Abstract
Although survival from breast cancer has improved significantly over the past 20 years, disease recurrence remains a significant clinical problem. The concept of stem-like cells in cancer has been gaining currency over the last decade or so, since evidence for stem cell activity in human leukaemia and solid tumours, including breast cancer, was first published. Evidence indicates that this sub-population of cells, known as cancer stem-like cells (CSCs), is responsible for driving tumour formation and disease progression. In breast cancer, there is good evidence that CSCs are intrinsically resistant to conventional chemo-, radio- and endocrine therapies. By evading the effects of these treatments, CSCs are held culpable for disease recurrence. Hence, in order to improve treatment there is a need to develop CSC-targeted therapies. Interleukin-8 (IL-8), an inflammatory cytokine, is upregulated in breast cancer and associated with poor prognostic factors. Accumulating evidence demonstrates that IL-8, through its receptors CXCR1/2, is an important regulator of breast CSC activity. Inhibiting CXCR1/2 signalling has proved efficacious in pre-clinical models of breast cancer providing a good rationale for targeting CXCR1/2 clinically. Here, we discuss the role of IL-8 in breast CSC regulation and development of novel therapies to target CXCR1/2 signalling in breast cancer.
1. Introduction
Breast cancer is the most common cancer affecting women worldwide and although survival has improved significantly, disease recurrence remains a significant problem. Accumulating evidence indicates that tumour cells are organised in a hierarchical manner initiated by a population of self-renewing cancer stem cells (CSCs) Citation[1]. Akin to their normal counterparts, CSCs have the capacity to self-renew and differentiate, but the mechanisms which normally strictly regulate these processes are deregulated leading to their expansion and production of aberrantly differentiated progeny Citation[2].
CSCs are predicted to be responsible for tumour initiation, maintenance and metastases. CSCs have been identified in ductal carcinoma in situ (DCIS), a precursor to invasive disease, and invasive breast cancer. Importantly, their activity is reported to correlate with poor prognostic factors such as high tumour grade and over-expression of human epidermal growth factor receptor 2(HER2) Citation[3-5]. Furthermore, by virtue of their intrinsic resistance to chemotherapy and radiotherapy, they can survive conventional treatments to repopulate the tumour. Consequently, they are held culpable for disease recurrence Citation[6,7].
The first stage in developing novel therapies to target breast CSC is to identify key factors which regulate their activity. These are complex and multifaceted, and effective regimens are likely to involve a combination of approaches in tandem with existing therapies. There are at least three potential ways to target breast CSCs: i) inhibition of aberrant self-renewal signalling pathways thereby inducing differentiation or apoptosis, ii) targeting resistance mechanisms or iii) targeting factors within the tumour microenvironment which regulate CSC activity. We have previously reviewed these approaches Citation[8]; in this article, we focus on the role of interleukin-8 (IL-8), an inflammatory cytokine within the tumour microenvironment, and discuss the rationale for developing novel therapies to target this signalling pathway.
Increasing evidence demonstrates that IL-8, via its cognate receptors CXCR1 and CXCR2, is a key regulator of breast CSC activity Citation[9,10]. The association between inflammation and cancer is well established and deregulated expression of multiple inflammatory cytokines, including IL-8, in malignant breast disease is well recognised. Although IL-8 is known to be increased in breast cancer, the mechanisms by which IL-8 contributes to breast cancer progression have remained poorly understood. However, recent studies indicate that IL-8 may drive tumourigenesis by promoting CSC invasion, metastatic spread and treatment resistance. Targeting CXCR1/2 signalling experimentally has proved efficacious in pre-clinical models of breast cancer providing a good rationale for targeting CXCR1/2 signalling to improve treatment and outcomes in breast cancer.
2. IL-8 signalling and breast cancer
IL-8, originally described as a potent neutrophil chemoattractant Citation[11], is a member of the CXC chemokine family. The biological effects of IL-8 are mediated by two G-protein coupled receptors (GPCRs), CXCR1 and CXCR2 Citation[12,13]. Whereas CXCR1 is activated by IL-8 and granulocyte chemotactic protein-2 (GCP-2)/CXCL6 Citation[14], CXCR2 is more promiscuous as in addition to IL-8, it can be activated by several other chemokines such as growth regulated oncogene (GRO)-α/CXCL1, GRO-β/CXCL2, GRO-γ/CXCL3, CXCL5, GCP-2 and neutrophil-activating protein-2 (NAP-2)/CXCL7 Citation[14].
IL-8 is upregulated in breast cancer compared to normal breast tissue and serum IL-8 levels are elevated in breast cancer patients Citation[15,16]. IL-8 has been implicated in disease progression since patients with metastatic breast cancer are reported to have higher serum IL-8 levels compared to those with localised disease Citation[17], and serum IL-8 level is an independent prognostic factor for post-relapse survival in patients with metastatic breast cancer Citation[18]. Over-expression of HER2, occurring in up to 20% of breast cancers and associated with poor survival, is reported to upregulate IL-8 and may explain the elevated levels observed in HER2-enriched tumours Citation[19]. Increased levels of IL-8 are also associated with loss or inactivation of estrogen receptor (ER), another poor prognostic factor in breast cancer. Elevated levels of IL-8 in HER2 over-expressing and ER negative cancers may contribute to the poor outcomes of these tumour subtypes.
Constitutive activation of CXCR1/2 through ligand upregulation may serve as an adaptive response to protect breast cancer cells from the cytotoxic effect of conventional chemotherapy agents and thereby limit their clinical efficacy. Studies using breast cancer cell lines demonstrate that conventional chemotherapy can lead to the upregulation of IL-8 and other CXCR1/2 ligands. Indeed multidrug resistant breast cancer cell lines are reported to produce significantly higher IL-8 protein levels compared to non-resistant controls Citation[20,21]. Failure of novel targeted therapies, such as PI3K/mammalian target of rapamycin inhibitors, has also been attributed to upregulation of IL-8 through alternative signalling pathways Citation[22].
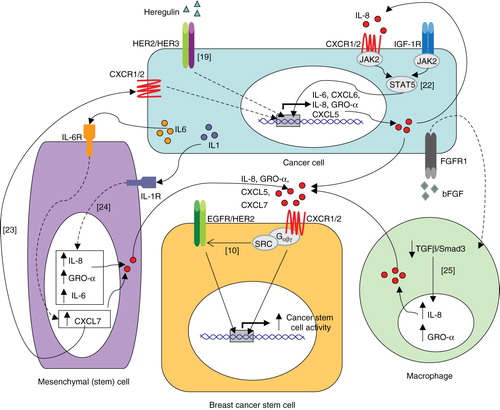
Regulation of IL-8 within the tumour micro-environment is complex, not only because of the variety of cells which can secrete it, but also the multitude of factors which can effect IL-8 expression by these different cell types. Important interactions between breast cancer cells and other cell types that comprise the tumour micro-environment, such as mesenchymal cells Citation[23,24] and macrophages Citation[25], which lead to upregulation of IL-8 are summarised in .
Figure 1. Model of cytokine networks depicting the proposed interactions between cancer cells and stromal cells: production of IL-8 by cancer cells, mesenchymal cells and macrophages increases cancer stem cell activity via CXCR1/2 by triggering EGFR/HER2-dependent and -independent signalling pathways. Numbers in square brackets refer to relevant reference.
3. Regulation of breast CSC activity through CXCR1/2
The failure of standard therapies to eradicate breast cancer is postulated to be due to the intrinsic resistance of CSCs which survive to repopulate the tumour. It is becoming increasingly evident that CXCR1/2 signalling is important in regulating breast CSCs activity underscoring the need to develop CXCR1/2 targeted therapies. Gene expression studies demonstrate that CXCR1 is over-expressed in breast CSCs compared to bulk tumour cells, and IL-8 can increase their invasive capacity as well as increase the proportion of CSCs in vitro Citation[10,26]. Importantly, in xenograft mouse models of breast cancer, inhibition of CXCR1/2 is reported to synergise with chemotherapy agents to retard tumour growth and inhibit metastases Citation[9]. Interestingly, we recently reported a significant direct correlation between metastatic fluid IL-8 level and CSC activity when cells from such fluid were cultured ex vivo, suggesting that cancers with elevated IL-8 levels have greater CSC activity Citation[10]. IL-8 may also promote a state of ‘stemness' by inducing epithelial-mesenchymal transition (EMT) Citation[27], a process which is implicated in the acquisition of stem cell traits through the regulation of EMT transcription factors, such as ZEB1 Citation[28-31].
Recent in vivo studies indicate that tumour growth can be accelerated by recruitment of bone marrow-derived mesenchymal stem cells (MSCs) to sites of developing tumours. Co-localisation of MSCs with breast CSCs is reported to increase the proportion of breast CSCs; an effect which is proposed to be initiated by cancer cell-derived IL-6 and sustained by IL-8 and other CXCR1/2 ligands released from both the cancer cells and mesenchymal cells Citation[23]. Cancer cell derived IL-1 can induce expression of IL-8 and CXCL1/GRO-α by MSCs thereby contributing to the formation and maintenance of CSCs () Citation[24].
Like other GPCRs, ligand activation of CXCR1/2 is known to activate multiple signalling cascades such as PI3K/AKT, PLC/PKC, Ras/Raf/ERK1/2, FAK, Rho and Rac Citation[32]. We recently reported new insights into CXCR1/2 signalling in breast cancer by demonstrating transactivation of HER2 on ligand activation of CXCR1/2 () Citation[10]. This was SRC-dependent and led to the activation of AKT and ERK1/2 signalling pathways which are known to be critical in regulating breast CSC activity Citation[10,33]. Inhibition of HER2 activity abrogated the CSC promoting effect of IL-8 in both HER2-positive and -negative primary breast cancers indicating that the functional effects of CXCR1/2 are, at least in part, dependent on HER2. In HER2 positive cancers, CXCR1/2 inhibition added to the efficacy of HER2 inhibition Citation[10]. Since HER2 is known to be important in regulating CSC activity, aberrant activation of this pathway through CXCR1/2 could have important biological consequences especially in tumours expressing high levels of IL-8 and other CXCR1/2 agonists.
4. Expert opinion: development of novel therapies to target CXCR1/2 signalling in breast cancer
Targeting CXCR1/2 signalling can be broadly approached by either interfering with ligand function/sequestration or receptor function. A summary of clinical trials utilising IL-8 and CXCR1/2 targeting agents in breast cancer and other diseases is presented in . Antibodies against IL-8 have demonstrated efficacy in preclinical models of bladder cancer Citation[34] and melanoma Citation[35]. Clinical trials in patients with chronic inflammatory diseases associated with increased production of IL-8, such as palmoplantar pustulosis, report that monoclonal humanised antibodies to IL-8 are clinically effective and well tolerated Citation[36]. In breast cancer, targeting IL-8 alone may be of limited benefit since other CXCR1/2 agonists are co-regulated with IL-8, such as CXCL1/GRO-α, CXCL2/GRO-β and CXCL5 Citation[37]. This problem can be overcome by inhibiting CXCR1/2.
Table 1. Summary of clinical trials of IL-8 and CXCR1/2 targeting agents in breast cancer and other diseases.
Several orally active, small molecule, non-competitive antagonists of CXCR1 and CXCR2 are currently available. SCH563705 (Merck) has particularly high binding affinities to CXCR1 and CXCR2 and proved effective at inhibiting primary human breast CSC activity ex vivo Citation[10]. Others such as reparixin (Dompé spa), SCH479833 (Merck) and SCH527123 (Merck) have demonstrated anti-tumour effects in xenograft models of breast cancer Citation[9], colorectal cancer Citation[38] and melanoma Citation[39]. Other pharmaceutical companies, including AstraZeneca and GlaxoSmithKline, are also developing novel CXCR1/2 inhibitors (). These compounds are, however, still in the early stages of drug development. Phase I trials demonstrate that reparixin, originally developed to prevent IL-8-induced reperfusion injury, is well tolerated in healthy volunteers Citation[40]. Similarly, SCH527123 has proved to be safe when trialled in patients with severe asthma Citation[41]. Based on recent evidence that CXCR1/2 inhibition can inhibit breast CSC self-renewal and metastases in vivo Citation[9], clinical trials are underway to determine the safety and efficacy of reparixin in combination with chemotherapy agents in patients with advanced breast cancer Citation[42-44]. Due to the heterogeneous levels of IL-8 and other CXCR1/2 ligands in breast cancer, tumours with higher cytokine levels, such as ER-negative or triple negative tumours may be more responsive to CXCR1/2 inhibitors than those with lower levels. CXCR1/2 inhibitors could improve outcomes in these specific subtypes which are notoriously less responsive to standard chemotherapy regimens. Consequently, ligand burden needs be considered when recruiting and evaluating the efficacy of such inhibitors as only subgroups of patients may derive benefit. Moreover, in triple negative breast cancers, recent evidence indicates that IL-8 expression is coordinated with IL-6, another pro-inflammatory cytokine which has also been shown to induce and promote breast CSC activity Citation[45-47]. Dual inhibition of IL-8/IL-6 was more effective at inhibiting colony formation and tumour engraftment compared to either alone Citation[45]. Hence, combined inhibition of multiple cytokine signalling pathways important in regulating CSC activity may increase the efficacy of CXCR1/2 targeted therapies.
As described above, CXCR1/2 signalling is dependent on HER2 activity and inhibition of CXCR1/2 has been shown to add to the efficacy of inhibiting HER2 in HER2 positive breast CSCs Citation[10]. Targeting CXCR1/2 in combination with HER2-targeted therapies has the potential to deliver a more effective treatment strategy to eradicate HER2 positive CSCs which may help to improve the survival of HER2 positive patients and warrants clinical evaluation.
Due to the pleiotropic effects of IL-8 it is possible that CXCR1/2 inhibitors could have unexpected toxicities. In addition to promoting tumourigenesis via multiple mechanisms, IL-8 can exert anti-tumour effects through neutrophil recruitment Citation[48]. Neutrophils, cytotoxic T cells, T helper cells and natural killer cells form part of the immune surveillance system which operates to detect and eradicate cancer cells Citation[49]. Hence, targeting the IL-8 signalling pathway could inadvertently promote tumour growth by abrogating the anti-tumour effects of neutrophil infiltration; it remains unknown whether this could have an effect on breast cancer progression. Whilst this primarily remains a theoretical concern, there is some evidence that IL-8 suppression can increase ER negative tumour growth in vivo in association with reduced tumour neutrophil recruitment Citation[50]. Depending on the clinical context within which they are used, the anti-inflammatory effect of CXCR1/2 inhibitors may in fact help to prevent lung damage during radiotherapy which could be beneficial for some patients Citation[51]. CXCR1/2 inhibitors have been shown to reduce circulating neutrophil counts with the potential for synergistic myelo-toxicity when combined with chemotherapeutic agents Citation[41]. Targeted drug delivery specifically to the cancer cells using novel technologies could minimise these effects.
In conclusion, recent advances reveal that IL-8 signalling is a key regulator of breast CSC activity representing a promising therapeutic target. Results from clinical trials investigating the efficacy of CXCR1/2 inhibitors with chemotherapy agents in breast cancer are eagerly awaited and will hopefully pave the way for future trials combining CXCR1/2 inhibitors with other therapies to improve outcomes in both the adjuvant and advanced settings.
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
- Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105-11
- Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea–a paradigm shift. Cancer Res 2006;66:1883-90; discussion 1895-1886
- Pece S, Tosoni D, Confalonieri S, et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell 2010;140:62-73
- Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003;100:3983-8
- Farnie G, Clarke RB, Spence K, et al. Novel cell culture technique for primary ductal carcinoma in situ: role of Notch and epidermal growth factor receptor signaling pathways. J Natl Cancer Inst 2007;99:616-27
- Diehn M, Cho RW, Lobo NA, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009;458:780-3
- Li X, Lewis MT, Huang J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 2008;100:672-9
- Ablett MP, Singh JK, Clarke RB. Stem cells in breast tumours: are they ready for the clinic? Eur J Cancer 2012;48:2104-16
- Ginestier C, Liu S, Diebel ME, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest 2010;120:485-97
- Singh JK, Farnie G, Bundred NJ, et al. Targeting CXCR1/2 significantly reduces breast cancer stem cell activity and increases the efficacy of inhibiting HER2 via HER2-dependent and -independent mechanisms. Clin Cancer Res 2013;19:643-56
- Schroder JM, Mrowietz U, Morita E, et al. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol 1987;139:3474-83
- Holmes WE, Lee J, Kuang WJ, et al. Structure and functional expression of a human interleukin-8 receptor. Science 1991;253:1278-80
- Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science 1991;253:1280-3
- Ahuja SK, Murphy PM. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J Biol Chem 1996;271:20545-50
- Green AR, Green VL, White MC, et al. Expression of cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin-8 as a potential regulatory factor in breast tumours. Int J Cancer 1997;72:937-41
- Chavey C, Bibeau F, Gourgou-Bourgade S, et al. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res 2007;9:R15
- Derin D, Soydinc HO, Guney N, et al. Serum IL-8 and IL-12 levels in breast cancer. Med Oncol 2007;24:163-8
- Benoy IH, Salgado R, Van Dam P, et al. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res 2004;10:7157-62
- Aceto N, Duss S, Macdonald G, et al. Co-expression of HER2 and HER3 receptor tyrosine kinases enhances invasion of breast cells via stimulation of interleukin-8 autocrine secretion. Breast Cancer Res 2012;14:R131
- Shi Z, Yang WM, Chen LP, et al. Enhanced chemosensitization in multidrug-resistant human breast cancer cells by inhibition of IL-6 and IL-8 production. Breast Cancer Res Treat 2012;135:737-47
- De Larco JE, Wuertz BR, Manivel JC, et al. Progression and enhancement of metastatic potential after exposure of tumor cells to chemotherapeutic agents. Cancer Res 2001;61:2857-61
- Britschgi A, Andraos R, Brinkhaus H, et al. JAK2/STAT5 inhibition circumvents resistance to PI3K/mTOR blockade: a rationale for cotargeting these pathways in metastatic breast cancer. Cancer Cell 2012;22:796-811
- Liu S, Ginestier C, Ou SJ, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res 2011;71:614-24
- Li HJ, Reinhardt F, Herschman HR, et al. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer Discov 2012;2:840-55
- Bohrer LR, Schwertfeger KL. Macrophages promote fibroblast growth factor receptor-driven tumor cell migration and invasion in a CXCR2-dependent manner. Mol Cancer Res 2012;10:1294-305
- Charafe-Jauffret E, Ginestier C, Iovino F, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res 2009;69:1302-13
- Fernando RI, Castillo MD, Litzinger M, et al. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res 2012;71:5296-306
- Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133:704-15
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74
- Morel AP, Lievre M, Thomas C, et al. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One 2008;3:e2888
- Chaffer CL, Marjanovic ND, Lee T, et al. Poised Chromatin at the ZEB1 Promoter Enables Breast Cancer Cell Plasticity and Enhances Tumorigenicity. Cell 2013;154:61-74
- Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res 2008;14:6735-41
- Korkaya H, Paulson A, Charafe-Jauffret E, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol 2009;7:e1000121
- Mian BM, Dinney CP, Bermejo CE, et al. Fully human anti-interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kappaB. Clin Cancer Res 2003;9:3167-75
- Huang S, Mills L, Mian B, et al. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol 2002;161:125-34
- Skov L, Beurskens FJ, Zachariae CO, et al. IL-8 as antibody therapeutic target in inflammatory diseases: reduction of clinical activity in palmoplantar pustulosis. J Immunol 2008;181:669-79
- Bieche I, Chavey C, Andrieu C, et al. CXC chemokines located in the 4q21 region are up-regulated in breast cancer. Endocr Relat Cancer 2007;14:1039-52
- Ning Y, Labonte MJ, Zhang W, et al. The CXCR2 antagonist, SCH-527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer models. Mol Cancer Ther 2012;11:1353-64
- Singh S, Sadanandam A, Nannuru KC, et al. Small-molecule antagonists for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing tumor cell proliferation, survival, and angiogenesis. Clin Cancer Res 2009;15:2380-6
- Leitner JM, Mayr FB, Firbas C, et al. Reparixin, a specific interleukin-8 inhibitor, has no effects on inflammation during endotoxemia. Int J Immunopathol Pharmacol 2007;20:25-36
- Nair P, Gaga M, Zervas E, et al. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: a randomized, placebo-controlled clinical trial. Clin Exp Allergy 2012;42:1097-103
- Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest 2011;121:3804-9
- Korkaya H, Wicha MS. breast cancer stem cells: we've got them surrounded. Clin Cancer Res 2013;19:511-13
- Schott A, Wicha M, Cristofanilli M, et al. Phase Ib pilot study to evaluate reparixin in combination with chemotherapy with weekly paclitaxel in patients with HER-2 Negative Metastatic Breast Cancer (MBC) [abstract]. Cancer Res 2012;72:571s
- Hartman ZC, Poage GM, den Hollander P, et al. Growth of Triple-Negative Breast Cancer Cells Relies upon Coordinate Autocrine Expression of the Proinflammatory Cytokines IL-6 and IL-8. Cancer Res 2013;73:3470-80
- Iliopoulos D, Hirsch HA, Wang G, et al. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci USA 2011;108:1397-402
- Korkaya H, Kim GI, Davis A, et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell 2012;47:570-84
- Hirose K, Hakozaki M, Nyunoya Y, et al. Chemokine gene transfection into tumour cells reduced tumorigenicity in nude mice in association with neutrophilic infiltration. Br J Cancer 1995;72:708-14
- Teng MW, Swann JB, Koebel CM, et al. Immune-mediated dormancy: an equilibrium with cancer. J Leukoc Biol 2008;84:988-93
- Yao C, Lin Y, Chua M-S, et al. Interleukin-8 modulates growth and invasiveness of estrogen receptor-negative breast cancer cells. Int J Cancer 2007;121:1949-57
- Fox J, Gordon JR, Haston CK. Combined CXCR1/CXCR2 antagonism decreases radiation-induced alveolitis in the mouse. Radiat Res 2011;175:657-64