Abstract
Objective: The mechanism responsible for the lipid-lowering effect of dipeptidyl peptidase-4 (DPP-4) inhibitors remains unknown in humans. We evaluated the effect of anagliptin on serum lipid profiles, including cholesterol synthesis and absorption markers, in Japanese patients with type 2 diabetes.
Methods: Thirty patients with type 2 diabetes (20 – 70 years old, low-density lipoprotein cholesterol (LDL-C) level over 120 mg/dl, and no history of treatment with antidiabetic or antihyperlipidemic drugs) were enrolled. One hundred milligrams of anagliptin were administered twice a day for a month.
Results: After treatment of anagliptin, the LDL-C and total cholesterol (TC) levels did not decrease overall, but the TC level decreased significantly in 28 patients whose HbA1c levels decreased. Lathosterol decreased significantly, whereas no changes in campesterol, sitosterol or cholestanol were observed.
Conclusion: These results of our study show no significant change in LDL-C, a tendency of decrease in TC and non-high-density lipoprotein cholesterol (non-HDL-C) after treatment of anagliptin for 1 month. Anagliptin therapy decreased the cholesterol synthesis marker lathosterol without changing cholesterol absorption markers.
1. Introduction
Controlling the lipid profiles in patients with diabetes is very important for preventing cardiovascular events, as reported previously Citation[1-3]. Dipeptidyl peptidase-4 (DPP-4) inhibitors increase active glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) by inhibiting DPP-4 enzymatic activity, and they improve hyperglycemia in a glucose-dependent fashion by increasing serum insulin and decreasing serum glucagon levels in diabetic patients Citation[4]. Takihata et al. reported that the administration of sitagliptin, but not pioglitazone, decreased low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) in Japanese patients with type 2 diabetes Citation[5]. Shigematsu et al. also reported that sitagliptin caused a significant decrease in total cholesterol (TC), LDL-C and non HDL-C in patients with type 2 diabetes Citation[6]. Kutoh et al. reported that the administration of alogliptin reduced the serum LDL-C levels, but not the HDL-C levels, in Japanese patients with type 2 diabetes Citation[7]. Especially, a meta-analysis of DPP-4 inhibitors suggested that treatment with DPP-4 inhibitors was associated with a significant reduction in TC Citation[8].
Anagliptin is a highly selective DPP-4 inhibitor that has been available in Japan since November 2012. Anagliptin reportedly suppresses the proliferation of vascular smooth muscle and inflammatory reactions of monocytes in apo E-deficient mice Citation[9]. Recently, Kakuda et al. reported that the administration of anagliptin decreased serum triglyceride (TG), TC and LDL-C levels in patients with type 2 diabetes Citation[10]. The mechanism responsible for this lipid lowering effect, however, has not yet been evaluated in humans. Lathosterol has been reported to be a marker of hepatic cholesterol synthesis and sitosterol, campesterol and cholestanol are markers of cholesterol absorption Citation[11,12]. Therefore, we evaluated the effects of anagliptin on the serum lipid profiles, including these markers, of Japanese patients with type 2 diabetes.
2. Patients and methods
2.1 Subjects
The study was approved by the Institutional Ethics Review Committee of Yokohama City University Hospital, and the protocol was registered in the UMIN Clinical Trial Registry as UMIN000013878. Informed consent was obtained from each of the subjects before the start of the study. As no previous reports evaluating the lipid-lowering effect over the course of a month were available, the exploratory sample size was determined to be 30 prior to the study. As the target LDL-C levels of < 120 mg/dl for primary prevention of ischemic heart disease in Japanese patients with type 2 diabetes, we enrolled male and female patients with type 2 diabetes mellitus (20 – 70 years of age, LDL-C level over 120 mg/dl, and no history of treatment with antidiabetic or antihyperlipidemic drugs).
2.2 Study design
One hundred milligrams of anagliptin were administered twice a day for 1 month. We instructed the subjects not to change the daily lives, including their food intake and exercise habits, for the month. Each patient was asked to check whether the medicine had been taken every day and to write any side effects in a diary. The laboratory data obtained before and after treatment were provided to the subjects after the treatment to prevent any changes in diet or exercise habits based on knowledge of the laboratory results. The subjects were requested to fast for at least 12 h before eating breakfast, and blood samples were collected before breakfast at 4 weeks after the start of treatment.
2.3 Measurements
The fasting plasma glucose (FPG), HbA1c, serum insulin, TC, LDL-C, HDL-C and TG levels were measured in the clinical laboratory of Yokohama City University Hospital. Nonesterified fatty acids (NEFA), lathosterol, sitosterol, campesterol, cholestanol and plasma active GLP-1 levels were measured at SRL, Inc. (Tokyo, Japan). The cholesterol synthesis and absorption markers were measured using gas chromatography, while the active GLP-1 levels were measured using an ELISA kit (Millipore Corp, MA, USA). A lathosterol level of less than 1 µg/ml and an active GLP-1 level of less than 2 pmol/l were evaluated as 0.9 µg/ml and 1.9 pmol/l, respectively.
2.4 Statistical analysis
Data were expressed as the mean ± SD. Data were compared by paired t-test between baseline and week 4. We performed univariate regression analysis to identify a correlation between the change in LDL-C and the changes in cholesterol synthesis or absorption markers. The statistical analyses were conducted using Ekuserutoukei 2012 software (Social Survey Research Information Co., Ltd., Tokyo, Japan).
3. Results
Twenty male and 10 female patients with a mean body height of 163.6 ± 8.5 cm, a body weight of 73.3 ± 16.3 kg and a body mass index (BMI) of 27.2 ± 5.2 kg/m2 were enrolled in the present study. The duration of diabetes was 6 ± 8 years. Three patients had a history of hypertension. The results for all the patients are shown in . The HbA1c level of two patients had not changed. In contrast, the HbA1c levels of the remaining (28) patients had decreased. The FPG level had decreased, and the active GLP-1 level had increased significantly. The BMI, serum LDL-C, HDL-C, TG and NEFA levels were not changed by the treatment. A tendency towards a decrease in the TC and non HDL-C levels after treatment was observed, but the difference was not significant. The TC level had decreased significantly at the end of the treatment period in the 28 patients whose HbA1c were decreased (from 243 ± 51 mg/dl – 232 ± 51 mg/dl, p = 0.0469). The lathosterol level had decreased significantly. In contrast, the campesterol, sitosterol and cholestanol levels were not changed by the anagliptin treatment.
Table 1. Comparison of parameters before and after treatment with anagliptin.
We compared the levels of FPG, HbA1c, active GLP-1, cholesterol synthesis and absorption markers between the patients whose LDL-C was decreased and increased (). In both the decreased and increased groups, the FPG and HbA1c levels were improved and the active GLP-1 level had increased significantly. In the LDL-C decreased group, the lathosterol level was significantly decreased by the treatment, but the sterol absorption markers were not changed. In the LDL-C increased group, the lathosterol level was not decreased significantly, and the campesterol levels tended to be increased, but the difference was not statistically significant.
Table 2. Comparison of FPG, HbA1c, active GLP-1, and cholesterol synthesis and absorption markers in patients whose LDL-C levels were decreased or increased.
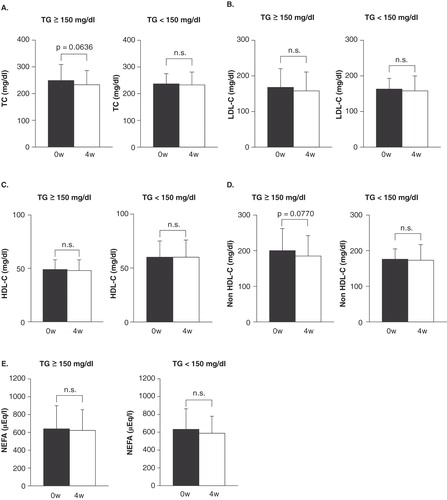
As shown in , there was significant positive correlation between the change in LDL-C and the changes in cholesterol absorption markers, but not cholesterol synthesis marker. We also compared the lipid profile based on the baseline TG level in this study. A tendency towards a decrease in the TC and non-HDL-C levels in the high baseline TG level patients after treatment was observed, but the difference was not significant ().
Figure 1. The correlation between the change of LDL-C and the change in cholesterol synthesis or absorption markers.
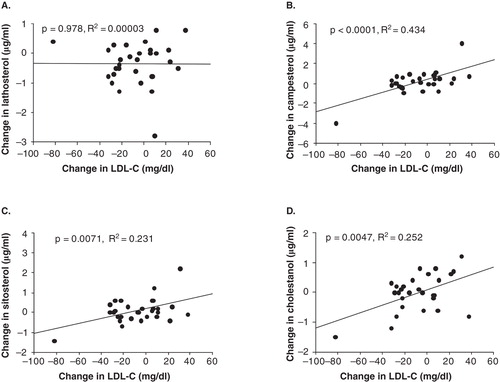
Figure 2. The comparison of (A)TC, (B) LDL-C, (C) HDL-C, (D) Non HDL-C and (E) NEFA at 0 and 4 weeks based on the baseline TG level.
As for adverse effects, three patients experienced mild constipation and three patients had mild diarrhea. No other adverse events were observed.
4. Discussion
The most important finding in this study was that the administration of anagliptin for a month decreased the cholesterol synthesis-related marker lathosterol by 12% (the degree was small, but significant) but did not change the level of a cholesterol absorption marker. In addition, there was significant positive correlation between the change in LDL-C and the changes in cholesterol absorption markers, but not cholesterol synthesis marker (). These results suggest that reduction in cholesterol absorption markers was not caused by the direct effect of anagliptin, but associated with the reduction in LDL-C. Indeed, while the LDL-C level did not decrease, the TC level did decrease in 28 patients whose HbA1c levels were decreased by the administration of anagliptin. Therefore, the cholesterol-lowering effect of anagliptin may be associated with the inhibition of cholesterol synthesis.
Some reports have indicated the existence of mechanisms for improving cholesterol metabolism through the actions of DPP-4 inhibitors and GLP-1 analogues in animal models. In a mouse study, the administration of the DPP-4 inhibitor vildagliptin decreased the expressions of genes involved in hepatic cholesterol biosynthesis, such as phospho-mevalonate kinase (Mvk), acyl-coenzyme dehydrogenase medium chain (Acadm), mevalonate (diphospho)decarboxylase (Mvd), and Acyl-CoA synthetase (Acsl1) Citation[13]. shRNA for adiponectin downregulated the mRNA expression of insulin-induced gene 2 (INSIG-2), LDL-receptor and PPARα in liver of ApoE–/– mice Citation[14]. Liraglutide restored the mRNA expression of INSIG-2, LDL-receptor and PPARα and also upregulated hydroxymethylglutaryl-CoA reductase (HMGCR) and sterol regulatory element-binding protein-2 (SREBP-2) in the liver of their mice. Thus, in animal model study, DPP-4 inhibitor decreases cholesterol synthesis. These findings agree with our results in human. We also would like to evaluate the mechanism to reduce the cholesterol synthesis by the treatment of anagliptin using animal model in the future.
Because the LDL-C level was not decreased by the treatment as a whole, we compared glycemic control, active GLP-1, and cholesterol synthesis and absorption markers in the patients whose LDL-C was decreased or increased ().
The lathosterol level was decreased only in the LDL-C decreased group, the glycemic control was improved in both groups, and the active GLP-1 levels were increased in both groups. Therefore, the cholesterol-lowering effect of anagliptin was probably not associated with the degree of improvement in glycemic control or the increase in the active GLP-1 levels. The reduction in lathosterol was small and a tendency toward an increase in the campesterol and sitosterol levels after treatment was observed in the increased group, but the difference was not significant. As a result, the LDL-C level may not be decreased in the increased group.
As high baseline TG levels and the administration of strong stains were independent predictors of greater TC and LDL-C reduction in the patients with type 2 diabetes treated with sitagliptin, the patients with increased synthesis of chylomicron and remnant lipoproteins may be the best response to sitagliptin therapy Citation[6]. We also compared the lipid profile based on the baseline TG level in this study. A tendency towards a decrease in the TC and non HDL-C levels in the high baseline TG level patients after treatment was observed, but the difference was not significant (). Further studies are needed in order to evaluate the lipid profiles in the patients with high baseline TG.
DPP-4 inhibitors other than anagliptin reportedly decrease serum cholesterol levels in humans Citation[5-8]. We would also like to evaluate the effects of other DPP-4 inhibitors on cholesterol synthesis and absorption to elucidate whether this effect is specific to anagliptin in patients with type 2 diabetes.
The present study had several limitations. We collected patients without history of treatment with antidiabetic or antihyperlipidemic drugs to evaluate the true effect of anagliptin on lipid profile in the daily clinical practice. Therefore, we were unable to collect sufficient number of subjects to assign the placebo-control group. In addition, this study had an open-label design. Kakuda et al. reported that the LDL-C and TC levels were decreased in patients with type 2 diabetes after treatment with angliptin for 3 or 6 months Citation[10]. As the duration of anagliptin treatment was only 1 month in the present study, the LDL-C level had not fully decreased. Therefore, a larger-scale, double-blinded study in which the drugs are administered to patients with type 2 diabetes for a longer period is needed in the future.
5. Conclusion
These results of our study show no significant change in LDL-C, a tendency of decrease in TC and non HDL-C after treatment of anagliptin for one month. Anagliptin therapy decreased the cholesterol synthesis marker lathosterol without changing cholesterol absorption markers.
Acknowledgments
Trial Registration: UMIN000013878.
Declaration of interest
This study was funded by Sanwa Kagaku Kenkyusho Co., Ltd. This work was supported in part by Grants-in-Aid for Scientific Research (B) 21390282 and (B) 24390235 from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, and a Medical Award from the Japan Medical Association. Yasuo Terauchi has received honoraria for lectures from MSD K.K.; Ono Pharmaceutical Co., Ltd.; Nippon Boehringer Ingelheim Co., Ltd.; Novartis Pharma K.K.; Takeda Pharmaceutical Co., Ltd.; Mitsubishi Tanabe Pharma Corp.; Daiichi Sankyo Co., Ltd.; Sanwa Kagaku Kenkyusho Co., Ltd.; Kowa Pharmaceutical Co., Ltd.; Novo Nordisk Pharma Ltd.; Eli Lilly Japan K.K.; Sanofi K.K.; Shionogi & Co., Ltd.; Bayer Yakuhin, Ltd.; and AstraZeneca K.K. and has obtained research support from MSD K.K.; Ono Pharmaceutical Co., Ltd.; Nippon Boehringer Ingelheim Co., Ltd.; Novartis Pharma K.K.; Takeda Pharmaceutical Co., Ltd.; Mitsubishi Tanabe Pharma Corp.; Daiichi Sankyo Co., Ltd.; Sanwa Kagaku Kenkyusho Co., Ltd.; Novo Nordisk Pharma Ltd.; Eli Lilly Japan K.K.; Sanofi K.K.; Dainippon Sumitomo Pharma Co., Ltd.; Shionogi & Co., Ltd.; Bayer Yakuhin, Ltd.; Astellas Pharma, Inc.; Pfizer Japan, Inc.; and AstraZeneca K.K. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
Bibliography
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685-96
- Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003;361:2005-16
- Tajima N, Kurata H, Nakaya N, et al. Pravastatin reduces the risk for cardiovascular disease in Japanese hypercholesterolemic patients with impaired fasting glucose or diabetes: diabetes subanalysis of the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA) Study. Atherosclerosis 2008;199:455-62
- Herman GA, Bergman A, Stevens C, et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab 2006;91:4612-19
- Takihata M, Nakamura A, Tajima K, et al. Comparative study of sitagliptin with pioglitazone in Japanese type 2 diabetic patients: the COMPASS randomized controlled trial. Diabetes Obes Metab 2013;15:455-62
- Shigematsu E, Yamakawa T, Kadonosono K, et al. Effect of sitagliptin on lipid profile in patients with type 2 diabetes mellitus. J Clin Med Res 2014;6:327-35
- Kutoh E, Ukai Y. Alogliptin as an initial therapy in patients with newly diagnosed, drug naive type 2 diabetes: a randomized, control trial. Endocrine 2012;41:435-41
- Monami M, Lamanna C, Desideri CM, et al. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Ther 2012;29:14-25
- Ervinna N, Mita T, Yasunari E, et al. Anagliptin, a DPP-4 inhibitor, suppresses proliferation of vascular smooth muscles and monocyte inflammatory reaction and attenuates atherosclerosis in male apo E-deficient mice. Endocrinology 2013;154:1260-70
- Kakuda H, Kobayashi J, Kakuda M, et al. The effect of anagliptin treatment on glucose metabolism and lipid metabolism, and oxidative stress in fasting and postprandial states using a test meal in Japanese men with type 2 diabetes. Endocrine 2015;48(3):1005-9
- Kempen HJ, Glatz JF, Gevers Leuven JA, et al. Serum lathosterol concentration is an indicator of whole-body cholesterol synthesis in humans. J Lipid Res 1988;29:1149-55
- Miettinen TA, Tilvis RS, Kesaniemi YA. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol 1990;131:20-31
- Flock G, Baggio LL, Longuet C, et al. Incretin receptors for glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide are essential for the sustained metabolic actions of vildagliptin in mice. Diabetes 2007;56:3006-13
- Li L, Miao Z, Liu R, et al. Liraglutide prevents hypoadiponectinemia- induced insulin resistance and alterations of gene expression involved in glucose and lipid metabolism. Mol Med 2011;17:1168-78
