Abstract
Development of personalized medicine involves integration of several biotechnologies. This editorial stresses the important role that biological therapies such as cell and gene therapies, recombinant proteins and vaccines play in personalization of treatment. Cell-based therapies, particularly vaccines made from the patient's own tumor cells, were the first therapeutic vaccines for cancer. Adoptive cell therapy is an immunotherapy based on ex vivo expansion of autologous T lymphocytes and subsequent administration to cancer patients. Stem cells as well as genetic modification of cells has been used for in vivo production of therapeutic substances best suited for individual patients. Besides cell therapy, RNAi has been used for personalized therapy of cancer. Monoclonal antibodies, designed to bind specifically to receptors in certain tumors, are also personalized medicines.
1. Introduction
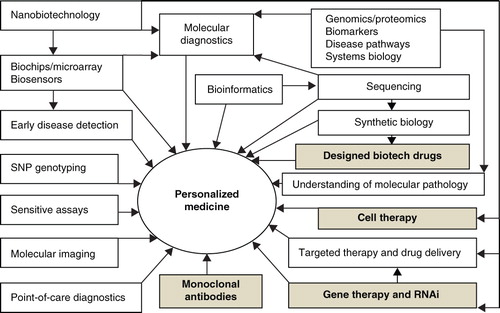
Personalized medicine is simply defined as the right drug for the right patient. A more comprehensive definition of personalized medicine is the prescription of specific therapeutics best suited for an individual taking into consideration both genetic and environmental factors that influence response to therapy Citation[1]. The term ‘genomic medicine’ implies that the sequencing of the human genome has enabled the practice of medicine to enter an era in which the individual patient's genome will help determine the optimal approach to care, whether it is preventive, diagnostic or therapeutic. Genomic medicine is not an adequate synonym for personalized medicine as other factors are also taken into consideration. Besides genomics, proteomic technologies have facilitated the development of personalized medicines and other technologies such as metabolomics are also contributing to this effort. Historically blood transfusion and organ transplantation were the first personalized therapies as they were matched to the individuals. Some cell therapies that use patient's own cells are considered to be personalized medicines, particularly vaccines prepared from the individual patient's tumor cells. More recently recombinant human proteins have enabled individualization of treatment. Biological therapies were personalized before the human genome was sequenced. Personalized medicine is the best way to integrate new biotechnologies into practice of medicine for improving the management of patients ().
2. Recombinant human proteins
A large number of therapeutic proteins have been approved for clinical use and many more are undergoing preclinical studies and clinical trials in humans. Most of these proteins are human or 'humanized' recombinant molecules. Immune response to therapeutic proteins in conventional animal models is usually not predictive of the response in humans and immunogenicity of therapeutic proteins can cause adverse effects. In order to assess immunogenicity of these molecules, appropriate detection, quantitation and characterization of antibody responses are necessary. There has been a considerable progress in development of computational methods for prediction of epitopes in protein molecules that have the potential to induce an immune response in a recipient. Such tools are already being applied in the development of therapeutic proteins. It is expected that prediction based on bioinformatics followed by in vitro and/or in vivo testing of any potentially immunogenic epitopes will help in avoiding, or at least minimizing, immune responses to therapeutic proteins. It is possible to develop recombinant proteins in combination with diagnostic tests to limit their use to patients where they are not only effective but also unlikely to induce immune reactions. Another approach to protein therapy is in vivo production of proteins by genetically engineered cells where delivery of proteins can be matched to the needs of the patient by in vivo production.
3. Therapeutic monoclonal antibodies
Compared with small-molecule drugs, mAbs are very specific and are less likely to cause toxicity based on factors other than the mechanism of action. Orally available small molecules have many targets but they may also be hepatotoxic and are involved in drug–drug interactions. They may interfere with cytochrome P-450. From the point of view of a clean safety profile, mAbs are extremely attractive. They can be designed to be very specific with high affinity for the target.
mAbs have been viewed as ideal molecules for cancer therapy for several years. Genetic engineering of mAbs to produce chimeric or humanized mAbs has greatly advanced their utility in molecular targeting therapies. Many clinical trials of mAbs as a single agent, or in combination protocol with current standard chemotherapy or immunoconjugates have shown promise in the treatment of specific diseases such as cancer.
4. Cell therapy
Cell therapy is the prevention or treatment of human disease by the administration of cells that have been selected, multiplied and pharmacologically treated or altered outside the body (ex vivo). A patient's own stem cells can be mobilized in vivo for therapeutic purposes and several drugs including biological agents are under investigations for this purpose Citation[2]. The aim of cell therapy is to replace, repair or enhance the function of damaged tissues or organs. The cells used can originate from the patient or from a donor or from another species. Other sources include cell lines and cells from patients' tumors to make personalized cancer vaccines. Cells can be encapsulated in selectively permeable membranes that block entry of immune mediators but allow outward diffusion of active molecules produced by the cells. Genetic engineering of cells is part of ex vivo gene therapy. The cells may be introduced by various routes into the body and selectively implanted at the site of action.
Several types of stem cells including embryonic stem cells can be used for developing personalized cell therapies or screening personalized medicines. One example is that of mesenchymal stem cells (MSCs). With the ability to isolate, expand and study MSCs in vitro, individual patient's MSCs can be tested for their sensitivity to various drugs. Potential applications are:
1. Selection of individual dosing regimens based on the in vitro responsiveness in a simple assay performed using a patient's own MSCs.
2. Optimized treatment plans can be created that efficiently and precisely match the host's expected biological response.
3. Hematopoietic stem cell transplantation has been combined with MSCs to enhance the effectiveness of the transplant and to suppress graft-versus-host disease, which has a potential for personalized treatment, but a major concern is the dual immune effects of MSCs: stimulator as well as suppressor effects Citation[3].
5. Personalized vaccines
The next era in vaccinology will be ushered in by vaccinomics that will enable the development of personalized vaccines, based on an increasing understanding of immune response phenotype/genotype information by applications of various ‘omics’ technologies. Two important areas for application of personalized vaccines are viral infections and cancer.
Many factors can contribute to the heterogeneity of vaccine-induced immune responses, including polymorphisms of immune response genes. Identification of genes involved directly or indirectly in the generation of the immune response to vaccines is important as polymorphisms may influence vaccine failure and vaccine-associated adverse events Citation[4]. Rapid advances in developing personalized vaccines are already occurring for hepatitis B, influenza, measles, mumps, rubella, anthrax and smallpox.
Cancer vaccines attempt to harness the specificity and resistance potentials of the human immune system. The aim of cancer vaccines is to stimulate the immune system to recognize, attack and destroy tumor cells. In contrast to vaccines for prophylaxis of infectious diseases, cancer vaccines are therapeutic and most of the personalized cancer vaccines are cell-based Citation[5]. Adoptive cell therapy (ACT) is the isolation of antigen-specific T lymphocytes, their ex vivo expansion and activation, and subsequent administration in large numbers to the autologous host. Clinical trials have shown the effectiveness of ACT for the treatment of patients with selected metastatic cancers, for example metastatic melanoma.
6. Gene therapy
Gene therapy is defined as the transfer of defined genetic material to specific target cells of a patient for the ultimate purpose of preventing or altering a particular disease state. The broad scope of gene therapy includes replacement of absent or defective DNA with functional DNA to modulate gene expression (increase or decrease) by the delivery of engineered genes or other genetic material via vectors, including naked plasmid DNA, viruses and cells. Cells may be genetically modified to secrete therapeutic substances such as neurotrophic factors. Ex vivo gene therapy involves the genetic modification of the patient's cells in vitro, mostly by use of viral vectors, prior to reimplanting these cells into the tissues of the patient's body. Additional DNA elements may be used that turn on the healthy gene in the right cells and at the right levels. This is a form of personalized therapy.
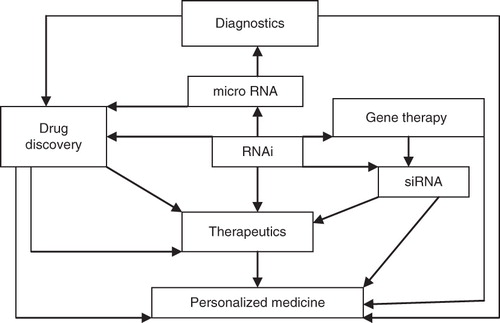
Antisense therapy, which involves the use of antisense oligonucleotides (AOs) to block abnormal disease-related proteins, is considered to be form of gene therapy because it is modulation of gene function for therapeutic purposes. However, AOs differ from standard gene therapies because they cannot give rise to proteins but can only block the expression of existing genes. As new technologies are overcoming some of the drug delivery problems of AOs, therapeutic applications are more promising for personalized medicine. RNA interference (RNAi) is a refined version of antisense to regulate the expression of genes. siRNAs can not only be used as a tool to study gene function but might also be used as genotype-specific drugs to mediate allele-specific inhibition. siRNA has been shown to produce genotype-specific inhibition of tumor growth in vivo, by targeting an SNP in POLR2A (gene of the large subunit of RNA polymerase II located in close proximity to the tumor suppressor gene p53, which frequently shows loss of heterozygosity in cancer cells Citation[6]. Thus RNAi may play an important role in personalized medicine. siRNAs or short hairpin RNAs (shRNAs) can be targeted to tumors via bispecific antibodies attached to the surface of minicells. Minicell-based therapy has shown highly effective targeted delivery with tumor regression in dogs with late-stage endogenous tumors and has potential therapeutic applications in personalized cancer medicine Citation[7]. The role of RNAi in the development of personalized medicine is shown in .
7. Expert opinion
Biological therapies are not only playing an important role in new therapeutics, they are also providing opportunities for developing personalized medicine. Biomarkers will serve as a common reference point for diagnosis as well as development of personalized biological therapies Citation[8]. Cell and gene therapies are most promising among biological therapies.
Cell therapies will play an important role in the development of personalized medicine. Adult stem cells of the individual patient are more suitable for personalized therapy and also avoid some of the ethical issues involved in the use of embryonic stem cells. Availability of technologies to derive induced pluripotent stem cells from adult somatic cells will enhance the potential of personalized cell-based therapy.
Gene therapy will be increasingly used for personalizing cancer treatment. Although biomarkers based on single-gene mutations are commonly used in clinical oncology practice currently, gene-expression signatures and imaging technologies have the potential to play important roles as biomarkers in the future Citation[9].
Antisense therapies lend themselves to personalization more readily than many other drugs. The reasons are as follows:
1. Antisense compounds target a disease at its genetic origin and modulate expression of the gene product whereas conventional pharmaceuticals merely counteract the manifestations of the disease by inhibiting gene products (proteins).
2. Antisense compounds can be easily designed and only require information on the nucleic acid sequence encoding a given protein without prior knowledge of the function of that protein.
3. Antisense DNA and RNA have an extremely high specificity for their target which cannot be usually achieved by conventional pharmaceuticals.
4. Antisense may also provide more disease-specific therapies and have less adverse reactions than conventional pharmaceuticals.
Vaccines can be personalized and the most promising ones are for treatment of cancer. Recombinant proteins have potential for personalized therapeutics. Furthermore, novel mAb designs and improved understanding of the mode of action of current mAbs indicates a promising future for this therapeutic approach. The accumulating results from many basic, clinical and translational studies may lead to more individualized therapeutic strategies using these agents directed at specific genetic and immunological targets. In the future, it is expected that synthetic biology will enable construction of cells that can be used for designing personalized biological therapies.
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Notes
Bibliography
- Jain KK. Textbook of Personalized Medicine. Springer; New York: 2009
- Jain KK. Cell therapy: technologies, markets & Co. Jain PharmaBiotech Publications; Basel: 2011
- Patel SA, Rameshwar P. Stem Cell transplantation for hematological malignancies: prospects for personalized medicine and co-therapy with mesenchymal stem cells. Curr Pharmacogenomics Pers Med 2011;9:229-39
- Poland GA, Ovsyannikova IG, Jacobson RM. Application of pharmacogenomics to vaccines. Pharmacogenomics 2009;10:837-52
- Jain KK. Personalized cancer vaccines. Expert Opin Biol Ther 2010;10:1637-47
- Mook OR, Baas F, de Wissel MB, Fluiter K. Allele-specific cancer cell killing in vitro and in vivo targeting a single-nucleotide polymorphism in POLR2A. Cancer Gene Ther 2009;16:532-8
- Macdiarmid JA, Brahmbhatt H. Minicells: versatile vectors for targeted drug or si/shRNA cancer therapy. Curr Opin Biotechnol 2011: published online 6 May 2011; doi:10.1016/j.copbio.2011.04.008
- Jain KK. A Handbook of Biomarkers. Springer; New York: 2010
- Wistuba II, Gelovani JG, Jacoby JJ, Methodological and practical challenges for personalized cancer therapies. Nat Rev Clin Oncol 2011;8:135-41