Abstract
Tumor angiogenesis is one of the main pathways targeted to treat cancer. Bevacizumab added survival benefit when combined with platinum-based chemotherapy in NSCLC. Recently, Phase III trials showed survival benefit when anti-angiogenic drugs are added to docetaxel as second-line treatment for NSCLC. These anti-angiogenic agents include nintedanib and ramucirumab, a tyrosine-kinase inhibitor and a monoclonal antibody, respectively, which target receptors involved in angiogenesis. These studies have some similarities and differences. We propose a new algorithm for treatment sequences in performance status 0-1 patients with non-oncogene-addicted NSCLC type adenocarcinoma. Indeed clearer scientific evidences are available for this subgroup of patients.
The discovery of oncogenic drivers in NSCLC represented the most important innovation over the last 20 years, leading to the advent of targeted therapies, which have radically changed our therapeutic strategies. The EGFR-tyrosine-kinase inhibitors (TKIs) Afatinib, Gefitinib and Erlotinib and the ALK-inhibitors Crizotinib, Ceritinib and Alectinib have shown a clear superiority, both in terms of survival and quality of life, compared to platinum-based chemotherapy, in patients whose tumors harbor EGFR-activating mutations Citation[1] and ALK-rearrangements Citation[2], respectively, becoming the new standard of treatment worldwide. The lung cancer mutation consortium (LCMC) study identified an oncogenic driver in about 60% of lung adenocarcinoma, showing that patients with a driver mutation who received a targeted agent, lived longer than patients who did not Citation[3], and highlighting the crucial role of the tumor molecular profile in the era of personalized therapy. We are really witnessing exciting times in translational lung cancer research, but we are still far from translating these findings into clinical practice. Indeed, only a small subgroup of NSCLC patients, approximately 15 – 20%, may receive a genotype-directed therapy during their disease course, while most of them are EGFR/ALK wild type, so non-candidate to receive any targeted agents outside the clinical trials. In this scenario, angiogenesis-directed therapies have always represented an alternative and appealing strategy, because of its crucial role in tumor growing, progression and metastasis. The identification of the VEGF pathway as a key mediator of tumor-induced angiogenic processes has led to the development of several anti-angiogenic agents in NSCLC, including both monoclonal antibodies (moAbs) and TKIs. Bevacizumab was the first anti-VEGF moAb that has shown an overall survival (OS) benefit in combination with Carboplatin-Paclitaxel regimen for six cycles, in first-line setting (12.3 vs 10.3 months, hazard ratio [HR]: 0.79, p = 0.003) Citation[4], and then continued as maintenance therapy (HR: 0.75, p = 0.03) Citation[5] until tumor progression, and is currently included among the standard first-line treatments recommended by the international guidelines Citation[6]. Since the approval of Bevacizumab, several studies investigated the potential role of different anti-angiogenic agents in NSCLC, including the small molecules sorafenib, sunitinib, vandetanib and motesanib, as single agent or in combination with chemotherapy, both in first- and second-line settings, all failing to demonstrate any OS benefit, despite an improvement of response rate and/or progression-free survival Citation[7]. Recently, other two different molecules, Nintedanib and Ramucirumab, have been combined with Docetaxel, as second-line treatment of NSCLC patients. Nintedanib (BIBF 1120) is an oral triple TKI, which targets the ATP-binding site in the kinase domain of VEGF receptor (VEGFR-1-2-3), fibroblast growth factor receptor (FGFR-1-2-3) and platelet-derived growth factor receptor (PDGFR-α-β), inducing the dimerization of the receptor and subsequently inhibiting the downstream, pro-angiogenic, signaling pathways. Early Phase I – II studies Citation[8,9] have shown a great activity of Nintedanib in combination with chemotherapy and a generally manageable safety profile, with a lower incidence of the classical side effects described with other anti-angiogenic drugs, probably due to its different mechanism of action. On the basis of these encouraging data, the Phase III, LUME-Lung 1 trial investigated Nintedanib in combination with docetaxel as second-line treatment for NSCLC patients who progressed to first-line therapy. Ramucirumab is a fully humanized IgG1 moAb that targets the VEGFR-2 extracellular domain with high affinity. As a receptor antagonist, it prevents the binding of all VEGF ligands to VEGFR-2 and the subsequent receptor activation, blocking the most important, VEGF-induced, angiogenic signaling pathways. Ramucirumab is currently approved in combination with Paclitaxel for the treatment of gastric cancer after prior chemotherapy and is also under investigation in several solid tumors. The REVEL trial compared Ramucirumab plus docetaxel versus docetaxel alone in pre-treated NSCLC patients. The positive results of both REVEL and LUME-Lung 1 studies have shown a modest but significant OS advantage, from the addiction of an anti-angiogenic agent to the standard chemotherapy Citation[10,11], leading to the approval of both drugs in the second-line setting. However, a comparison between these two clinical trials can help to highlight some differences worth of stress.
First of all, the OS benefit obtained with Ramucirumab is extended to the overall NSCLC population included in the REVEL trial (HR: 0.86, 95% CI: 0.75 – 0.98; p = 0.023), including both squamous and non-squamous histology, while Nintedanib demonstrated an OS advantage limiting to the subgroup of patients with adenocarcinoma histology (HR: 0.83, 95% CI: 0.70 – 0.99; p = 0.0359). The criteria by which the authors selected patients include small differences between the two trials (% of patients with stage IIIB, % of patients who had a first-line chemotherapy not containing platinum, % of non-measurable lesions) maintaining a substantial overlap between the percentage of patients not treated with previous antiangiogenic moAbs (85 vs 95%, respectively) and the criteria used to select which patients undergo such treatment. In particular, elderly patients (> 65 years), represented about one-third of the overall included population, while those with performance status (PS) of two were not eligible in both the studies, limiting any conclusions regarding this setting of patients. A substantial discrepancy was in the proportion of patients with squamous NSCLC enrolled in the two trials (42% in the LUME-Lung 1 and 25% in REVEL) because, although REVEL trial was not designed with a pre-planned subgroup analysis to assess the formal effectiveness of Ramucirumab in this setting of patients, the benefit in terms of OS in the experimental arm (9.5 vs 8.2 months) would seem not to reach statistical significance only because underpowered.
Furthermore, these two large clinical trials show some methodological differences, such as the choice of the primary end point. Both research groups also argued that it is difficult to choose the best treatment in the second-line setting due to the scarcity of data from large prospective randomized trials that assess the impact of these molecules considering major clinical end points as OS. Nevertheless, only the REVEL trial, but not the LUME-Lung 1 trial, identifies OS as the primary end point. On the other hand, it must be underlined that the results of the subgroup analysis of the REVEL trial, although rationally plausible, come from a non pre-planned analysis. Therefore, it has to be considered as partially informative.
As regards the toxicity profile, a similar increased incidence of hematological (decreased white blood cell count and neutropenia) and non-hematological events (mainly fatigue) is recorded in both studies. Hypertension should be mentioned because its increased incidence is reported in the REVEL study (11 vs 5% in the control arm) and not also reported in the LUME-Lung 1 trial, despite it should be considered as a specific toxicity of this class of drugs, probably because it was not considered as a criterion for exclusion from treatment with Nindetanib. Finally, attention must be paid to the assessment of EGFR mutation status. This analysis was not considered routine until 2008, when the LUME-Lung 1 trial was begun, but it had already been considered since 2010, when the REVEL trial was begun. Despite this, only in the 35% of patients enrolled in the last one it was possible to obtain a correct information about EGFR status (33% EGFR wild-type in the experimental arm). Therefore, it did not provide convincing results to better understand the behavior of Ramucirumab in patients with EGFR-mutated status, but these results suffice to insert Ramucirumab in the treatment algorithm of patients with advanced EGFR wild-type NSCLC.
Nowadays, the available therapeutic options for NSCLC patients allow to suggest treatment sequences to achieve the maximum benefit in survival. These sequential strategies should be deduced through the scientific evidences provided by clinical trials. In NSCLC, the identification of oncogenic drivers (EGFR, EML4-ALK, ROS1), which are targetable by new anticancer molecules, yielded the classification in oncogene-addicted and non-oncogene-addicted lung cancers. The first ones should be addressed to targeted therapy as front-line management. Non-oncogene-addicted NSCLC have to be distinguished basing on PS and age, since PS ≥ 2 and elderly patients could not tolerate platinum-based regimens as first-line treatment. Various clinical trials and meta-analyses highlighted controversial data about these subgroups of patients Citation[12]. Another classification for NSCLC regards the histology, namely squamous versus non-squamous histotypes. The new anti-angiogenic agents, Ramucirumab and Nintedanib, seem to add survival benefit in non-oncogene-addicted non-squamous NSCLC PS 0-1 and non-elderly patients. We wonder whether their introduction into clinical practice could change the sequential strategies for the treatment of this subgroup, who represents the greater population among all NSCLC patients.
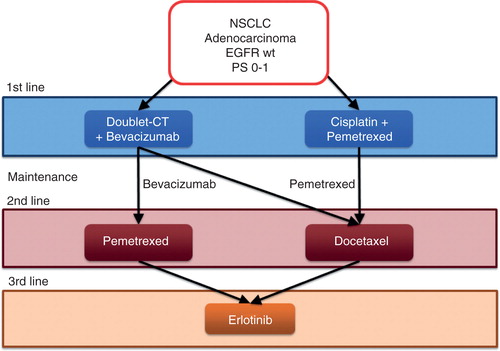
All guidelines and clinical recommendations for first-line treatment of these patients agree that a platinum-based doublet chemotherapy plus bevacizumab or the combination of cisplatin and pemetrexed, including maintenance therapy, represent the best options to achieve the highest survival benefit Citation[4,5]. Until now, docetaxel, pemetrexed and erlotinib have been the only valid options as second-line treatment in this subgroup. Docetaxel and pemetrexed have been demonstrated to be equivalent in efficacy, even though pemetrexed has a better tolerability profile Citation[13]. Erlotinib was compared with chemotherapy, in various clinical trials with docetaxel and pemetrexed. However, conflicting results do not allow to state if this TKI is equal or inferior to cytotoxic agents in second-line treatment. Various meta-analyses tried to clarify this comparison, but also pooled data are controversial Citation[14]. For these reasons, it is not sure if erlotinib is the best option for second-line treatment, whereas it represents the best option for third-line treatment as highlighted in the BR.21 trial Citation[1]. On the basis of these evidences and relative considerations, we think that the most worthwhile algorithm for sequences of treatment from first- to third-line is illustrated in . No scientific evidences are available to support anticancer treatment along with supportive care beyond the third-line erlotinib.
Figure 1. Algorithm of treatment sequences for PS 0-1 patients with NSCLC adenocarcinoma without EGFR or ALK mutations before the approval of Nintedanib and Ramucirumab.
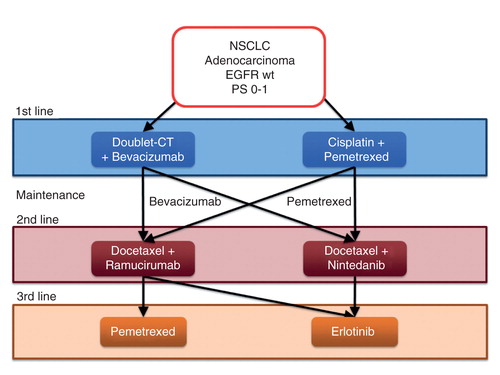
Our insights in REVEL and LUME-Lung 1 trials led us to suppose a sweeping change in the scenario for the sequences of treatment from first- to third-line of non-elderly and PS 0-1 non-oncogene-addicted NSCLC patients. In the new algorithm that we propose here () both doublet chemotherapy plus bevacizumab and cisplatin and pemetrexed combination keep their position as the best options for front-line treatment. In patients treated with each first-line regimen, docetaxel could be combined with Ramucirumab or Nintedanib as second-line therapy, according to data from REVEL and LUME-Lung 1 trials, respectively Citation[10,11]. In both these cases, erlotinib remains the best option for third-line treatment. For those patients who did not receive pemetrexed within first-line regimen, it can be considered as alternative to erlotinib. This choice could be deduced from evidences of comparison of docetaxel and pemetrexed. In this study, the patients undergoing a crossover to third-line pemetrexed after second-line docetaxel achieved an OS similar to patients receiving the sequence pemetrexed-docetaxel Citation[13].
Figure 2. Algorithm of treatment sequences for PS 0-1 patients with NSCLC adenocarcinoma without EGFR or ALK mutations after the approval of Nintedanib and Ramucirumab.
Recent data about another treatment strategy such as the anti-programmed cell death-1 (PD-1), nivolumab, in second-line treatment showed to be superior to docetaxel in both squamous and non-squamous NSCLC Citation[15], leading to its approval by the FDA limiting to the squamous histotype. Probably much more time has to be waited to introduce it into clinical practice, for the treatment of adenocarcinoma. Furthermore, a direct comparison between such anti-PD-1 agent and the new standard second-line therapy, represented by Ramucirumab/Nintedanib plus Docetaxel, could be useful in order to detect the best treatment strategy, and also identify predictive biomarkers for the selection of patients candidate to the described approaches. This flow-chart could be taken into account to identify the best sequence from first to third line, once Ramucirumab and Nintedanib are fully approved and labeled for second-line treatment. The algorithm could also help to find the worth setting for further studies with new agents or combinations. Our proposal aims to suggest that these new anti-angiogenic drugs are not just two further pawns on the chessboard of our fight against lung cancer. Rather they will be able to play a key role in the treatment sequences and to change the whole strategy for advanced NSCLC at least in non-squamous types.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Notes
Bibliography
- Bronte G, Rolfo C, Giovannetti E, et al. Are erlotinib and gefitinib interchangeable, opposite or complementary for non-small cell lung cancer treatment? Biological, pharmacological and clinical aspects. Crit Rev Oncol Hematol 2014;89(2):300-13
- Rolfo C, Passiglia F, Castiglia M, et al. ALK and crizotinib: after the honeymoon…what else? Resistance mechanisms and new therapies to overcome it. Transl Lung Cancer Res 2014;3(4):250-61
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311(19):1998-2006
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355(24):2542-50
- Lopez-Chavez A, Young T, Fages S, et al. Bevacizumab maintenance in patients with advanced non-small-cell lung cancer, clinical patterns, and outcomes in the eastern cooperative oncology group 4599 study: results of an exploratory analysis. J Thorac Oncol 2012;7(11):1707-12
- Reck M, Popat S, Reinmuth N, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25(Suppl 3):iii27-39
- Majem M, Pallarès C. An update on molecularly targeted therapies in second- and third-line treatment in non-small cell lung cancer: focus on EGFR inhibitors and anti-angiogenic agents. Clin Transl Oncol 2013;15(5):343-57
- Mross K, Stefanic M, Gmehling D, et al. Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors. Clin Cancer Res 2010;16(1):311-19
- Reck M, Kaiser R, Eschbach C, et al. A phase II double-blind study to investigate efficacy and safety of two doses of the triple angiokinase inhibitor BIBF 1120 in patients with relapsed advanced non-small-cell lung cancer. Ann Oncol 2011;22(6):1374-81
- Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014;15(2):143-55
- Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384(9944):665-73
- Bronte G, Rolfo C, Passiglia F, et al. What can platinum offer yet in the treatment of PS2 NSCLC patients? A systematic review and meta-analysis. Crit Rev Oncol Hematol 2015. [Epub ahead of print]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22(9):1589-97
- Li N, Yang L, Ou W, et al. Meta-analysis of EGFR tyrosine kinase inhibitors compared with chemotherapy as second-line treatment in pretreated advanced non-small cell lung cancer. PLoS One 2014;9(7):e102777
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373(2):123-35
