pain intensity scores at 24, 48 and 72 h.
Conclusions: In this meta-analysis, fentanyl ITS was as efficacious as morphine IV PCA and may offer additional benefits as demonstrated by its ‘excellent’ PGA ratings.
1. Introduction
The fentanyl iontophoretic transdermal system (ITS) offers a non-invasive patient-controlled approach for the short-term management of acute postoperative pain by administering the analgesic via iontophoresis (i.e., transdermal delivery of fentanyl through the skin via the application of a low-intensity electrical field) Citation[1]. The fentanyl drug delivery system consists of two components: a top-half (‘Controller’) containing all electronics packaged separately from a bottom-half (‘Drug Unit’) containing the hydrogels (‘Drug Unit’). The Controller has a recessed on-demand dose-activation button, red light-emitting diode (LED), green LED and dose counting liquid crystal display, while the Drug Unit contains fentanyl HCl (10.8 mg of fentanyl HCl is equivalent to 9.7 mg of fentanyl). The healthcare provider assembles the system by snapping the Drug Unit and Controller together immediately prior to application to the patient. An adhesive covers the bottom of the drug component housing and allows the system to be attached to the patient’s skin. Fentanyl ITS delivers preprogrammed analgesic doses based on patient control. Fentanyl ITS eliminates the potential for programming errors, and minimizes the potential for medication errors Citation[2]. Additionally, staff time spent on Patient controlled analgesia (PCA) administration may be reduced with fentanyl ITS Citation[3,4].
The efficacy and safety of fentanyl ITS in postoperative pain management has been established in four 72-h active-controlled RCTs, which were based on a comparison with morphine intravenous patient-controlled analgesia (IV PCA) Citation[5-8]. We have previously reported results using a simple pooled analysis of three of the four Phase III active comparator clinical trials that compared fentanyl ITS with morphine IV PCA Citation[9]. The analysis was performed as if the data were derived from a single sample in this approach. However, by utilizing a meta-analysis, one can detect treatment effects with greater power and estimate these effects with greater precision Citation[10]. While existing meta-analyses have demonstrated the advantages of PCA over non-PCA administration Citation[11,12], they have not yet included efficacy data for fentanyl ITS. To allow an indirect comparison with existing evidence on the efficacy of different postoperative pain management options, a meta-analysis of all four studies comparing fentanyl ITS versus morphine IV PCA was performed.
2. Methods
The four multicenter studies included postoperative patients at least 18 years of age who underwent a variety of different surgeries, including orthopedic, gynecological and abdominal procedures () Citation[5-8]. Three of the studies included centers from North-America Citation[6-8] (mainly the USA), while one study was conducted in Europe Citation[5]. The meta-analysis was conducted according to the Cochrane methodology Citation[13]. Altogether, these four RCTs included 2569 patients (fentanyl ITS, n = 1271; morphine IV PCA, n = 1298; evaluable patient population). Data from the four RCTs were extracted from the clinical study reports by one author and reviewed independently by at least one additional author. Meta-analysis was performed for each end point if at least two of the trials provided head-to-head comparisons.
Table 1. Overview of included studies and subject characteristics*.
Analogous to the Cochrane review Citation[13], the primary analysis applied a random effects model and was based on the evaluable patient populations. To be considered evaluable for efficacy population analysis, patients had to have at least 3 h of study treatment. The evaluable population analysis was chosen because it gives the treatment effect a better opportunity to be noticed by eliminating the noise associated with patients who did not complete at least 3 h of study treatment. As secondary analyses, the intent-to-treat population (i.e., all randomized patients) was analyzed, and fixed effects models were also tested.
The primary efficacy end point of the active-controlled trials was the 24-h treatment success rate, determined as a rating of ‘excellent’ or ‘good’ on the validated end point of the patient global assessment (PGA) of the method of pain control (assessed as the last PGA recorded in the first 24 h) Citation[14]. Secondary efficacy end points included PGA assessments at 48 and 72 h, the investigator global assessment (IGA) completed at last assessment and pain intensity at 24, 48 and 72 h.
The PGA of the method of pain control was based on a categorical 4-point scale (excellent, good, fair or poor). Investigators used the same categorical scale in their global assessments, which were recorded when the patient completed (72 h) or terminated the study. PGA and IGA responses of ‘excellent’ or ‘good’ were considered a treatment success.
Patients reported pain intensity on a verbal numerical rating scale (0 – 10) or a visual analog scale (VAS, 0 – 100) at 24, 48 and 72-h time points. The scales were anchored at ‘no pain’ and ‘worst possible pain’. For the purpose of this meta-analysis, pain scales were transformed to a 100-mm VAS following the example of a Cochrane meta-analysis Citation[11].
2.1 Statistical analysis
The meta-analysis was conducted using random effects models Citation[13], with fixed effect models being explored as secondary analyses. Analyses using random effects models assume that the results are to be generalized to the entire patient population (i.e., the patients included in the four studies are treated as a sample from the entire patient population). Analyses using fixed effects models assume that the results are not intended to be generalized beyond the studies included in the analysis (i.e., they treat the included studies as if they were the only possible studies that could ever be performed). The random effects method is usually more conservative, with CIs that are likely to be wider than those from a fixed effects analysis, and has been used previously in Cochrane evaluations of this and other clinical areas Citation[11,13].
For dichotomous variables, odds ratios (ORs) indicating the probability of the outcome to occur for a patient receiving fentanyl ITS versus morphine IV PCA were calculated. For continuous variables, the weighted mean difference (WMD) between treatments was calculated. Statistical tests were performed at the 0.05 significance level, with no multiplicity adjustments. Ninety-five percent CIs were provided for all parameters. I2 values describe the percentage of total variation across studies, which is due to heterogeneity rather than chance. I2 is calculated from basic results obtained from a typical meta-analysis as I2 = 100% X (Q – df)/Q, where Q is Cochran’s heterogeneity statistic and df is the degrees of freedom. Negative values of I2 are put equal to zero so that I2 lies between 0 and 100%. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity.
Only patients with observations at the given time points were included in the calculations. Any missing values were excluded from analyses.
3. Results
A total of 1271 fentanyl ITS and 1298 morphine IV PCA patients were included in the primary analysis of evaluable patient populations. Across all four studies, the mean age of patients ranged from 50.3 to 62.8 years for the fentanyl ITS and morphine IV PCA treatment groups (), with the majority (> 60%) being female. Treatment success according to the PGA was comparable between fentanyl ITS and morphine IV PCA at 24 h (OR = 0.95, p = 0.66) () and 48 h (OR = 1.37, p = 0.21) postoperatively. At 72 h, a higher percentage of fentanyl ITS patients (90.0%) rated their method of postoperative pain treatment as ‘excellent’ or ‘good’ compared with morphine IV PCA (83.6%) patients (OR = 1.76, p = 0.03) (). (The OR of 1.76 indicates that the odds of an ‘excellent’ or ‘good’ rating on the PGA at 72 h were 76% higher with fentanyl ITS than with morphine IV PCA). When only ‘excellent’ PGA ratings were assessed, fentanyl ITS was found to be statistically superior to morphine IV PCA at 24 h (OR = 1.53, p < 0.0001) (), 48 h (OR = 2.20, p < 0.0001) and 72 h (OR = 2.58, p < 0.0001) ().
Figure 1. (A) PGA ratings of Success at 24 h. (B) PGA ratings of Excellent at 24 h. For PGA Success missing assessments were treated as failures and for PGA Excellent missing assessments were treated as non-excellent response. Odds ratio (95% CI) and p-values were based on logistic regression with treatment as effect for individual studies, based on random effect model for meta-analysis for ‘Total’ in the figure.
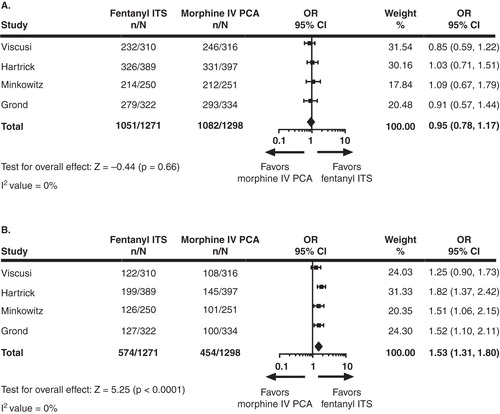
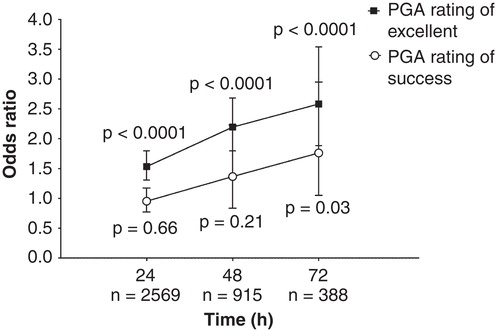
Figure 2. PGA responder rates at 24, 48 and 72 h. Plot shows estimated odds ratios and 95% CIs at each time point. p-values shown are for test for overall treatment effect (HO: OR = 1 vs HA: OR ≠ 1) at each time point between the fentanyl ITS and morphine IV PCA treatment groups. I2 values for PGA success at 24, 48 and 72 h were 0, 62 and 11%, respectively; I2 values for PGA ratings of ‘excellent’ at 24, 48 and 72 h were 0, 0 and 0%, respectively.
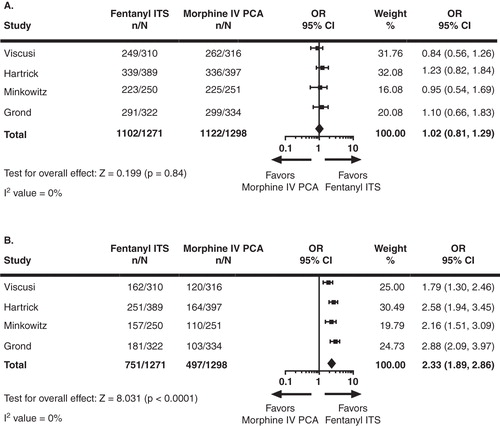
IGA ratings of treatment success were comparable for fentanyl ITS and morphine IV PCA (OR = 1.023, p = 0.84) (). However, a significantly higher percentage of investigators rated fentanyl ITS as ‘excellent’ on the IGA than morphine IV PCA (59.1 vs 38.3%, respective; OR = 2.33, p < 0.001).
Figure 3. (A) IGA ratings of Success at last assessment. (B) IGA ratings of Excellent at last assessment. For IGA Success missing assessments were treated as failures and for IGA Excellent missing assessments were treated as non-excellent response.Odds ratio (95% CI) and p-values were based on logistic regression with treatment as effect for individual studies, based on random effect model for meta-analysis for ‘Total’ in the figure.
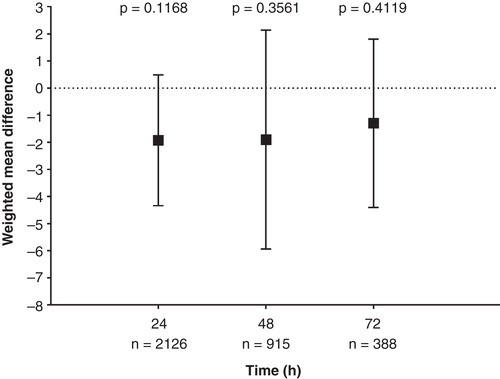
The WMDs in patient-reported pain intensity were comparable at 24 h (WMD = –1.93, p = 0.12), 48 h (WMD = –1.9, p = 0.36) and 72 h (WMD = –0.82, p = 0.41) between fentanyl ITS and morphine IV PCA (). The WMD is the difference between the weighted means of both treatments with weighting determined by a study’s relative sample size and the variability (e.g., standard deviation) within studies. Although not significant, the WMD of –1.93 at 24 h indicated that fentanyl ITS patients had on average a 1.93 point lower pain score (on a 100-point scale) than morphine IV PCA patients.
Figure 4. Weight mean difference is mean pain intensity at 24, 48 and 72 h. Plot shows estimated weighted mean differences and 95% CIs at each time point. p-Values shown are for test for overall treatment effect (HO: WMD = 0 vs HA: WMD ≠ 0) at each time point between the fentanyl ITS and morphine IV PCA treatment groups. Pain was assessed on a scale from 0 (no pain) to 100 (worse possible pain). A negative weighted mean difference results in less pain in patients treated with fentanyl ITS compared with morphine IV PCA. I2 values at 24, 48 and 72 h were 55, 67 and 0%, respectively.
Results of the secondary analysis using the ITT populations were comparable to those from the primary analysis of evaluable patient populations (data not shown). I2 values are 0% for the end points that we used in random effect models, which indicate no heterogeneity.
4. Discussion
Findings of this meta-analysis of four active controlled RCTs indicate that fentanyl ITS and morphine IV PCA provide similar efficacy as assessed by the primary end point, PGA treatment success at 24 h using the last PGA recorded in the 24 h period as well as the secondary end point of PGA treatment success at 48 h. However, at 72 h, PGA success rates favored fentanyl ITS over morphine IV PCA (p = 0.03). In addition, fentanyl ITS provided statistically significantly better results than morphine IV PCA in terms of ‘excellent’ response rates by patients and investigators on the PGA (at 24, 48 and 72 h) and IGA (at last assessment), respectively.
The PGA of the method of pain control is a validated scale in patients with postoperative pain that is a useful measure for assessing pain control provided by different drug delivery systems Citation[12]. In the validation of this scale, patients who reported ‘excellent’ or ‘good’ on the PGA of the method of pain control had lower pain intensity scores and better satisfaction with pain relief compared with patients who reported ‘fair’ or ‘poor’. In fact, a clear delineation between ratings of ‘good’ and ‘excellent’ has been shown. ‘Excellent’ ratings are consistently associated with lower pain intensity scores.
Published meta-analyses have shown that treatment with IV PCA leads to less postoperative pain than nurse-administered analgesia (i.e., IV, intramuscular and subcutaneous opioids) Citation[11,12]. In this meta-analysis, patient-reported pain intensity was found to be similar between fentanyl ITS and morphine IV PCA at 24, 48 and 72 h. Because of the negative WMD in pain between fentanyl ITS and morphine IV PCA, it can therefore be deduced that fentanyl ITS is likely to improve pain compared with conventional analgesia.
The results of this meta-analysis are consistent with the previously published pooled analysis Citation[9]. In the pooled analyses, fentanyl ITS was equally as effective as morphine IV PCA on the primary end point (PGA treatment success at 24 h). The strength of a meta-analysis is that the individual studies are weighted first and then combined, which avoids the problems seen with simple pooling.
This meta-analysis is limited in that only four trials comparing fentanyl ITS and morphine IV PCA were available for inclusion. In addition, the population was not homogenous in that there were many different types of surgeries. While this is a limitation, the Phase III studies were intentionally designed to reflect real world practice in patients who would need PCA across many different types of surgery. Therefore, the results from these studies and this meta-analysis are more generalizable to all types of patients with postoperative pain.
Results of this meta-analysis demonstrate that fentanyl ITS is an effective alternative to morphine IV PCA in the treatment of postoperative pain control. In this meta-analysis, fentanyl ITS may offer additional benefits over IV PCA, as demonstrated by findings of PGA ratings of ‘excellent’.
Declaration of interest
The Medicines Company (Parsippany, NJ, USA) sponsored the analysis and writing of this paper. RS Sinatra serves as consultant to The Medicines Company and Pacira Pharmaceuticals (San Diego, CA, USA). ER Viscusi has research funded to his institution by AcelRx, piacere, Medtronic. He has consulted for AcelRx, Pacira The Medicines Company, Mallinckrodt, Cubist, Trevena, Pacira and has received speaking honoraria from AstraZeneca, Mallinckrodt, Cubist, Salix and Pacira. S Groud serves as a consultant to The Medicines Company and Jansen-Cilag. L Ding and JB Jones and H Danesi are employees of The Medicines Company. S Grundy of SD Scientific, Inc. provided technical writing assistance and was funded by The Medicines Company. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
Bibliography
- Batheja P, Thakur R, Michniak B. Transdermal iontophoresis. Expert Opin Drug Deliv 2006;3(1):127-38
- Viscusi ER, Schechter LN. Patient-controlled analgesia: Finding a balance between cost and comfort. Am J Health Syst Pharm 2006;63(8 Suppl 1):S3-13
- Bonnet F, Eberhart L, Wennberg E, et al. Fentanyl HCl iontophoretic transdermal system versus morphine IV-PCA for postoperative pain management: survey of healthcare provider opinion. Curr Med Res Opin 2009;25(2):293-301
- Evans C, Schein J, Nelson W, et al. Improving patient and nurse outcomes: a comparison of nurse tasks and time associated with two patient-controlled analgesia modalities using Delphi panels. Pain Manag Nurs 2007;8(2):86-95
- Grond S, Hall J, Spacek A, et al. Iontophoretic transdermal system using fentanyl compared with patient-controlled intravenous analgesia using morphine for postoperative pain management. Br J Anaesth 2007;98(6):806-15
- Hartrick CT, Bourne MH, Gargiulo K, et al. Fentanyl iontophoretic transdermal system for acute-pain management after orthopedic surgery: a comparative study with morphine intravenous patient-controlled analgesia. Reg Anesth Pain Med 2006;31(6):546-54
- Minkowitz HS, Rathmell JP, Vallow S, et al. Efficacy and safety of the fentanyl iontophoretic transdermal system (ITS) and intravenous patient-controlled analgesia (IV PCA) with morphine for pain management following abdominal or pelvic surgery. Pain Med 2007;8(8):657-68
- Viscusi ER, Reynolds L, Chung F, et al. Patient-controlled transdermal fentanyl hydrochloride vs intravenous morphine pump for postoperative pain: a randomized controlled trial. JAMA 2004;291(11):1333-41
- Viscusi ER, Siccardi M, Damaraju CV, et al. The safety and efficacy of fentanyl iontophoretic transdermal system compared with morphine intravenous patient-controlled analgesia for postoperative pain management: an analysis of pooled data from three randomized, active-controlled clinical studies. Anesth Analg 2007;105(5):1428-36
- Petrie A, Sabin C. Systematic reviews and meta-analysis. Medical Statistics at a Glance. 3rd edition. Wiley-Blackwell; Oxford, UK: 2009
- Hudcova J, McNicol E, Quah C, et al. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database Syst Rev 2006(4):CD003348
- Walder B, Schafer M, Henzi I, Tramer MR. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. A quantitative systematic review. Acta Anaesthesiol Scand 2001;45(7):795-804
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; The Cochrane Collaboration. 2011. Available from: www.cochrane-handbook.org [Last Accessed 3rd April 2014]
- Rothman M, Vallow S, Damaraju CV, Hewitt DJ. Using the patient global assessment of the method of pain control to assess new analgesic modalities in clinical trials. Curr Med Res Opin 2009;25(6):1433-43
