Abstract
Hepatocellular carcinoma (HCC) is an aggressive malignancy with approximately half a million cases diagnosed each year. Although strategies in surgical interventions have been investigated and applied, the prognosis is still poor. Novel chemotherapy for advanced stage HCC patients is still greatly in need. Hippo–Yes-associated protein (YAP) signaling pathway controls organ size by regulating both cell proliferation and apoptosis during normal development. The pathway also has a prominent role in suppressing tumor growth, with the most evident contribution in HCC. In recent years, regulators of this pathway have gradually been revealed, providing new information for understanding this complex yet important growth-control signaling. This knowledge provides a basis for rational design of therapeutics against cancer that depends upon Hippo–YAP signaling for growth.
1. Introduction
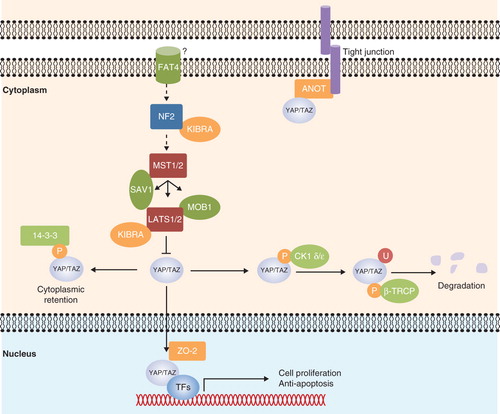
The Hippo–Yes-associated protein (YAP) signaling pathway was first discovered in Drosophila and controls organ size. Core components of the pathway are highly conserved in mammals. They act in a kinase cascade, in which kinases large tumor suppressor homolog 1/2 (LATS1/2) are phosphorylated by mammalian Sterile 20-like kinase 1/2 (MST1/2), and the phosphorylation is coupled by an adaptor protein Salvador homolog 1 (SAV1 or WW45). The cascade promotes phosphorylation and cytoplasmic retention of a transcriptional co-activator — YAP, attenuating its ability to mediate transcription of genes (). Neurofibromatosis 2 (NF2) and Kidney and brain expressed protein (KIBRA) are considered to be upstream components of the Hippo –YAP signaling. They transduce signals from membrane receptors (presumably FAT tumor suppressor homolog 4) to the core Hippo kinases, although the precise mechanism by which the receptor is connected to the upstream components remains unclear (reviewed in Citation[1]).
Figure 1. Hippo-YAP signaling in mammals. Upon attenuation of the Hippo-YAP signaling, Yes-associated protein (YAP) and the transcriptional coactivator with PDZ-binding motif (TAZ) translocate into the nucleus with the aid of zona occludens-2 (ZO-2). YAP/TAZ partners with DNA-binding transcription factors (TFs) to initiate transcription of genes that are involved in cell proliferation or anti-apoptosis. YAP/TAZ also forms complex with angiomotin (AMOT), and the interaction recruits YAP/TAZ to the tight junction. Neurofibromatosis 2 (NF2) and kidney and brain expressed protein (KIBRA) are considered to be upstream components of the pathway. When Hippo signaling is activated, they transduce signals from a receptor, presumably FAT tumor suppressor homolog 4 (FAT4), to the core kinases. Mammalian Ste20-like kinase 1/2 (MST1/2) autophosphorylate and also phosphorylate Salvador homolog 1 (SAV1), large tumor suppressor homolog 1/2 (LATS1/2) and Mps Once Binder (MOB1). Activated LATS1/2 then phosphorylate YAP/TAZ, leading to the 14-3-3 protein binding and cytoplasmic retention. Further phosphorylation of YAP/TAZ by LATS1/2 induces casein kinase 1 (CK1 δ/ε) binding, resulting in a subsequent polyubiquitination and degradation by beta-transducin repeat-containing protein (β-TRCP) E3 ubiquitin ligase. Arrowed and blunted ends of lines indicate activation and inhibition, respectively. Dashed lines indicate unknown mechanisms.
2. Tumor suppressive role of Hippo–YAP signaling
The tumor suppressive functions of the Hippo–YAP signaling components, including NF2, MST1/2, SAV1, and LATS1/2, were investigated in transgenic mice. Inactivation of the Nf2 gene in mice resulted in expansion of progenitor cells and liver enlargement, which eventually gave rise to HCC, cholangiocarcinoma (CC), or mixed histology liver tumors. EGFR inhibitors, such as Elotinib, could inhibit proliferation of Nf2 –/– liver progenitor cells Citation[2]. It is not known, however, if EGFR inhibitors provide clinical benefits for all HCC patients, as the NF2 deficiency in human is not significantly associated with hepatic tumorigenesis. Although in several Phase II clinical trials, the administration of Erlotinib alone or in combination with Bevacizumab (an antibody against VEGF-A ligand) improved overall survival in HCC patients with unresectable tumors Citation[3], such benefits were not seen with other EGFR inhibitors, like Gefitinib and Cetuximab. It is possible that the EGFR inhibitors are only beneficial to HCC patients with certain genetic background, such as NF2 defects. This awaits further evaluation.
Specific double-knockout of Mst1/2 in liver resulted in a loss of phospho-YAP and an increased nuclear accumulation of YAP Citation[4,5]. In human HCC, loss of cleaved MST1 (an active form) and reduced phospho-YAP were reported Citation[4], indicating that MST1/2 activation is important in controlling the downstream Hippo–YAP signaling. Therefore, modulating activities of MST1/2 or directly introducing the active forms of MST1/2 may promote YAP phosphorylation and thus reduce expression of Hippo-YAP target genes. Ras association domain-containing protein 1 (RASSF1A), for example, is a known tumor suppressor that activates MST1/2 Citation[6], although their interactions in HCC still need to be confirmed. The gene promoter of RASSF1A is frequently hypermethylated in cancer, therefore the administration of a DNA methylation inhibitor may help to restore RASSF1A expression, as well as to reactivate the attenuated Hippo–YAP signaling.
Liver-specific knockout of Sav1 resulted in modest liver enlargement, which eventually developed into liver tumors with marked expansion of hepatic progenitor cells (oval cells) with mixed HCC and CC histology in the tumor tissues Citation[5,7]. The data indicated that SAV1 has an important role in inhibiting hepatocarcinogenesis, but surprisingly, in some of the Sav1-deficient tumors, the levels of phospho-YAP or phospho-LATS1/2 were not modulated Citation[5]. It is possible that SAV1 is not required for the phosphorylation of LATS1/2 in hepatocytes. Indeed, the increased nuclear localization of YAP was only observed in the oval cell populations, but not in the hepatocytes Citation[7].
Mice with Lats1 knockout developed soft tissue sarcoma and ovarian tumors Citation[8], whereas mice with Lats2 knockout exhibited embryonic lethality Citation[9]. Lats2 mRNA was found to be regulated by miR-372 Citation[10] and miR-373 Citation[11], and whether these oncogenic microRNAs could be used as therapeutics are still not clear. Since each microRNA regulates multiple gene targets, off-target effects would be the primary concern.
YAP could be phosphorylated at multiple sites. Upon the activation of the signaling cascade, LATS1/2 phosphorylate YAP at Ser-127, leading to 14-3-3 protein binding and cytoplasmic retention. LATS1/2 may also further phosphorylate YAP at Ser-381, which leads to subsequent phosphorylation by casein kinase 1 (CK1 δ/ε), resulting in YAP polyubiquitination and degradation by beta-transducin repeat-containing protein (β-TRCP) E3 ubiquitin ligase Citation[12]. Indeed, LATS1/2 is able to phosphorylate YAP at five serine sites within conserved HXRXXS motifs, and the combination of phosphorylation-deficient mutations at Ser-127 and Ser-381 is sufficient to switch on the transforming properties of YAP. Moreover, restoration of either Ser-127 or Ser-381 mutation could suppress the oncogenic properties of the YAP-5SA mutant, in which the serine residues in all the five HXRXXS motifs are mutated to alanine Citation[12]. These results demonstrated that LATS1/2 phosphorylation on Ser-127 and Ser-381 are crucial for the inhibition of YAP.
3. Regulators of Hippo–YAP signaling
Lately, more regulators have been found to modulate phosphorylation status of YAP. The catalytic subunit of protein phosphoatase-1 (PP1A), for example, was shown to interact with and dephosphorylate YAP, which resulted in an increased nuclear accumulation and transcriptional activity of YAP Citation[13]. Importantly, the study showed that the use of okadiac acid could inhibit PPA, thereby increasing Ser-127 phosphorylation and cytoplasmic retention of YAP. The study indicated that the inhibition of YAP dephosphorylation could be an approach to modulate Hippo signaling activity.
The transcriptional co-activator YAP interacts with DNA-binding transcription factors, such as transcriptional enhancer activator domain (TEAD) family members, runt-domain transcription factors (RUNXs), small and mothers against decapentaplegic (SMAD) family members, p73, paired box 3 (PAX3), PAX8, thyroid transcription factor-1 (TTF-1), T-box transcription factor 5 (TBX5), and PPARγ, to regulate target gene expression (reviewed in Citation[14]). The YAP–TEAD interaction appears to have fundamental importance in mediating oncogenic properties of YAP. Using X-ray crystallography and site-directed mutagenesis, a three-dimensional structure of the YAP–TEAD complex was generated. The binding requires a surface pocket that has both hydrophobic and hydrophilic characters Citation[15]. The Y406 in TEAD1 forms a hydrogen bond with S94 in YAP, and mutations of either of the residues strongly disrupted the binding and diminished the expression of the downstream targets of YAP Citation[16]. Therefore, small inhibitors that bind to the binding pocket may disrupt the YAP–TEAD interaction and suppress the YAP-mediated oncogenic transformation. To date, several cellular screening assays have been be used to detect transcriptional activity of YAP, for example, luciferase reporter assay and gene expression analysis of the downstream targets of YAP Citation[15,16]. These assays could help to screen for small-molecule inhibitors that interfere with the binding.
Recently, YAP has been found to interact with tight-junction proteins, such as angiomotin and zona occludens-2 (ZO-2). Angiomotin family proteins, including AMOT, AMOTL1, AMOTL2, bind to YAP and recruit it to the tight junction, attenuating its ability to induce gene expression Citation[17-19]. It has long been a question how YAP shuttles between cytoplasm and nucleus, as it has neither nuclear localization signal (NLS) nor nuclear export signal (NES). A recent study proposed that ZO-2 is responsible for the shuttling. ZO-2 has both the NLS and NES, and it has the ability to bind to YAP to regulate its subcellular localization Citation[20]. Unlike angiomotin, which promotes cytoplasmic localization of YAP, ZO-2 enhances YAP nuclear localization Citation[21]. However, it is unclear if ZO-2 also promotes nuclear localization of YAP in HCC. If such an interaction holds, then a small-molecular inhibitor that interferes with the binding should be able to control the growth of HCC cells.
4. Expert opinion
Targeted therapeutics against Hippo–YAP signaling may help in addressing challenge of treating advanced-stage HCC, especially when standard chemotherapy is largely ineffective. Along with the development of Hippo–YAP targeted therapy, a gene signature or biomarker is needed to identify individual tumors that depend upon Hippo–YAP signaling for growth. Recent studies have shown YAP as a direct target of miR-375 Citation[22], and their expressions were largely anti-correlated Citation[23]. As such, miR-375 might be used as a marker to identify tumors that have enhanced YAP expression. Given the fact that microRNAs are very stable in blood, it is possible to develop a circulating microRNA biomarker that is able to select the subpopulation of patients who are most likely to respond to the targeted therapy.
The oncogenic functions of YAP in HCC are largely depended upon its subcellular localization. Small molecules that disrupt YAP dephosphorylation or nuclear translocation would be beneficial, as they will allow YAP to be tethered in the cytoplasm for subsequent ubiquitination and degradation. The gradually discovered molecular regulators that affect phosphorylation status of YAP will provide the basis for rational drug design to modulate the Hippo–YAP signaling pathway in HCC.
Declaration of interest
The work was supported by grants from the National Natural Science Foundation of China to Dr. J Luk (Grant No. 81128010) and Dr. Z Xu (Grant No. 81000880). Dr. J Luk is an employee of Roche. The manuscript was written prior to Roche employment.
Bibliography
- Liu AM, Xu MZ, Chen J, Targeting YAP and Hippo signaling pathway in liver cancer. Expert Opin Ther Targets 2010;14:855-68
- Benhamouche S, Curto M, Saotome I, Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev 2010;24:1718-30
- Thomas MB, Morris JS, Chadha R, Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol 2009;27:843-50
- Zhou D, Conrad C, Xia F, Mst1 and Mst2 maintain hepatocytequiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 2009;16:425-38
- Lu L, Li Y, Kim SM, Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci USA 2010;107:1437-42
- Guo C, Tommasi S, Liu L, RASSF1A is part of a complex similar to the Drosophila Hippo/Salvador/Lats tumor-suppressor network. Curr Biol 2007;17:700-5
- Lee KP, Lee JH, Kim TS, The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci USA 2010;107:8248-53
- St John MA, Tao W, Fei X, Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat Genet 1999;21:182-6
- McPherson JP, Tamblyn L, Elia A, Lats2/Kpm is required for embryonic development, proliferation control and genomic integrity. Embo J 2004;23:3677-88
- Cho WJ, Shin JM, Kim JS, miR-372 regulates cell cycle and apoptosis of ags human gastric cancer cell line through direct regulation of LATS2. Mol Cells 2009;28:521-7
- Lee KH, Goan YG, Hsiao M, MicroRNA-373 (miR-373) post-transcriptionally regulates large tumor suppressor, homolog 2 (LATS2) and stimulates proliferation in human esophageal cancer. Exp Cell Res 2009;315:2529-38
- Zhao B, Li L, Tumaneng K, A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCFbeta-TRCP. Genes Dev 2010;24:72-85
- Wang P, Bai Y, Song B, PP1A-mediated dephosphorylation positively regulates YAP2 activity. PLoS One 2011;6:e24288
- Mauviel A, Nallet-Staub F, Varelas X. Integrating developmental signals: a Hippo in the (path)way. Oncogene 2011; published online 29 August 2011; doi:10.1038/onc.2011.363
- Tian W, Yu J, Tomchick DR, Structural and functional analysis of the YAP-binding domain of human TEAD2. Proc Natl Acad Sci USA 2010;107:7293-8
- Li Z, Zhao B, Wang P, Structural insights into the YAP and. TEAD complex. Genes Dev 2010;24:235-40
- Zhao B, Li L, Lu Q, Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev 2011;25:51-63
- Chan SW, Lim CJ, Chong YF, Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem 2011;286:7018-26
- Paramasivam M, Sarkeshik A, Yates JR III, Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor. Mol Biol Cell 2011;22:3725-33
- Oka T, Remue E, Meerschaert K, Functional complexes between YAP2 and ZO-2 are PDZ domain-dependent, and regulate YAP2 nuclear localization and signalling. Biochem J 2010;432:461-72
- Oka T, Schmitt AP, Sudol M. Opposing roles of angiomotin-like-1 and zona occludens-2 on pro-apoptotic function of YAP. Oncogene 2012;31:128-34
- Liu AM, Poon RT, Luk JM. MicroRNA-375 targets Hippo-signaling effector YAP in liver cancer and inhibits tumor properties. Biochem Biophys Res Commun 2010;394:623-7
- Kowalik MA, Saliba C, Pibiri M, Yes-associated protein regulation of adaptive liver enlargement and hepatocellular carcinoma development in mice. Hepatology 2011;53:2086-96