Abstract
Human epidermal growth factor receptor 2 (HER2) was acknowledged as an important therapeutic target in breast cancer more than 25 years ago. Subsequently, significant basic science and translational discoveries have resulted in the approval of four HER2-targeted therapies over the past 15 years. This editorial discusses future challenges regarding selection and development of treatments for HER2-positive breast cancer, which can only be met by continuing to support research efforts into the basic mechanisms by which cancer cells escape targeted therapies. Identifying specific molecular mechanisms underlying the sensitivity or resistance to each HER2-targeted agent will ultimately allow individualized therapy for each patient.
1. Reviewing the relevance of HER2 as a target in breast cancer
Human epidermal growth factor receptor 2 (HER2/erbB2) is a member of the epidermal growth factor receptor (EGFR) family of tyrosine kinases. This family consists of four receptors: EGFR, HER2, HER3, and HER4. Binding of ligands to EGFR, HER3, and HER4 has been associated with conformational changes that result in the release of an intramolecular tether between loops in subdomains II and IV of HER3 Citation[1]. The resulting open conformation allows receptors to form homo- or heterodimers. HER2 plays a critical role as a preferred interaction partner in these dimers. HER2 is normally fixed in an open conformation that resembles a ligand-activated state despite the absence of ligand Citation[2]. This extended conformation allows HER2 to readily interact with other receptors, which appears to increase the affinity of dimer pairs for ligands, resulting in an even further increase in kinase activity. The HER2-HER3 heterodimer is the most potent signaling pair in this family partly due to the presence of multiple PI3K docking sites in the intracellular tail of HER3.
Amplification and overexpression of the HER2 gene was first reported in 1987 in approximately 20 – 30% of breast cancers Citation[3]. HER2 was established as a relevant therapeutic target based on its unique overexpression at the cell surface and its association with high tumor grade and metastatic disease. Strategies to target HER2 resulted in the development and clinical approval of the monoclonal antibody (mAb) trastuzumab, which binds to the juxtamembrane region in domain IV of HER2 Citation[2,4]. Trastuzumab achieves significant response rates in patients with HER2-positive metastatic breast cancer in combination with chemotherapy. However, as is the case with many targeted cancer therapies, tumors eventually escape growth inhibition. Changing the chemotherapeutic agent in the regimen is often enough to yield an improvement in response. However, additional HER2-targeted drugs are now available, which may result in longer progression-free survival rates for patients whose breast tumors overexpress HER2.
Lapatinib is a dual tyrosine kinase inhibitor (TKI) of EGFR and HER2 and is approved in combination with capecitabine for trastuzumab-refractory disease. Many patients show resistance to lapatinib, raising the concern that mechanisms that are responsible for resistance to trastuzumab may also contribute to cross-resistance to other HER-targeted agents.
Pertuzumab is a mAb that binds to domain II of HER2, sterically hindering interactions between HER2 and other receptors Citation[4]. As a result, pertuzumab has been shown to disrupt HER2-HER3 and HER2-EGFR heterodimer formation and block ligand-stimulated activation of HER2-containing receptor complexes Citation[5]. The combination of pertuzumab and trastuzumab was shown to synergistically inhibit growth of HER2-overexpressing breast cancer Citation[6] and to enhance tumor regression in xenografts of HER2-positive breast cancer Citation[7]. Both antibodies appear to share some mechanisms of action, such as inducing antibody-dependent cellular cytotoxicity. However, the combined benefit of the antibodies may be due to distinct mechanisms of action, such as trastuzumab-mediated inhibition of HER2 extracellular domain cleavage and pertuzumab-mediated disruption of HER2 dimerization Citation[7].
Clinical trials have validated the benefit of combining trastuzumab with pertuzumab for treating HER2-positive metastatic breast cancers. Treatment with pertuzumab and trastuzumab achieved an objective response rate of 24.2% and clinical benefit of 50% against trastuzumab-refractory breast cancers Citation[8]. In another trial, single-agent pertuzumab did not suppress disease progression in contrast to trastuzumab plus pertuzumab Citation[9]. The clinical benefit rate for pertuzumab alone versus the combination was 10.3 vs 41.2% Citation[9]. Pathologic complete response rates of 45.8 vs 29% were also reported in patients treated with trastuzumab, pertuzumab, and docetaxel vs trastuzumab plus docetaxel Citation[10]. Finally, Phase III data showed that trastuzumab plus pertuzumab with chemotherapy improved progression-free (18.5 months vs 12.4 months) and overall survival relative to trastuzumab plus chemotherapy Citation[11]. The combination of pertuzumab, trastuzumab, and docetaxel is now approved for patients with HER2-positive metastatic breast cancers who have not received prior HER2 therapy, i.e., as a first-line treatment for HER2-over-expressing breast cancer.
2. Expert opinion on pertuzumab
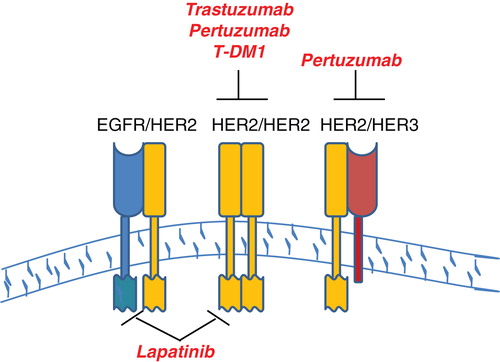
Drug development efforts targeted against HER2-positive breast cancer have shown tremendous success over the past decade, resulting in multiple treatment options for patients. Trastuzumab-emtansine (T-DM1) was approved in February 2013 for trastuzumab-refractory metastatic HER2-positive breast cancer. This antibody-chemotherapy conjugate represents a new effort to chemically link a targeted drug with a cytotoxic chemotherapeutic agent, resulting in selective delivery of a microtubule inhibitor plus anti-HER2 therapy to HER2-overexpressing tumors. Approval of T-DM1 was based on the Phase III EMILIA trial, which showed improved progression-free and overall survival with T-DM1 vs lapatinib and capecitabine in patients with trastuzumab-refractory disease. Thus, there are now four selective HER2-targeted agents approved for patients with HER2-overexpressing breast cancer, which are trastuzumab, lapatinib, pertuzumab, and trastuzumab-emtansine ().
Figure 1. Schematic of HER2-targeted therapies. There are four selective HER2-targeted agents currently approved by the FDA for patients with HER2-over-expressing breast cancer. The schematic shows EGFR, HER2, and HER3 at the cell surface. Trastuzumab is a monoclonal antibody against extracellular domain IV of HER2. Pertuzumab binds extracellular domain II of HER2, interfering with and disrupting interactions between HER2 and other receptors, such as HER3. Trastuzumab-emtansine is a novel antibody-drug conjugate, in which trastuzumab is chemically linked to a microtubule inhibiting chemotherapeutic agent. Lapatinib is a dual EGFR/HER2 TKI.
One of the major issues that will face clinical and scientific investigators is how to decide which HER2-targeted therapy to use. Trastuzumab plus chemotherapy has been the front-line choice for HER2-overexpressing metastatic breast cancer for more than a decade. Now, trastuzumab plus pertuzumab is approved for use in the front-line setting. T-DM1 is likely to replace lapatinib as the treatment of choice for trastuzumab-refractory disease based on the compelling results of the EMILIA trial. The decision of whether to include pertuzumab in a first-line or refractory setting may warrant additional biomarker screening. Currently, HER2 immunohistochemistry (IHC) and/or fluorescent in situ hybridization are performed on breast tumor biopsies. It is reasonable to propose that elevated co-expression of HER3 or EGFR may predict for the subset of tumors that are more likely to respond to pertuzumab. In fact, pre-clinical testing showed that trastuzumab disrupted HER2 homodimer-driven signaling, whereas pertuzumab disrupted heregulin-stimulated signaling, which is dependent upon HER3 Citation[12]. Thus, the decision to treat with trastuzumab plus pertuzumab may be based upon rational selection of patients whose tumors over-express HER2 and HER3. In addition to predicting for response to pertuzumab Citation[12], HER3 signaling was associated with resistance to lapatinib in pre-clinical studies Citation[13]. Thus, biomarker analysis of HER3 expression by IHC may serve as a useful test for deciding whether to include pertuzumab in an individualized treatment regimen.
The sequence in which the drugs are administered may also alter the response or toxicities related to the combination. Although simultaneous treatment with pertuzumab and trastuzumab has shown significant benefit, the possibility remains that sequencing these drugs may improve benefit even further. For example, treatment with pertuzumab and subsequent disruption of HER2-HER3 interactions may allow increased access of trastuzumab or T-DM1 to HER2. Further, sequential treatment with pertuzumab followed by a TKI may reduce toxicities related to this combination. Pertuzumab-mediated disruption of HER2 dimerization may reduce downstream kinase signaling enough that a reduced dose of TKI may be required. Thus, studies should be designed to investigate the potential benefits of sequencing HER2-targeted therapies.
Another important consideration is the mechanism of resistance to trastuzumab. If a tumor that has progressed on trastuzumab treatment shows up-regulation of other cell surface receptors, such as insulin-like growth factor-I receptor (IGF-IR), MUC4, or MET, there may be rationale for treating with pertuzumab. Pertuzumab binds to the dimerization domain of HER2 and sterically blocks interaction with other receptors. Thus, in addition to impeding HER2-HER3 heterodimerization, pertuzumab may disrupt HER2-IGF-IR and HER2-MUC4 interactions. Indeed, cells with acquired resistance to trastuzumab have been shown to develop a unique interaction and cross-talk between HER2 and IGF-IR Citation[14]. Pertuzumab was shown to partially disrupt this interaction Citation[14]. Further, trastuzumab resistant cells showed a trend toward increased sensitivity to pertuzumab relative to sensitive cells Citation[14]. Thus, there is rationale for treating with pertuzumab in the presence of co-overexpression of HER2 and other cell surface receptors. However, the converse may also be true, i.e., interaction of HER2 with other receptors could actually impede response to pertuzumab. This possibility is based on the fact that these receptors have the potential to mask the pertuzumab-binding site of HER2. Thus, additional pre-clinical and clinical correlative investigations must be performed to determine if co-expression of HER2 with IGF-IR, MET, and MUC4, amongst other receptors, predicts sensitivity to pertuzumab. In addition, characterization of the minimal region of HER2 that is required for interactions with these receptors may facilitate development of a peptidomimetic that could disrupt HER2 heterodimerization and effectively treat trastuzumab-refractory disease.
In addition to increased signaling due to over-expression of HER2, new evidence shows that HER2 activating mutations occur in breast cancers in the absence of HER2 amplification Citation[15]. These findings warrant investigation into which therapies may benefit this small subset of patients. Tumors with these mutations were sensitive to HER2 tyrosine kinase inhibition with neratinib Citation[15]. Thus, the presence of HER2 activating mutations serves as a potential basis for deciding between a HER2 TKI and HER2 mAb. The evolutionary changes that accumulate in a tumor as it progresses through treatment with various targeted therapies will also have to be considered. The overexpression or mutational status of HER2 may change as the tumor acquires resistance to a HER2-targeted agent and may differ between metastatic cells and primary tumor cells.
We have reached a new era in the treatment of HER2-positive metastatic breast cancer due to the success of translational cancer research. The availability of multiple targeted therapies holds overwhelming promise for potential improvements in progression-free and overall survival for patients with this subtype of breast cancer. Treatment with a cocktail of HER2-targeting drugs is likely to improve benefit even further. However, this benefit will have to be balanced with the potential for increased toxicity due to multiple drugs. In addition, because most HER2-targeted therapies that are currently available are antibodies, which cannot cross the blood-brain barrier, a major challenge will be developing effective therapies to eliminate brain metastases from HER2-positive breast cancer. The present success stories in the field of HER2-overexpressing breast cancer research reflect decades of basic, translational, and clinical cancer research efforts. Similar efforts are being applied to other subtypes of breast cancer and other tumor types. The future challenges that face us can only be met by continuing to support efforts to understand the basic mechanisms by which cancer cells respond to or escape from targeted inhibition and by supporting efforts to translate these findings into the clinic.
Declaration of interest
The source of funding for this paper is NIH R01CA157754.
Bibliography
- Cho HS, Leahy DJ. Structure of the extracellular region of HER3 reveals an interdomain tether. Science 2002;297:1330-3
- Cho HS, Mason K, Ramyar KX, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 2003;421:756-60
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82
- Franklin MC, Carey KD, Vajdos FF, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 2004;5:317-28
- Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2002;2:127-37
- Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res 2004;64:2343-6
- Scheuer W, Friess T, Burtscher H, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 2009;69:9330-6
- Baselga J, Swain SM. CLEOPATRA: a phase III evaluation of pertuzumab and trastuzumab for HER2-positive metastatic breast cancer. Clin Breast Cancer 2010;10:489-91
- Cortes J, Fumoleau P, Bianchi GV, et al. Pertuzumab monotherapy after trastuzumab-based treatment and subsequent reintroduction of trastuzumab: activity and tolerability in patients with advanced human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2012;30:1594-600
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25-32
- Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109-19
- Ghosh R, Narasanna A, Wang SE, et al. Trastuzumab has preferential activity against breast cancers driven by HER2 homodimers. Cancer Res 2011;71:1871-82
- Garrett JT, Olivares MG, Rinehart C, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci USA 2011;108:5021-6
- Nahta R, Yuan LX, Zhang B, et al. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res 2005;65:11118-28
- Bose R, Kavuri SM, Searleman AC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov 2013;3:224-37