Abstract
Type 2 diabetes is usually associated with a number of metabolic and cardiovascular (or cardiometabolic) risk factors that contribute to a high rate of vascular events in these patients. Adipose tissue is now known to secrete a number of pro-inflammatory adipokines that are thought to mediate the link between obesity, insulin resistance and atherosclerosis. Therefore, not only is abdominal obesity a major cardiometabolic risk factor per se, it has the potential to give rise to other emerging risk factors. Plasma concentrations of inflammatory markers, such as C-reactive protein, may provide additional information to guide management and may even represent therapeutic targets. Reducing the risk of cardiovascular events in patients with Type 2 diabetes will involve targeting traditional risk factors and probably novel cardiometabolic factors.
1. Introduction
Patients with diabetes are at high risk of a range of comorbid complications, including cardiovascular disease (CVD). Up to 80% of patients with Type 2 diabetes will develop and possibly die from macrovascular disease Citation[1] and the risk of CVD is two- to fourfold higher in patients with Type 2 diabetes than in non-diabetics Citation[2]. Indeed, a patient with diabetes has as high a risk of experiencing a first myocardial infarction (MI) as someone without diabetes who has already had an MI Citation[2] and up to 23% of diabetic patients with coronary heart disease (CHD) were found to have silent ischaemia and experienced poor outcomes following acute events Citation[3].
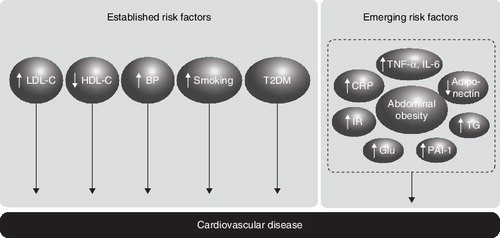
The increased risk of cardiovascular events seen in patients with Type 2 diabetes is associated with a cluster of risk factors for cardiovascular and metabolic disorders that tend to coexist in these patients, including hypertension, dyslipidaemia and abdominal obesity. Patients who have these cardiometabolic risk factors are at greater risk of CVD than diabetic patients who do not. As treatment has proven effective in reducing vascular events in patients with Type 2 diabetes, assessment of the cardiometabolic risk of a patient and appropriate management is warranted Citation[1-3].
Inflammation is increasingly recognised as playing an important role in the development of insulin resistance and atherosclerosis Citation[4,5]. The abnormal inflammatory response associated with obesity may, therefore, represent a link between excess adipose tissue and the development of Type 2 diabetes and CVD Citation[5]. Therefore, in addition to the established cardiometabolic risk factors, increased levels of circulating pro-inflammatory markers, such as TNF-α, IL-6 and C-reactive protein (CRP), as well as anti-inflammatory markers, such as adiponectin, may provide additional information about the cardiometabolic risk of a patient () Citation[4,6,7].
This review focuses on the cardiometabolic risk associated with obesity and the usefulness of CRP as a novel marker for assessing cardiometabolic risk. In addition, present approaches to the management of cardiovascular risk in patients with Type 2 diabetes and their effects on inflammatory markers are discussed.
2. Obesity and waist circumference
The prevalence of obesity is reaching epidemic levels, with over one billion adults worldwide being overweight and 300 million clinically obese Citation[101]. In England, almost 24% of men and women were classified as obese in 2004, compared with ∼ 13% of men and 16% of women in 1993 Citation[102].
Overweight or obesity has traditionally been defined using body mass index (BMI). The World Health Organization classifies a person as being overweight if they have a BMI of 25 – 29.9 kg/m2 and as obese with a BMI ≥ 30 kg/m2. However, the suitability of BMI as a measure of obesity has recently been questioned, partly because the risk of hypertension, dyslipidaemia and diabetes can increase from a BMI as low as 21 kg/m2 Citation[8]. In addition, the complications commonly found in obese patients are more closely related to the location of excess fat rather than excess weight per se, a concept that was first proposed in the mid-1940s by Jean Vague Citation[9,10]. Several studies have since confirmed that a high proportion of abdominal adipose tissue – in particular, visceral adipose tissue – is a major risk factor for Type 2 diabetes and CHD Citation[9].
Waist circumference, as a direct measure of abdominal obesity, therefore, represents a more meaningful method than BMI for assessing obesity and, thereby, cardiometabolic risk. Imaging techniques, such as MRI and CT, which can distinguish between visceral and subcutaneous adiposity, have shown that waist circumference measurement correlates closely with the amount of visceral fat Citation[11]. Waist circumference is also more practical in the clinical setting than measuring the waist/hip ratio. For these reasons, the International Diabetes Federation defines abdominal (or central) obesity as a waist circumference ≥ 94 cm for Europid men and ≥ 80 cm for Europid women (with ethnicity-specific values for other groups), and places this as the central feature of the metabolic syndrome () Citation[103].
3. Adipose tissue and inflammation
Once viewed as a passive reservoir for energy storage, adipose tissue is actually a complex and highly active metabolic and endocrine organ. Adipose tissue secretes a range of bioactive proteins that act both within adipose tissue (paracrine/autocrine action) and at distant sites (endocrine function) Citation[12]. These secreted proteins are generally referred to as adipokines. The expression, production and release of a number of these adipokines are altered during obesity, including leptin, TNF-α, IL-6, plasminogen activator inhibitor 1 (PAI-1) and adiponectin (). The inflammatory state of obesity and the production of pro-inflammatory adipokines are increasingly being implicated in the aetiology of the development of Type 2 diabetes and atherosclerosis.
3.1 Leptin
Leptin is secreted in direct proportion to adipose tissue mass and is an indicator of nutritional status Citation[13]. The main role of leptin is to signal energy sufficiency rather than energy excess and has a major role in the regulation of appetite and energy balance. It exerts its effects on energy homeostasis via hypothalamic pathways and direct action on peripheral tissues, such as muscle and pancreatic β-cells. In the West of Scotland Coronary Prevention Study, higher plasma leptin concentrations were associated with increased risk of a future coronary event Citation[14]. The significance of leptin was maintained following adjustment for BMI and classic risk factors, such as lipid levels and systolic blood pressure. However, this is in contrast to results from the Quebec Cardiovascular Study, where high plasma leptin levels were not predictive of ischaemic heart disease Citation[15].
The role of leptin as a risk factor for vascular disease remains speculative. One of the reasons for this controversy is that leptin may act through various mechanisms. For example, leptin receptors in normal vessel-wall segments and neointimal or atherosclerotic lesions have been reported Citation[16]. Leptin has also been shown to stimulate endothelin-1 production Citation[17]. Endothelin-1 is a powerful vasoconstrictor and it can activate platelets Citation[17,18].
3.2 Adiponectin
Adiponectin is expressed by adipocytes and circulates in the bloodstream Citation[19]. It plays an important role in modulating glucose and lipid metabolism in insulin-sensitive tissues. In the liver, adiponectin improves insulin sensitivity, increases influx of non-esterified fatty acids (NEFAs), increases fatty acid oxidation and reduces hepatic glucose production. In muscle, adiponectin stimulates glucose use and fatty acid oxidation Citation[19]. Adiponectin also has several anti-atherogenic effects on the vasculature, including inhibition of monocyte adhesion to endothelial cells and macrophage transformation into foam cells Citation[20]. Therefore, adiponectin has antidiabetic, anti-inflammatory and anti-atherogenic effects. In contrast to most other adipokines, plasma adiponectin levels are reduced in obesity and Type 2 diabetes, contributing to peripheral insulin resistance in these patients Citation[19]. Increasing plasma adiponectin concentrations may, therefore, be useful in treating insulin resistance, diabetes and CVD.
3.3 Plasminogen activator inhibitor 1
PAI-1 is the primary inhibitor of fibrinolysis and has been implicated in angiogenesis and atherogenesis because it promotes arterial thrombus formation Citation[21]. Plasma levels of PAI-1 are elevated in obesity and insulin resistance, are predictive of future risk of Type 2 diabetes and CVD, and may represent a very early marker for these diseases Citation[21,22].
3.4 Interleukin-6
Expression of IL-6 by adipocytes and circulating IL-6 levels increase with obesity, insulin resistance and impaired glucose tolerance. IL-6 stimulates the hypothalamic–pituitary–adrenal axis, activation of which is associated with abdominal obesity, insulin resistance and hypertension, and plasma concentrations of IL-6 predict the development of Type 2 diabetes and CVD Citation[23]. IL-6 inhibits adipogenesis, decreases adiponectin secretion and is a powerful inducer of the hepatic acute-phase response, resulting in elevated concentrations of acute-phase reactants such as CRP Citation[23]. A direct role for IL-6 in the pathogenesis of atherosclerosis has been suggested, as it decreases lipoprotein lipase (LPL) activity and levels of monomeric LPL in the plasma, which increases macrophage uptake of lipids Citation[23].
3.5 Tumour necrosis factor-α
Synthesis and secretion of the pro-inflammatory cytokine TNF-α by adipocytes is increased in obesity and is thought to play an important role in the development of insulin resistance Citation[24]. TNF-α affects gene expression in metabolically important tissues such as adipose tissue and the liver. In adipose tissue, TNF-α suppresses genes involved in the uptake and storage of NEFAs. In the liver, TNF-α increases expression of genes involved in de novo synthesis of cholesterol and fatty acids, and suppresses expression of genes involved with glucose uptake and metabolism, and fatty acid oxidation Citation[24]. These changes in gene expression result in increased levels of circulating NEFAs, which contribute to the development of insulin resistance Citation[24,25]. TNF-α also impairs insulin signalling via activation of serine kinases that increase phosphorylation of insulin receptor substrate 1, making it a poor substrate for insulin receptor kinases and, thus, disrupting the insulin signalling cascade Citation[5].
4. C-reactive protein
CRP has attracted attention as a biomarker for inflammation and, as such, is thought to play a role in the innate immune response. In healthy people, levels of CRP remain very low; however, levels can rise rapidly in response to a wide variety of stimuli, including infection and trauma. Although not produced in significant amounts, if at all, by adipose tissue, elevated production of TNF-α and IL-6 by adipose tissue leads to an acute-phase response that triggers production of CRP by the liver Citation[26].
Several large, population-based studies, including the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) trial Citation[27], the Women's Health Study Citation[28], the Honolulu Heart Study Citation[29] and the NHANES (National Health and Nutrition Examination Survey) Citation[30] have shown a strong linear relationship between CRP levels and risk of cardiovascular events. Indeed, one study suggests that CRP is a stronger predictor of cardiovascular events than low-density lipoprotein cholesterol (LDL-C) levels Citation[28].
A population-based study in men and women showed CRP to be strongly related to the cardiometabolic risk factors of hypertension, obesity (as measured by both BMI and waist circumference), insulin resistance, raised fasting plasma glucose levels, raised triglycerides and low high-density lipoprotein cholesterol (HDL-C) levels Citation[31]. CRP levels were also found to be strongly related to the number of cardiometabolic risk factors present in recently diagnosed, drug-naive patients with Type 2 diabetes Citation[32]. This relationship appeared to be mediated by obesity, as it became nonsignificant after adjusting for BMI.
CRP levels may provide additional prognostic information for identifying patients with Type 2 diabetes who are at high risk of cardiovascular events. One study found that patients with diabetes and high CRP levels were seven-times more likely to have CVD than those without diabetes (or the metabolic syndrome) and low CRP levels Citation[33]. CRP levels are elevated in diabetic patients with CAD Citation[34] and strongly predictive of death and cardiovascular events in patients with Type 2 diabetes Citation[35].
In 2004, the American Heart Association and the Centers for Disease Control issued joint guidelines for the use of CRP as a marker for assessing the risk of CVD Citation[36]. They suggest that CRP (as measured by high-sensitivity [hs] assay) should be evaluated in patients judged by global assessment to be at intermediate risk; that is, a 10 – 20% risk of CVD over 10 years, to provide additional information to direct further evaluation or treatment.
A hsCRP level < 1.0 mg/l denotes low risk, 1.0 – 3.0 mg/l intermediate risk and > 3.0 mg/l high risk Citation[37]. Those at intermediate and high risk would probably benefit from aggressive risk-lowering treatment strategies. People in the high-risk category have an approximately twofold greater relative-risk of experiencing a coronary event than those in the low-risk category.
In addition to being a predictor of vascular events, increasing evidence suggests that CRP may contribute directly to atherogenesis through effects on monocytes/macrophages and modulating endothelial dysfunction Citation[38,39]. However, the direct contribution of CRP to atherosclerosis needs to be confirmed.
5. Management of cardiometabolic risk
5.1 Non-pharmacological options
Diet modification and increased physical activity can be beneficial, as even moderate weight loss (≤ 10%) can improve glycaemic control, reduce blood pressure and lower cholesterol levels Citation[40]. Lifestyle modifications also have beneficial effects on CRP concentrations. Increasing the amount of fruit and vegetables in the diet can lower CRP levels. Higher intake of fruit and vegetables was associated with lower plasma CRP concentrations in a cross-sectional study of 486 women Citation[41]. The effect of lifestyle modification, including exercise, on CRP levels has been investigated in a small study involving 47 overweight or obese adults Citation[42]. Regular exercise and diet instruction improved hsCRP levels, which were associated with weight loss and improved aerobic capacity. A recent review of 33 studies investigating the effects of weight loss through lifestyle, dietary and/or exercise measures or surgery, showed that weight loss was associated with a decline in CRP levels Citation[43]. Each 1 kg of weight loss resulted in a mean change in CRP level of -0.13 mg/l.
Adhering to these recommendations presents a significant challenge for many individuals. Long-term weight loss and modification of other cardiometabolic risk factors may, therefore, require pharmacological and/or surgical intervention to achieve targets. It is likely that a number of pharmacological and/or surgical options will need to be considered depending on the individual risk-profile of each patient.
5.2 Treatment of diabetic dyslipidaemia
Dyslipidaemia in Type 2 diabetic patients is characterised by raised triglyceride levels, reduced HDL-C levels and an increase in the proportion of small, dense LDL-C Citation[44]. Pharmacological therapies available for the treatment of dyslipidaemia include statins, fibrates, niacin and cholesterol absorption inhibitors.
The 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, or statins, lower LDL-C levels and are effective in reducing the risk of cardiovascular events in patients with diabetes Citation[45]. The greatest benefit of statin therapy in terms of the lowest number needed to treat is seen in those with metabolic risk factors. In one post-hoc analysis of a statin trial (GREACE [Greek atorvastatin and coronary-heart-disease evaluation]) the maximum benefit was seen in those with CHD and metabolic syndrome and lowering triglyceride levels was associated with a reduction in vascular events Citation[46]. In the PRINCE (Pravastatin Inflammation/CRP Evaluation) trial, pravastatin reduced CRP levels after 24 weeks of treatment in patients with no prior history of CVD (-16.9%) and with known CVD (-13.3%) Citation[47]. The effect of CRP appeared to be independent of the effect of pravastatin on LDL-C levels, suggesting that statins have anti-inflammatory effects in addition to lipid-lowering effects. This was supported by the PROVE-IT (Pravastatin or Atorvastatin Evaluation and Infection Therapy) trial in which patients treated with a statin (atorvastatin or pravastatin) who achieved a target level of CRP < 2 mg/l experienced a significant reduction in cardiovascular events at all levels of LDL-C achieved Citation[48]. CRP levels have also been shown to correlate with progression of atherosclerosis. In the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) trial, reduced rates of progression of atherosclerosis were seen in patients who received intensive statin treatment and who achieved greater reductions in LDL-C and CRP levels Citation[49]. However, trials conducted using statin therapies had no significant effect on CRP after adjusting for the change in LDL; 89 – 98% of CRP change was related to LDL lowering and 2 – 11% to non-LDL effects of statins Citation[50].
Fibrates, which target the PPAR-α, can be used to lower triglyceride levels and raise HDL-C levels in patients with diabetes. Like statins, the greatest benefit of fibrate therapy is seen in patients with metabolic risk factors. In the BIP (Bezafibrate Infarction Prevention) study, conducted in patients with CHD, no benefit of bezafibrate was demonstrated on the combined primary end point of fatal or non-fatal MI and sudden death. However, a significant reduction in cardiovascular events was seen with bezafibrate in the subgroup that had elevated triglyceride and low HDL-C levels Citation[51]. The benefit of fibrate therapy on cardiovascular mortality in the FIELD (Fenofibrate Intervention in Event Lowering in Diabetes) study was unclear Citation[52]. However, in an analysis of the subgroup of patients with Type 2 diabetes in the VA-HIT (Department of Veterans Affairs High-Density Lipoprotein Intervention Trial), gemfibrozil reduced the risk of death from CHD, stroke or MI by 32% compared with placebo (p = 0.004) Citation[53]. Fenofibrate has been shown to reduce the progression of CHD in patients with Type 2 diabetes and reduce plasma concentrations of IL-6 and CRP in hyperlipidaemic patients Citation[54,55]. Ciprofibrate can also significantly reduce plasma CRP levels independently of its effect on lipid levels Citation[56]. Taken together, these results indicate a beneficial effect of fibrates in reducing CVD in patients with diabetes.
Niacin inhibits adipose tissue lipolysis and synthesis of triglycerides in the liver, leading to increased HDL-C levels and reduced triglyceride and LDL-C levels. Although niacin has proven efficacy in reducing the risk of cardiovascular events Citation[57], patient compliance is poor – largely due to flushing as a side effect. The use of niacin in patients with diabetes is associated with a modest increase in blood glucose, which is a disadvantage in this population Citation[58].
Ezetimibe inhibits cholesterol absorption in the intestine by binding cholesterol transport pumps (ATP-binding cassette proteins) and has shown additional benefits on LDL-C and hsCRP levels when added to existing statin therapy Citation[59]. A recent meta-analysis involving 5039 patients where ezetimibe was added to a statin showed a decrease of 10.7% in triglyceride levels and a rise in HDL-C levels of 1.7% Citation[60]. There is also evidence that ezetimibe can lower triglyceride levels to a greater extent when baseline values are ‘high’ Citation[61], and in these patients, small, dense LDL may be preferentially decreased Citation[62].
5.3 Antihyperglycaemic therapies
Intensive glycaemic control has been shown to benefit macrovascular outcomes in patients with Type 1 diabetes Citation[63]. Although reducing the risk of microvascular disease in patients with Type 2 diabetes by 25%, the effect of tight blood-glucose control on the risk of macrovascular disease is less clear Citation[64]. Furthermore, it appears that different antihyperglycaemic agents have different effects. Intensive blood glucose control with metformin in overweight patients with Type 2 diabetes produced a greater reduction in the risk of stroke compared with chlorpropamide, glibenclamide or insulin Citation[65], and the thiazolidinediones have demonstrated anti-atherogenic and anti-inflammatory effects Citation[66-70].
The anti-atherogenic effects of pioglitazone were first demonstrated in a study involving 136 Japanese patients with Type 2 diabetes Citation[66]. Treatment with pioglitazone significantly reduced CRP and increased adiponectin levels, but had no effect on leptin. These actions were independent of reductions in glycated haemoglobin (HbA1c), suggesting that the anti-atherogenic effects of pioglitazone are mediated by a separate mechanism to glycaemic control. In patients with advanced diabetic nephropathy, pioglitazone reduced levels of CRP by 41% and IL-6 by 38% Citation[67]. In patients whose diabetes was not adequately controlled by insulin therapy, adding pioglitazone significantly reduced hsCRP levels compared with placebo and improved glycaemic control Citation[68].
Concerns have recently been raised regarding the safety of rosiglitazone, which is widely used to treat patients with Type 2 diabetes. The results of the RECORD (Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes) interim analysis and other meta-analyses showed that the risk of MI was significantly increased with rosiglitazone Citation[71-74]. At present, it is not clear if there are differences between the two glitazones and whether or not any such potential variability is clinically relevant Citation[71-74].
5.4 Antihypertensive therapies
Tight blood-pressure control reduces the risk of both macrovascular and microvascular complications of Type 2 diabetes. In the UKPDS (UK Prospective Diabetes Study), hypertensive patients with Type 2 diabetes were randomised to receive either tight blood-pressure control (blood pressure of < 150/85 mmHg; n = 758) or less tight control (blood pressure of < 180/105 mmHg; n = 390) for a median follow up of 8.4 years Citation[75]. Tight control of blood pressure reduced the risk of stroke by 44% and microvascular disease by 37% compared with less tight control. Available therapies for reducing blood pressure include diuretics, calcium channel blockers, β-blockers, ACE inhibitors and angiotensin II receptor blockers (ARBs).
The effects of the ARB irbesartan on biomarkers for low-grade inflammation were investigated in the IRMA 2 (Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria) study Citation[76]. Irbesartan reduced hsCRP by 5.4%/year for 2 years compared with a 10% increase per year in control patients (p < 0.001), and slowed the rise in IL-6 concentration to 1.8%/year compared with 6.5%/year with placebo (p = 0.005). Similarly, the rise in plasma fibrinogen was significantly slowed down (p = 0.027). This decrease in low-grade inflammation may reduce the risk of microvascular and macrovascular disease in patients with Type 2 diabetes.
The third-generation β-blocker carvedilol has both antioxidant and anti-inflammatory effects. In the GEMINI (Glycemic Effects in Diabetes Mellitus: Carvedilol-Metoprolol Comparison in Hypertensives) trial, carvedilol was compared with metoprolol in the presence of rennin–angiotensin system blockade. Carvedilol was superior in improving insulin sensitivity, preserving HbA1c and preventing the progression to microalbuminuria, despite similar blood pressure responses in patients with diabetes mellitus and hypertension Citation[77].
Microalbuminuria is a predictor of vascular risk and progressive impaired renal function, possibly at levels now considered as normal Citation[78]. This applies to patients with and without diabetes. Other drugs such as ACE inihibitors, angiotensin II receptor inhibitors and statins as well as good glycaemic control can help decrease the progression of microalbuminuria Citation[78].
5.5 Pharmacological treatment of obesity
Pharmacological agents used to treat obesity include orlistat and sibutramine. Orlistat is an intestinal lipase inhibitor that reduces the breakdown and absorption of triglycerides across the intestine, reducing dietary fat uptake by ∼ 30% Citation[79]. Sibutramine is a combined serotonin and noradrenaline re-uptake inhibitor that acts centrally to promote satiety – a feeling of fullness that results in reduced food intake Citation[79].
In a meta-analysis of long-term, placebo-controlled trials, overweight and obese patients treated with orlistat experienced weight loss of 2.89 kg over 1 year compared with 4.45 kg for those treated with sibutramine Citation[80]. In a large study (n = 3305) aimed at preventing Type 2 diabetes in obese patients with normoglycaemia or impaired glucose tolerance, orlistat reduced systolic blood pressure by 1.8 mmHg, diastolic blood pressure by 1.6 mmHg, LDL-C levels by 0.27 mmol/l (with no effect on HDL or triglycerides) and fasting blood glucose by 0.8 mmol/l in patients with diabetes Citation[81]. Sibutramine has shown little effect on LDL-C levels, a modest effect on HbA1c (reducing by an average 0.3%) Citation[82] and conflicting effects on concentrations of triglycerides and HDL-C Citation[81].
The combination of orlistat and fenofibrate can improve body weight, waist circumference, blood pressure and lipid levels in overweight or obese patients with metabolic syndrome Citation[83]. The combination reduced the number of patients who fulfilled diagnostic criteria for the metabolic syndrome more than either monotherapy, but this difference was not significant. Furthermore, the effects of these two agents on LDL-C and triglyceride levels appeared to be additive.
5.6 A new therapy for managing cardiometabolic risk
Rimonabant is a recent addition to the pharmacological options for managing cardiometabolic risk. Rimonabant modulates the endocannabinoid system through selective blockade of the CB1 cannabinoid receptor, thus, suppressing appetite, decreasing lipogenesis and increasing adiponectin production Citation[79]. It has been licensed by the European Medicines Agency as an adjunct to diet and exercise for the treatment of overweight (BMI > 27 kg/m2) or obese (BMI ≥ 30 kg/m2) patients with associated risk factors, such as Type 2 diabetes or dyslipidaemia. It is presently approved for use in 13 European countries Citation[104]. However, approval of rimonabant for use in the US was rejected by the FDA due to increased depression rates and psychiatric-related adverse-effect reporting compared with placebo in the RIO (Rimonabant in Obesity) trials Citation[84-87,105]. More information on these adverse events has been requested by the FDA.
The efficacy of rimonabant was demonstrated in four 1- to 2-year, double-blind clinical trials encompassing the RIO clinical trial programme Citation[84-87]. A review of these studies suggested that rimonabant is associated with apparently superior weight loss compared with orlistat and sibutramine (5.4 versus 2.89 and 4.85 kg, respectively), however, no direct comparisons have been carried out so far Citation[88]. The RIO-Lipids study investigated the effects of rimonabant on metabolic risk factors Citation[86]. In patients receiving rimonabant, adiponectin levels increased by an average 57.7%, which was significantly greater than the increase in patients receiving placebo (p < 0.001). Furthermore, 57% of this increase could not be attributed to weight loss. Plasma leptin levels decreased in patients receiving rimonabant in a dose-dependent fashion and CRP levels decreased by 0.9 mg/l Citation[86].
Significant decreases in hsCRP and leptin concentrations compared with placebo (-1.4 versus 0 mg/l, p = 0.02; and -0.3 versus +3.1 ng/ml, p < 0.0001, respectively), were also observed in the RIO-Diabetes study Citation[87]. This study assessed the efficacy and safety of rimonabant in 1047 overweight or obese patients with Type 2 diabetes that were inadequately controlled by metformin or sulfonylureas Citation[87]. In addition to facilitating weight loss, rimonabant positively affected a number of cardiometabolic risk factors, significantly improving glycaemic control (HbA1c reduced by 0.7% compared with placebo, p < 0.0001), HDL-C, non-HDL-C, triglyceride concentrations and systolic blood pressure compared with placebo ().
A post-hoc analysis of data from the RIO clinical trial programme investigated the effects of rimonabant on systolic and diastolic blood pressure. In the pooled intent-to-treat population, rimonabant (20 mg/day) for 1 year reduced both systolic and diastolic blood pressure by 0.8 mmHg, compared with +0.3 mmHg (p = 0.007) and -0.3 mmHg (p = 0.029), respectively, for placebo Citation[89]. Reductions in blood pressure were greater in patients who had hypertension at the start of the studies.
Rimonabant has also demonstrated efficacy as initial therapy for drug-naive patients with Type 2 diabetes in the recent SERENADE (Study Evaluating Rimonabant Efficacy in Drug-Naive Diabetic patients) trial. After 6 months, treatment with rimonabant 20 mg/day reduced HbA1c by a further -0.5% compared with placebo (-0.8 versus -0.3%, p = 0.0009) Citation[90]. As seen in the RIO-Diabetes trial, rimonabant also significantly improved other cardiometabolic risk factors compared with placebo, such as HDL-C (10.1 versus 3.2%, p < 0.0001) and triglyceride levels (-16.3 versus 4.4%, p < 0.003).
The positive effects on HDL-C, triglycerides and HbA1c and the potentially positive effects on emerging cardiometabolic risk factors, such as CRP, leptin and adiponectin, indicate rimonabant may represent an effective treatment approach to reducing the cardiovascular complications of Type 2 diabetes. Further investigation into the effects of these emerging risk factors is warranted. There is also a need for event-based studies and long-term safety records to evaluate the full potential of rimonabant Citation[91].
Studies are ongoing in several countries evaluating the effect of rimonabant in overweight or obese patients on various end points including plasma glucose in patients with impaired fasting glucose, change in HDL and triglyceride levels in dyslipidaemic patients, change in carotid intima-media thickness, improvement in glycaemic control in patients with Type 2 diabetes already on insulin, cardiovascular morbidity and mortality in patients with cardiovascular risk factors.
6. Managing multiple cardiometabolic risk factors
The Joint British Societies' guidelines on the prevention of CVD recommend a multidisciplinary approach to managing risk factors in patients at high-risk of developing CVD, which includes patients with Type 2 diabetes Citation[92]. Identifying cardiometabolic risk and aggressively treating all modifiable risk factors simultaneously can reduce the complications associated with Type 2 diabetes. Intensive intervention, consisting of behavioural therapy and pharmacotherapy aimed at multiple risk factors, could reduce the risk of cardiovascular and microvascular events by ∼ 50% compared with a more traditional management approach Citation[93].
7. Preventing or delaying the onset of Type 2 diabetes mellitus
This topic has been reviewed elsewhere Citation[94]. Early trials on Type 2 diabetes prevention were based on lifestyle measures; however, these goals are both difficult to achieve and sustain. Several trials based on pharmacological interventions using metformin, orlistat, nateglinide, acarbose, thiazolidinediones, hormone replacement therapy, statins or fibrates have been conducted Citation[94]. Their findings are either encouraging or require more extensive evaluation. Furthermore, studies using ACE inhibitors and angiotensin II receptor antagonists showed that when compared with other antihypertensive agents (e.g., β-blockers and calcium channel antagonists) these drugs can reduce the progression to Type 2 diabetes. The results of the HOPE (Heart Outcomes Prevention Evaluation) trial in patients at high-risk of cardiovascular events suggested that the ACE inhibitor ramipril prevented Type 2 diabetes (secondary outcome). However, this was not seen in the DREAM (Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication) trial, which looked at Type 2 diabetes prevention as a primary outcome Citation[95].
Measures, therefore, exist that could decrease the incidence of Type 2 diabetes. Preventing Type 2 diabetes is relevant because within trials new diabetes is associated with a risk of vascular events ranging between that of established diabetes and non-diabetic patients Citation[96]. In view of the action of rimonabant on metabolic variables, body weight and waist circumference (see above) it is possible to speculate that this drug will reduce the risk of developing Type 2 diabetes in high-risk populations. Partially correcting these factors has been shown to decrease the risk of developing diabetes in the XENDOS (Xenical in the prevention of diabetes in obese subjects) study Citation[97].
8. Conclusions
Patients with Type 2 diabetes are at increased risk of cardiovascular events compared with the non-diabetic population and this risk is further increased by the presence of cardiometabolic risk factors. The chronic state of inflammation associated with obesity may contribute to both insulin resistance and atherosclerosis. Plasma concentrations of inflammatory adipokines produced by adipose tissue and the inflammatory marker CRP may, therefore, provide additional prognostic information about cardiometabolic risk and help guide management.
In addition to providing prognostic information, raised levels of CRP may play a direct role in the progression of atherosclerosis. Many of the therapies presently used to manage individual cardiometabolic risk factors also modulate levels of adipokines and CRP. Treatment with drugs (e.g., statins, angiotensin receptor blockers and rimonabant) that exert beneficial effects on multiple risk factors represent a comprehensive approach to managing cardiometabolic risk in patients with Type 2 diabetes.
9. Expert opinion
The prevalence of obesity is increasing rapidly and is associated with huge costs to healthcare providers. The proportion of the population who are obese is projected to increase over the coming years; therefore, interventions that can help to alleviate this trend will be important.
Elevated waist circumference confers a substantially increased risk of having a cardiovascular event. However, studies such as the Shape of the Nations have shown that the perception of this among healthcare providers is not aligned with the evidence Citation[98,99]. Even if waist circumference is measured, the information may not be acted on. Patient awareness regarding the relevance of waist circumference as a predictor of cardiometabolic risk is also limited.
Many agents are available for the treatment of obesity, CVD and Type 2 diabetes. As these conditions often coexist, any one patient may be taking numerous drugs to treat the individual risk factors. Lifestyle modifications are an option, but sustaining these measures is an issue.
Orlistat, rimonabant and sibutramine all have an effect on weight loss. The rimonabant studies have shown that it also acts on other cardiometabolic risk factors and some of these actions appear to be weight independent. Orlistat also has beneficial effects on risk factors, but its actions may be largely dependent on the weight loss achieved. Sibutramine can raise blood pressure unlike orlistat and rimonabant. Direct comparisons between these three drugs as well as studies that include relevant clinical end points (e.g., vascular events and long-term safety) are needed to define what the ideal treatment is. Combining these treatment options also deserves further investigation.
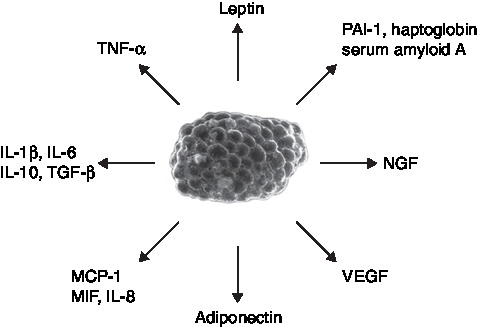
Table 1. International Diabetes Federation definition of the metabolic syndrome: ethnic-specific values for waist circumference.
Table 2. The effect of rimonabant on established and emerging cardiometabolic risk factors in patients with Type 2 diabetes.
Acknowledgements
The authors would like to thank Nicholas Gibbs, BSc, Discovery London, for editorial assistance.
Declaration on interest
Editorial support was funded by sanofi-aventis. The authors have attended conferences, participated in advisory boards and trials and given talks sponsored by various pharmaceutical companies, including sanofi-aventis. The authors were not remunerated for writing this review and they are the guarantors for this article. Open access of this article has been provided through an education grant from sanofi-aventis.
Notes
Bibliography
- BUSE J, GINSBERG H, BAKRIS G et al.: Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American heart association and the American diabetes association. Diabetes Care (2007) 30(1):162-172.
- HAFFNER SM, LEHTO S, RONNEMAA T, PYORALA K, LAAKSO M: Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. (1998) 339(4):229-234.
- ZELLWEGER M: Prognostic significance of silent coronary artery disease in type 2 diabetes. Herz (2006) 31(3):240-245.
- PRADHAN AD, RIDKER PM: Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur. Heart J. (2002) 23(11):831-834.
- HOTAMISLIGIL GS: Inflammatory pathways and insulin action. Int. J. Obes. Relat. Metab. Disord. (2003) 27(Suppl. 3):S53-S55.
- HOTAMISLIGIL GS, SHARGILL NS, SPIEGELMAN BM: Adipose expression of TNF-α: direct role in obesity-linked insulin resistance. Science (1993) 259(5091):87-91.
- DIAMANT M, LAMB HJ, VAN DE REE MA et al.: The association between abdominal visceral fat and carotid stiffness is mediated by circulating inflammatory markers in uncomplicated type 2 diabetes. J. Clin. Endocrinol. Metab. (2005) 90(3):1495-1501.
- HASLAM DW, JAMES WP: Obesity. Lancet (2005) 366(9492):1197-1209.
- DESPRES JP: Health consequences of visceral obesity. Ann. Med. (2001) 33(8):534-541.
- VAGUE J: La différenciation sexuelle, facteur déterminant des formes de l'obésité. Presse Med. (1947) 30:339-340.
- POULIOT MC, DESPRES JP, LEMIEUX S et al.: Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am. J. Cardiol. (1994) 73(7):460-468.
- KERSHAW EE, FLIER JS: Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. (2004) 89(6):2548-2556.
- PARASKEVAS KI, LIAPIS CD, MIKHAILIDIS DP: Leptin: a promising therapeutic target with pleiotropic action besides body weight regulation. Curr. Drug Targets (2006) 7(6):761-771.
- WALLACE AM, MCMAHON AD, PACKARD CJ et al.: Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation (2001) 104(25):3052-3056.
- COUILLARD C, LAMARCHE B, MAURIEGE P et al.: Leptinemia is not a risk factor for ischemic heart disease in men. Prospective results from the Quebec cardiovascular study. Diabetes Care (1998) 21(5):782-786.
- SCHROETER MR, SCHNEIDERMAN J, SCHUMANN B et al.: Expression of the leptin receptor in different types of vascular lesions. Histochem. Cell Biol. (2007).
- CHAO HH, HONG HJ, LIU JC et al.: Leptin stimulates endothelin-1 expression via extracellular signal-regulated kinase by epidermal growth factor receptor transactivation in rat aortic smooth muscle cells. Eur. J. Pharmacol. (2007).
- JAGROOP IA, DASKALOPOULOU SS, MIKHAILIDIS DP: Endothelin-1 and human platelets. Curr. Vasc. Pharmacol. (2005) 3(4):393-399.
- CHANDRAN M, PHILLIPS SA, CIARALDI T, HENRY RR: Adiponectin: more than just another fat cell hormone? Diabetes Care (2003) 26(8):2442-2450.
- OUCHI N, KIHARA S, ARITA Y et al.: Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation (1999) 100(25):2473-2476.
- KOHLER HP, GRANT PJ: Plasminogen-activator inhibitor type 1 and coronary artery disease. N. Engl. J. Med. (2000) 342(24):1792-1801.
- FESTA A, D'AGOSTINO R Jr, TRACY RP, HAFFNER SM: Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes (2002) 51(4):1131-1137.
- YUDKIN J, KUMARI M, HUMPHRIES S, MOHAMED-ALI V: Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis (2000) 148(2):209-214.
- RUAN H, MILES PD, LADD CM et al.: Profiling gene transcription in vivo reveals adipose tissue as an immediate target of TNF-α: implications for insulin resistance. Diabetes (2002) 51(11):3176-3188.
- ECKEL RH, GRUNDY SM, ZIMMET PZ: The metabolic syndrome. Lancet (2005) 365(9468):1415-1428.
- YUDKIN JS: Adipose tissue, insulin action and vascular disease: inflammatory signals. Int. J. Obes. Relat. Metab. Disord. (2003) 27(Suppl. 3):S25-S28.
- KOENIG W, SUND M, FROHLICH M et al.: C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring trends and determinants in cardiovascular disease) Augsburg Cohort study, 1984 to 1992. Circulation (1999) 99(2):237-242.
- RIDKER PM, RIFAI N, ROSE L, BURING JE, COOK NR: Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. (2002) 347(20):1557-1565.
- SAKKINEN P, ABBOTT R, CURB J et al.: C-reactive protein and myocardial infarction. J. Clin. Epidemiol. (2002):445-451.
- FORD ES, GILES WH: Serum C-reactive protein and self-reported stroke: findings from the third National health and nutrition examination survey. Arterioscler. Thromb. Vasc. Biol. (2000) 20(4):1052-1056.
- RUTTER MK, MEIGS JB, SULLIVAN LM, D'AGOSTINO RB Sr, WILSON PWF: C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham offspring study. Circulation (2004) 110(4):380-385.
- KAHN SE, ZINMAN B, HAFFNER SM et al.: Obesity is a major determinant of the association of C-reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes (2006) 55(8):2357-2364.
- MALIK S, WONG ND, FRANKLIN S et al.: Cardiovascular disease in US patients with metabolic syndrome, diabetes, and elevated C-reactive protein. Diabetes Care (2005) 28(3):690-693.
- PU LJ, LU L, XU XW et al.: Value of serum glycated albumin and high-sensitivity C-reactive protein levels in the prediction of presence of coronary artery disease in patients with type 2 diabetes. Cardiovasc. Diabetol. (2006) 5:27.
- LINNEMANN B, VOIGT W, NOBEL W, HU J: C-reactive protein is a strong independent predictor of death in type 2 diabetes: association with multiple facets of the metabolic syndrome. Exp. Clin. Endocrinol. Diabetes (2006) 114(3):127-134.
- SMITH SC Jr, ANDERSON JL, CANNON RO III et al.: CDC/AHA workshop on markers of inflammation and cardiovascular disease: application to clinical and public health practice: report from the clinical practice discussion group. Circulation (2004) 110(25):E550-E553.
- PEARSON TA, MENSAH GA, ALEXANDER RW et al.: Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American heart association. Circulation (2003) 107(3):499-511.
- PASCERI V, WILLERSON JT, YEH ETH: Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation (2000) 102(18):2165-2168.
- JIALAL I, DEVARAJ S, VENUGOPAL SK: C-reactive protein: risk marker or mediator in atherothrombosis? Hypertension (2004) 44(1):6-11.
- GOLDSTEIN D: Beneficial health effects of modest weight loss. Int. J. Obes. Relat. Metab. Disord. (1992) 16(6):397-415.
- ESMAILLZADEH A, KIMIAGAR M, MEHRABI Y et al.: Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am. J. Clin. Nutr. (2006) 84(6):1489-1497.
- JAE S, FERNHALL B, HEFFERNAN K et al.: Effects of lifestyle modifications on C-reactive protein: contribution of weight loss and improved aerobic capacity. Metabolism (2006) 55(6):825-831.
- SELVIN E, PAYNTER N, ERLINGER T: The effect of weight loss on C-reactive protein: a systematic review. Arch. Intern. Med. (2007) 167(1):31-39.
- PAPADAKIS JA, MILIONIS HJ, PRESS M, MIKHAILIDIS DP: Treating dyslipidaemia in non-insulin-dependent diabetes mellitus – a special reference to statins. J. Diabetes Complications (2001) 15(4):211-226.
- VIJAN S, HAYWARD RA: Pharmacologic lipid-lowering therapy in type 2 diabetes mellitus: background paper for the American college of physicians. Ann. Intern. Med. (2004) 140(8):650-658.
- ATHYROS VG, KAKAFIKA AI, PAPAGEORGIOU AA et al.: Atorvastatin decreases triacylglycerol-associated risk of vascular events in coronary heart disease patients. Lipids (2007).
- ALBERT MA, DANIELSON E, RIFAI N, RIDKER PM; FOR THE PRINCE INVESTIGATORS: Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA (2001) 286(1):64-70.
- RIDKER PM, CANNON CP, MORROW D et al.: C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. (2005) 352(1):20-28.
- NISSEN SE, TUZCU EM, SCHOENHAGEN P et al.: Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N. Engl. J. Med. (2005) 352(1):29-38.
- KINLAY S: Low-density lipoprotein-dependent and -independent effects of cholesterol-lowering therapies on C-reactive protein: a meta-analysis. J. Am. Coll. Cardiol. (2007) 49(20):2003-2009.
- Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the bezafibrate infarction prevention (BIP) study. Circulation (2000) 102(1):21-27.
- KEECH A, SIMES R, BARTER P et al.: Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet (2005) 366(9500):1849-1861.
- RUBINS HB, ROBINS SJ, COLLINS D et al.: Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the department of Veterans affairs high-density lipoprotein intervention trial (VA-HIT). Arch. Intern. Med. (2002) 162(22):2597-2604.
- STAELS B, KOENIG W, HABIB A et al.: Activation of human aortic smooth-muscle cells is inhibited by PPAR[α] but not by PPAR[γ] activators. (1998) 393(6687):790-793.
- TSIMIHODIMOS V, KOSTOULA A, KAKAFIKA A et al.: Effect of fenofibrate on serum inflammatory markers in patients with high triglyceride values. J. Cardiovasc. Pharmacol. Ther. (2004) 9:27-33.
- RIZOS E, KOSTOULA A, ELISAF M, MIKHAILIDIS DP: Effect of ciprofibrate on C-reactive protein and fibrinogen levels. Angiology (2002) 53(3):273-277.
- CANNER P, FURBERG C, MCGOVERN M: Benefits of niacin in patients with versus without the metabolic syndrome and healed myocardial infarction (from the coronary drug project). Am. J. Cardiol. (2006) 97(4):477-479.
- ELAM MB, HUNNINGHAKE DB, DAVIS KB et al.: Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: a randomized trial. Arterial disease multiple intervention trial. JAMA (2000) 284(10):1263-1270.
- PEARSON TA, DENKE MA, MCBRIDE PE et al.: A community-based, randomized trial of ezetimibe added to statin therapy to attain NCEP ATP III goals for LDL cholesterol in hypercholesterolemic patients: the ezetimibe add-on to statin for effectiveness (EASE) trial. Mayo Clin. Proc. (2005) 80(5):587-595.
- MIKHAILIDIS DP, SIBBRING GC, BALLANTYNE CM, DAVIES GM, CATAPANO AL: Meta-analysis of the cholesterol-lowering effect of ezetimibe added to ongoing statin therapy. Curr. Med. Res. Opin. (2007) 23(8):2009-2026.
- GAZI IF, DASKALOPOULOU SS, NAIR DR, MIKHAILIDIS DP: Effect of ezetimibe in patients who cannot tolerate statins or cannot get to the low density lipoprotein cholesterol target despite taking a statin. Curr. Med. Res. Opin. (2007).
- KALOGIROU M, TSIMIHODIMOS V, GAZI I et al.: Effect of ezetimibe monotherapy on the concentration of lipoprotein subfractions in patients with primary dyslipidaemia. Curr. Med. Res. Opin. (2007) 23(5):1169-1176.
- NATHAN DM, CLEARY PA, BACKLUND JY et al.: Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N. Engl. J. Med. (2005) 353(25):2643-2653.
- UK PROSPECTIVE DIABETES STUDY (UKPDS) GROUP: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet (1998) 352(9131):837-853.
- UKPDS GROUP: Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet (1998) 352(9131):854-865.
- SATOH N, OGAWA Y, USUI T et al.: Antiatherogenic effect of pioglitazone in type 2 diabetic patients irrespective of the responsiveness to its antidiabetic effect. Diabetes Care (2003) 26(9):2493-2499.
- AGARWAL R: Anti-inflammatory effects of short-term pioglitazone therapy in men with advanced diabetic nephropathy. Am. J. Physiol. Renal Physiol. (2006) 290(3):F600-F605.
- MATTOO V, ECKLAND D, WIDEL M et al.: Metabolic effects of pioglitazone in combination with insulin in patients with type 2 diabetes mellitus whose disease is not adequately controlled with insulin therapy: results of a six-month, randomized, double-blind, prospective, multicenter, parallel-group study. Clin. Ther. (2005) 27(5):554-567.
- REYNOLDS L, KINGSLEY F, KAROUNOS D, TANNOCK L: Differential effects of rosiglitazone and insulin glargine on inflammatory markers, glycemic control, and lipids in type 2 diabetes. Diabetes Res. Clin. Pract. (2007) 77(2):180-187.
- YU D, MURDOCH S, PARIKH S et al.: Rosiglitazone increases LDL particle size and buoyancy and decreases C-reactive protein in patients with type 2 diabetes on statin therapy. Diab. Vasc. Dis. Res. (2006) 3(3):189-196.
- HOME PD, POCOCK SJ, BECK-NIELSEN H et al.: Rosiglitazone evaluated for cardiovascular outcomes – an interim analysis. N. Engl. J. Med. (2007):NEJMoa073394.
- NISSEN SE, WOLSKI K: Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. (2007) 356(24):2457-2471.
- LINDBERG M, ASTRUP A: The role of glitazones in management of type 2 diabetes. A DREAM or a nightmare? Obes. Rev. (2007) 8(5):381-384.
- GERRITS CM, BHATTACHARYA M, MANTHENA S et al.: A comparison of pioglitazone and rosiglitazone for hospitalization for acute myocardial infarction in type 2 diabetes. Pharmacoepidemiol. Drug Saf. (2007).
- UK PROSPECTIVE DIABETES STUDY GROUP: Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ (1998) 317(7160):703-713.
- PERSSON F, ROSSING P, HOVIND P et al.: Irbesartan treatment reduces biomarkers of inflammatory activity in patients with type 2 diabetes and microalbuminuria: an IRMA 2 substudy. Diabetes (2006) 55(12):3550-3555.
- BAKRIS GL, FONSECA V, KATHOLI RE et al.: Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA (2004) 292(18):2227-2236.
- KARAGIANNIS A, MIKHAILIDIS DP, TZIOMALOS K, KAKAFIKA AI, ATHYROS VG: Has the time come for a new definition of microalbuminuria? Expert Opin. Pharmacother. (2007).
- FINER N: Does pharmacologically induced weight loss improve cardiovascular outcome? Impact of anti-obesity agents on cardiovascular risk factors. Eur. Heart J. (2005) 7(Suppl. 7):L32-L38.
- LI Z, MAGLIONE M, TU W et al.: Meta-analysis: pharmacologic treatment of obesity. Ann. Intern. Med. (2005) 142(7):532-546.
- PADWAL R, MAJUMDAR S: Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet (2007) 369:71-77.
- JAMES WP, ASTRUP A, FINER N et al.: Effect of sibutramine on weight maintenance after weight loss: a randomised trial. STORM study group. Sibutramine trial of obesity reduction and maintenance. Lancet (2000) 356(9248):2119-2125.
- FILIPPATOS TD, KIORTSIS DN, LIBEROPOULOS EN et al.: Effect of orlistat, micronised fenofibrate and their combination on metabolic parameters in overweight and obese patients with the metabolic syndrome: the FenOrli study. Curr. Med. Res. Opin. (2005) 21(12):1997-2006.
- PI-SUNYER FX, ARONNE LJ, HESHMATI HM, DEVIN J, ROSENSTOCK J: Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA (2006) 295(7):761-775.
- VAN GAAL LF, RISSANEN AM, SCHEEN AJ, ZIEGLER O, ROSSNER S: Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet (2005) 365(9468):1389-1397.
- DESPRES JP, GOLAY A, SJOSTROM L: Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N. Engl. J. Med. (2005) 353(20):2121-2134.
- SCHEEN AJ, FINER N, HOLLANDER P, JENSEN MD, VAN GAAL LF: Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet (2006) 368(9548):1660-1672.
- WIERZBICKI AS: Rimonabant: endocannabinoid inhibition for the metabolic syndrome. Int. J. Clin. Pract. (2006) 60(12):1697-1706.
- VAN GAAL L, SCHEEN A, DESPRES JP et al.: Effect of rimonabant on systolic and diastolic blood pressure in overweight/obese patients with/without co-morbidities. European Society of Hypertension Annual Meeting. Madrid, Spain (2006).
- IRANMANESH A, ROSENSTOCK J, HOLLANDER P; ON BEHALF OF THE SERENADE STUDY GROUP: SERENADE: rimonabant monotherapy for treatment of multiple cardiometabolic risk factors in treatment-naïve patients with type 2 diabetes. The International Diabetes Federation 19th World Diabetes Congress. Cape Town, South Africa (3 – 7 December 2006).
- KAKAFIKA AI, MIKHAILIDIS DP, KARAGIANNIS A, ATHYROS VG: The role of endocannabinoid system bockade in the treatment of the metabolic syndrome. J. Clin. Pharmacol. (2007) 47:642-652.
- JBS2: JBS 2: Joint British Societies' guidelines on prevention of cardiovascular disease in clinical practice. Heart (2005) 91(Suppl. 5):V1-V52.
- GAEDE P, VEDEL P, LARSEN N et al.: Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N. Engl. J. Med. (2003) 348(5):383-393.
- LIBEROPOULOS EN, TSOULI S, MIKHAILIDIS DP, ELISAF MS: Preventing type 2 diabetes in high risk patients: an overview of lifestyle and pharmacological measures. Curr. Drug Targets (2006) 7(2):211-228.
- SCHEEN AJ: DREAM study: prevention of type 2 diabetes with ramipril and/or rosiglitazone in persons with dysglycaemia but no cardiovascular desease. Revue medicale de Liege (2006) 61(10):728-732.
- AKSNES TA, KJELDSEN SE, ROSTRUP M et al.: Impact of new-onset diabetes mellitus on cardiac outcomes in the valsartan antihypertensive long-term use evaluation (VALUE) trial population. Hypertension (2007) 50(3):467-473.
- TORGERSON JS, HAUPTMAN J, BOLDRIN MN, SJOSTROM L: XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care (2004) 27(1):155-161.
- Shape of the Nations. World Heart Federation (2005).
- WIERZBICKI AS, GANOTAKIS ES, MIKHAILIDIS DP: Shape of the Nations survey and attitudes to cardiometabolic risk. Curr. Med. Res. Opin. (2007) 23(1):25-28.
Websites
- http://www.who.int/dietphysicalactivity/media/en/gsfs_obesity.pdf Obesity and overweight (2003).
- http://www.ic.nhs.uk/pubs/hlthsvyeng2004upd Health survey for England 2004. Updating of trend tables to include 2004 data (2005).
- http://www.idf.org/home/index.cfm?unode=32EF2063-B966-468F-928C-A5682A4E3910 The IDF worldwide definition of the metabolic syndrome (2005).
- http://www.emea.europa.eu/humandocs/PDFs/EPAR/acomplia/32982607en.pdf Press release: European Medicines Agency recommends Acomplia must not be used in patients on antidepressants or with major depression (2007).
- http://www.fda.gov/ohrms/dockets/AC/07/questions/2007-4306q-fda-questionstocommittee-draft.pdf Advisory Committee meeting for Rimonobant (Zimulti™) (2007).