Abstract
Introduction: Neuropathic pain and chronic inflammatory pain are large unmet medical needs. Over the past two decades, numerous ’pain targets’ have been identified for analgesic drug discovery. Despite promising results in rodent pain models, many compounds modulating such targets lacked efficacy in clinical trials. An exception is oral EMA401, a small-molecule angiotensin II type 2 receptor (AT2R) antagonist.
Areas covered: Herein, angiotensin II/AT2R signaling-induced hyperexcitability and abnormal sprouting of cultured dorsal root ganglion neurons, together with radioligand binding, pharmacokinetics, analgesic efficacy and mode of action of small-molecule AT2R antagonists in rodent models of peripheral neuropathic and chronic inflammatory pain, are reviewed. The findings of a successful Phase IIa clinical trial of EMA401 in patients with neuropathic pain are presented in brief.
Expert opinion: The functional importance of angiotensin II/AT2R signaling has remained enigmatic for decades, and there are no clinically available medications that target the AT2R. However, on the basis of preclinical findings and recent clinical trial data showing that the peripherally restricted, small-molecule AT2R antagonist, EMA401, successfully alleviated neuropathic pain in a Phase II clinical trial, the AT2R is receiving considerable attention as a new therapeutic target with human validation for the relief of peripheral neuropathic and chronic inflammatory pain conditions.
1. Introduction
Cross-sectional surveys in many countries over the past 15 years show that the prevalence of chronic pain is ∼ 15 – 20% of the adult population Citation[1]. Apart from adversely affecting the quality of life of patients and their caregivers, chronic pain imposes a high socioeconomic cost encompassing the direct costs of healthcare consumption and the indirect costs of reduced workforce participation Citation[2].
It is well understood that acute pain has a protective role to promote healing following an acute insult to tissue such as that which occurs in the surgical setting. By contrast, chronic pain that persists long after healing is complete is regarded as a maladaptive response and a ‘disease’ in its own right Citation[1]. Examples of chronic pain include persistent inflammatory pain and neuropathic pain. Currently recommended first-line treatments for the relief of neuropathic pain include the tricyclic antidepressants (e.g., amitriptyline and nortriptyline), anticonvulsants (e.g., gabapentin and pregabalin) and the antiarrhythmics (e.g., mexiletine) Citation[3]. For many patients, side effects are dose limiting. This is illustrated by the fact that the numbers needed to treat for gabapentin and pregabalin that are widely prescribed for the relief of neuropathic pain conditions are in the range of 2.5 – 4.0, whereas the numbers needed to produce minor harm including central nervous system (CNS) side effects, are similar in the range of 3.5 – 4.5 Citation[4]. Hence, there is a large unmet medical need for novel analgesics that are efficacious and well tolerated Citation[4].
Over the past two decades, numerous receptors, ion channels and enzymes have been identified as novel targets for use in analgesic drug discovery. However, despite promising preclinical studies, most new compounds that progressed to Phase IIa ‘proof of concept’ studies in patients with peripheral neuropathic pain or chronic inflammatory pain have failed due to lack of efficacy Citation[5]. Examples include chemokine receptor-2 antagonists Citation[6], neurokinin-1 receptor antagonists Citation[7] and fatty acid amide hydrolase inhibitors Citation[8]. A notable exception is the orally active, small-molecule, selective angiotensin II type 2 receptor (AT2R) antagonist, EMA401, that is in clinical development as a novel analgesic for the relief of both peripheral neuropathic pain and chronic inflammatory pain Citation[9]. In the following sections, we review studies to date on the pro-nociceptive effects of the angiotensin II/AT2R axis in peripheral components of the somatosensory system as well as the analgesic effects of small-molecule AT2R antagonists in rodent models of peripheral neuropathic pain and chronic inflammatory pain. Additionally, the findings of a successful, randomized, double-blind, placebo-controlled Phase IIa proof-of-concept clinical trial of EMA401, an orally active first-in-class AT2R antagonist, undertaken in patients with postherpetic neuralgia (PHN), a type of peripheral neuropathic pain notoriously difficult to treat, are briefly presented.
The clinical development and regulatory pathways for approval of novel pharmaceutical agents for human use are long and arduous Citation[10]. Hence, considerable development work remains on more fully defining the clinical utility, safety and tolerability profiles of peripherally restricted, small-molecule AT2R antagonists as novel analgesics for the relief of peripheral neuropathic and chronic inflammatory pain states in humans.
2. Renin–angiotensin system
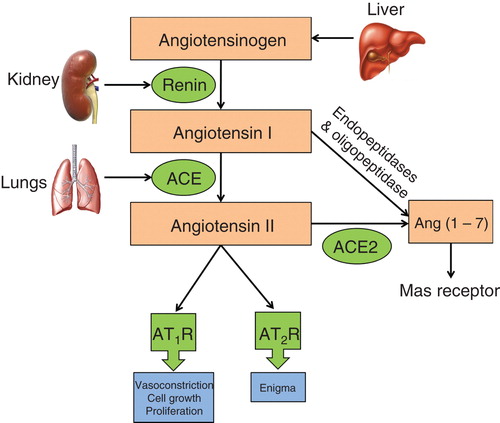
The renin–angiotensin system (RAS) was first discovered in 1898 by Tigerstedt and Bergmann Citation[11]. Since then, knowledge on the biochemical and physiological processes modulated by circulating and tissue RAS components has grown exponentially Citation[12]. In brief, angiotensinogen that is produced and secreted by the liver is enzymatically cleaved by the protease, renin, in the juxtaglomerular apparatus of the kidney to form the inactive decapeptide, angiotensin I Citation[12]. Angiotensin I is cleaved by angiotensin-converting enzyme as well as several other aminopeptidases and caboxypeptidases to form angiotensin II, the major endogenous ligand of the RAS Citation[12]. Although there are other bioactive angiotensin peptides, viz., angiotensin III, angiotensin IV and angiotensin 1 – 7, angiotensin II is considered to be the major biologically active endogenous ligand of the RAS () Citation[13].
Angiotensin II exerts its physiological actions by acting as an agonist at both the angiotensin II type 1 receptor (AT1R) and the AT2R, that are members of the seven transmembrane spanning region G-protein-coupled receptor (GPCR) superfamily Citation[14]. Although angiotensin II shows similar binding properties at the AT1R and AT2R, these two receptors differ in their genomic structure, tissue-specific expression and functional effects Citation[12]. The role of angiotensin II signaling via the AT1R is well characterized in the cardiovascular system, the renal system, tumorigenesis, tissue remodeling, cognition, embryonic development, reproduction and bone homeostasis Citation[12]. By contrast, the functional importance of the AT2R has remained enigmatic Citation[14], but in general it has been thought to functionally antagonize the AT1R Citation[14].
3. Angiotensin II/AT2R axis: implications in pro-nociceptive signaling
3.1 Angiotensin II and AT2R expression in the peripheral components of the somatosensory system
Angiotensin II and the AT2R are present in the lumbar dorsal root ganglia (DRGs) of adult rats Citation[15-17], consistent with the presence of AT2R mRNA in adult rat lumbar DRG neurons Citation[15,16]. Using specific immunofluorescently labeled antibodies and sections of adult rat lumbar DRGs, there was co-localization of the AT2R with substance P and neurofilament 200 kDa (NF-200) Citation[15-17], which are markers for small/medium and medium/large diameter sensory neurons, respectively. Similarly, there was co-localization of angiotensin II with substance P and a subset of NF-200-positive neurons in adult rat lumbar DRG neurons Citation[16,17]. In human postmortem and avulsion-injured DRGs, 60% of small/medium diameter neurons were immunopositive for the AT2R Citation[18]. In human tissue sections, the AT2R was present on nerve fibers in peripheral nerves, skin, bowel and urinary bladder Citation[18], suggesting the possibility that augmented angiotensin II/AT2R signaling may underpin visceral pain, but this requires investigation.
3.2 Insights from in vitro functional studies in cultured cells
Although the AT2R has long been considered as a ‘neglected child’ of the RAS Citation[19], multiple in vitro studies using cultured cells of neuronal origin Citation[20-23], as well as cultured human and rat DRG neurons Citation[13,18], optic nerve Citation[24], cerebellar explants Citation[25], brain Citation[26] and sciatic nerve Citation[27], show that angiotensin II signaling via the AT2R has a role in neuronal hyperexcitability Citation[18,26] and/or neurite outgrowth Citation[13,20-25]. This is particularly noteworthy as these effects are pathobiological hallmarks of various intractable chronic pain conditions Citation[28].
A role for augmented angiotensin II signaling via the AT2R in the peripheral components of the somatosensory system to contribute to peripheral neuropathic and/or chronic inflammatory pain states is supported by the fact that angiotensin II augments capsaicin-induced calcium influx to induce neuronal hyperexcitability and neurite outgrowth in cultured adult rat and human DRG sensory neurons Citation[18]. Importantly, both effects were attenuated in a concentration-dependent manner by EMA401 Citation[18], an orally active, small-molecule, AT2R antagonist with > 10,000-fold selectivity over the AT1R Citation[29]. These effects appear to be transduced by a GPCR mechanism as EMA401’s inhibitory effect on capsaicin responses was diminished in the presence of either forskolin to stimulate intracellular 3’-5’-cAMP formation, or 8-bromo-cAMP, a stable analog of cAMP, in cultured DRG neurons Citation[18].
In other work, angiotensin II signaling via the AT2R induced neurite outgrowth in cultured cells of neuronal origin via a mechanism involving nerve growth factor (NGF)-independent phosphorylation of the neurotrophic tyrosine kinase A receptor (TrkA) Citation[30]. This in turn produced sustained activation (phosphorylation) of p44/p42 mitogen-activated protein kinase (MAPK) Citation[22,23,30], a key enzyme in the phosphorylation (activation) of multiple receptors and ion channels implicated in the pathobiology of chronic pain Citation[28]. These include voltage-gated sodium and calcium channels Citation[28] as well as transient receptor potential vanilloid 1 (TRPV1), the expression of which is markedly increased in the neuronal membrane in an NGF-dependent manner Citation[28].
The aforementioned in vitro data are of particular interest as hyperexcitability and persistent ectopic firing of first-order sensory neurons contribute to the development and/or maintenance of a range of pathological pain states, including peripheral neuropathic pain and chronic inflammatory pain, as well as cancer-induced bone pain that is underpinned by both inflammatory and neuropathic components Citation[31-34]. Hence, it is plausible that angiotensin II signaling via the AT2R in the peripheral components of the somatosensory system may have a role in the pathobiology of peripheral neuropathic pain and/or chronic inflammatory pain.
4. AT2R antagonists: a novel class of analgesics for the relief of peripheral neuropathic pain
In support of the notion that angiotensin II signaling via the AT2R has a key role in the pathobiology of peripheral neuropathic pain, several small-molecule AT2R antagonists with > 1000-fold specificity for the AT2R over the AT1R, viz., EMA200 (also known as PD123319), EMA300 (also known as PD121981) and EMA400 (also known as PD126055) () produced dose-dependent analgesia in rats with a chronic constriction injury (CCI) of the sciatic nerve, a widely utilized rat model of peripheral neuropathic pain Citation[29]. These data and related work are described in more detail in the following sections.
Figure 2. Chemical structures of the small-molecule angiotensin II type 2 receptor antagonists, viz., EMA200 (also referred to as PD123319), EMA300 (also referred to as PD121981), EMA400 (also referred to as PD126055) and EMA401 ([S]-enantiomer of EMA400).
![Figure 2. Chemical structures of the small-molecule angiotensin II type 2 receptor antagonists, viz., EMA200 (also referred to as PD123319), EMA300 (also referred to as PD121981), EMA400 (also referred to as PD126055) and EMA401 ([S]-enantiomer of EMA400).](/cms/asset/0c80703f-c6ea-403e-886a-1901162b6873/iett_a_957673_f0002_b.jpg)
4.1 Radioligand binding
The binding affinities of EMA200, EMA300, EMA400, EMA401 (S-enantiomer of EMA400) and EMA402 (R-enantiomer of EMA400) for cloned rat AT1 and AT2 receptors are 3 – 4 orders of magnitude higher at the AT2R c.f. the AT1R () Citation[29]. Importantly, the binding affinity of EMA401 at the rat and human AT2R was similar at 39 nM (), whereas the binding affinity of EMA402 at the rat and human AT2R was 20 – 30-fold lower (). The binding affinities of both EMA401 and EMA402 at the rat and human AT1R were very low (IC50 values > 50 M) (), demonstrating the high specificity of these compounds for the AT2R over the AT1R.
Table 1. Radioligand binding affinities and relative selectivities at the cloned rat and human AT2R and AT1R.
4.2 Analgesic efficacy in rodent models of peripheral neuropathic pain
To date, the analgesic efficacy of small-molecule AT2R antagonists has been reported in rodent neuropathic pain models involving induction of a peripheral nerve injury by a mechanical or chemical insult (), as briefly described below.
Table 2. Analgesic efficacy of selective, small molecule, AT2R antagonists in rodent models of inflammatory and peripheral neuropathic pain.
4.2.1 CCI rat model of neuropathic pain
In CCI rats with fully developed mechanical allodynia in the ipsilateral (injured side) hindpaws, single intraperitoneal (i.p.) bolus doses of each of EMA200, EMA300 and EMA400 produced dose-dependent analgesia, whereas vehicle was inactive Citation[29]. The ED50s for EMA200, EMA300 and EMA400 were reportedly 3.2, 0.78 and 0.01 mg/kg, respectively. Hence, EMA400 is ∼ 250-fold more potent than EMA200 and ∼ 60-fold more potent than EMA300 in this model Citation[29].
4.2.2 Dideoxycytidine-induced rat model of peripheral neuropathy
In rats with mechanical hypersensitivity in the bilateral hindpaws secondary to peripheral neuropathy induced by the antiretroviral drug, dideoxycytidine (ddC), single i.p. bolus doses of EMA200 (0.3 − 10 mg/kg) produced dose-dependent analgesia; the corresponding ED50 was 3.2 mg/kg Citation[35], mirroring its analgesic potency in CCI rats. Additionally, twice daily i.p. administration of EMA300 at 30 mg/kg for three consecutive days produced significant analgesia in ddC rats in a manner similar to gabapentin at 30 mg/kg administered according to the same dosing regimen Citation[35].
4.3 Target validation and analgesic mode of action in peripheral neuropathic pain
4.3.1 Target validation: AT2R knockout mice
In female C57BL/6 mice, CCI of the sciatic nerve induced mechanical allodynia in the ipsilateral hindpaws to a similar extent in wild-type mice, mice with genetic deletion of the AT2R, as well as the hemizygotes Citation[17]. Importantly, the analgesic effects of single bolus i.p. doses of EMA300 were abolished with intermediate effects in the hemizygotes Citation[17]. This gene dose–response relationship affirms the AT2R as the target mediating the analgesic effects in peripheral neuropathic pain.
4.3.2 Analgesic mode of action in peripheral neuropathic pain
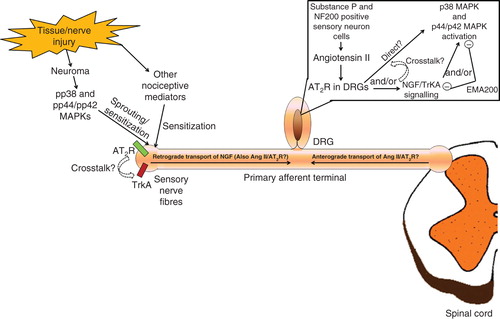
The molecular mechanisms through which the AT2R antagonist, EMA300, mediates its analgesic effects in peripheral neuropathic pain were investigated using immunohistochemical methods in the ipsilateral lumbar DRGs of CCI rats relative to sham controls Citation[17]. Use of specific immunofluorescently labeled antibodies showed that angiotensin II and the AT2R were co-localized with neuronal nuclei (NeuN) of substance P-containing small-to-medium sensory neurons in the lumbar DRGs. Additionally, the AT2R was co-localized with NeuN of NF200-positive medium-to-large sensory DRG neurons, whereas angiotensin II was co-localized with a subset of these neurons Citation[15-17]. In CCI rats with fully developed mechanical allodynia in the ipsilateral hindpaws, the ipsilateral lumbar DRG levels of angiotensin II, phosphorylated p38 (pp38) MAPK and phosphorylated p44/p42 (pp44/pp42) MAPK were increased c.f. the corresponding levels in the ipsilateral lumbar DRGs of sham-control rats Citation[17]. At the time of peak EMA300-mediated analgesia in CCI rats, blockade of augmented angiotensin II signaling via the AT2R in the ipsilateral lumbar DRGs resulted in inhibition of p38 MAPK and p44/p42 MAPK activation Citation[17], key mechanisms implicated in the pathobiology of peripheral neuropathic pain Citation[28].
4.4 AT2R antagonists: analgesic efficacy in rodent models of inflammatory pain
In a Freund’s Compete Adjuvant (FCA)-induced rat model of unilateral monoarthritis, mechanical hyperalgesia in the ipsilateral hindpaws was alleviated in a dose-dependent manner by single i.p. bolus doses of each of EMA300 and EMA400 () Citation[36]. In other work in an FCA-induced model of unilateral inflammatory pain in the rat hindpaw, chronic i.p. administration of EMA200 (5 mg/kg/day) for 7 days according to a prevention protocol blocked development of thermal and mechanical hypersensitivity, as well as cutaneous sensory hyperinnervation in the ipsilateral hindpaws () Citation[37]. Administration of single i.p. bolus doses of EMA200 at 10 mg/kg to FCA rats with fully developed hindpaw hypersensitivity produced full relief of thermal hyperalgesia and partial relief of mechanical hyperalgeisa in the ipsilateral hindpaws at 3 h postdosing Citation[37], with this latter effect likely due to the fact that the peak analgesia occurred at 0.5 – 1.0 h postdosing () Citation[36].
4.5 AT2R antagonism: analgesic efficacy in prostate cancer induced bone pain – a rat model of mixed peripheral neuropathic and inflammatory pain
4.5.1 Analgesic efficacy of EMA200 in prostate cancer-induced bone pain
Confirming the analgesic efficacy of small-molecule AT2R antagonists for the relief of both peripheral neuropathic and inflammatory pain, single intravenous bolus doses of EMA200 at 0.3 – 10 mg/kg produced dose-dependent analgesia in a rat model of prostate cancer-induced bone pain (PCIBP) () Citation[15,38], a type of pain underpinned by both peripheral neuropathic and inflammatory pain mechanisms Citation[39]. The mean ED50s for the relief of mechanical allodynia in the ipsilateral and contralateral hindpaws were reportedly 0.8 and 1.8 mg/kg, respectively. The corresponding mean ED50s for the relief of thermal hypersensitivity in the ipsilateral and contralateral hindpaws were 3.9 and 5.5 mg/kg, respectively Citation[15].
4.5.2 Analgesic mode of action in PCIBP
In PCIBP rats with fully developed bilateral hindpaw hypersensitivity, lumbar DRG levels of angiotensin II, NGF, TrkA, pp38 MAPK and pp44/pp42 MAPK were significantly increased compared with the corresponding levels in sham rats Citation[15]. By contrast, lumbar DRG expression levels of the AT2R did not differ significantly between PCIBP- and sham rats Citation[15]. Interestingly, in human peripheral nerve segments proximal to injury, AT2R expression levels were reduced, whereas in human neuromas that also express high levels of pp44/pp42 MAPK Citation[40], AT2R expression levels were preserved Citation[18].
At the time of peak EMA200 analgesia (0.5 h) in PCIBP rats, augmented angiotensin II/AT2R signaling was attenuated not only by the AT2R blockade but also due to a reduction in the elevated lumbar DRG levels of angiotensin II. Concurrently, augmented NGF signaling via TrkA in the lumbar DRGs was reduced, with the net effect being inhibition of the activation of p38 MAPK and p44/p42 MAPK Citation[15]. A schematic diagram illustrating these findings is shown in . At the time of peak analgesia evoked by EMA200, the PCIBP-induced increase in NGF levels in the lumbar DRGs was attenuated. This finding together with in vitro work showing that NGF-induced neurite outgrowth in cultured adult rat DRG neurons was unaffected by EMA200 Citation[13] suggests that augmented angiotensin II/AT2R signaling is upstream of the augmented NGF/TrkA signaling cascade in the lumbar DRGs, but this requires investigation.
4.6 Pharmacokinetics
A comparison of the in vivo pharmacokinetics and bioavailability of single bolus doses of each of EMA200, EMA300, EMA400 and its active S-enantiomer, EMA401, in male Sprague–Dawley rats, shows that the pharmacokinetics of EMA400/EMA401 are superior to those of EMA200 and EMA300 () Citation[29]. Specifically, the oral bioavailability of EMA400 and EMA401 is similar at 30%, which is superior to the poor oral bioavailability of EMA200 and EMA300 at 6 – 7% Citation[29]. The low volume of distribution (Vd) of EMA401 at 3.7l/kg Citation[29] is consistent with the fact that it does not cross the blood– brain barrier (BBB), confirmed by the lack of BBB penetration of 14C-labeled EMA401 in the rat Citation[18]. By contrast, the Vd values for EMA200 and EMA300 are an order of magnitude higher at 47 and 77 l/kg, respectively () Citation[29]. The systemic clearance of EMA401 at 1.1 l/h/kg is lower than that for EMA200 and EMA300 at 9.1 and 6.3 l/h/kg, respectively () Citation[29]. The superior pharmacokinetics and oral bioavailability of EMA401 c.f. EMA200 and EMA300 in rats, together with EMA401’s high analgesic potency in rodent pain models, are consistent with its selection as drug candidate for progression into formal nonclinical and early-phase clinical development.
Table 3. Mean (±SEM) pharmacokinetics and oral bioavailability of small-molecule AT2R antagonists in adult male Sprague–Dawley rats.
5. Clinical study of EMA401 in patients with PHN
The safety and tolerability of EMA401 have been assessed in several Phase I clinical studies undertaken in informed consenting healthy human subjects (ACTRN12608000335392, ACTRN12609000489291, ACTRN12610000620022, AC TRN12610000798066, ACTRN12610000961044). Additionally, the analgesic efficacy, safety and tolerability of EMA401 have been assessed in a multicenter, randomized, double-blind, placebo-controlled, Phase IIa proof-of-concept clinical study in 183 patients with PHN Citation[9]. In brief, patients were randomized to receive twice-daily oral doses of EMA401 at 100 mg, or placebo, for 4 weeks Citation[9]. For patients administered EMA401, there was a significant reduction in mean daily pain intensity scores c.f. placebo such that in the final treatment week the mean reduction in pain intensity from baseline was -2.34 compared with -1.64 in the placebo group Citation[9]. EMA401 was reportedly well tolerated, and there were no serious renal, cardiovascular or CNS-related adverse events Citation[9]. The absence of CNS side effects is consistent with the inability of EMA401 to cross the BBB Citation[18].
Although pregabalin and tapentadol have been approved by regulatory agencies for the relief of neuropathic pain in the past decade, the mechanisms underpinning their analgesic actions are not novel as they are more potent analogs of gabapentin and tramadol, respectively Citation[41,42]. Over the same period, many new ‘pain targets’ have been reported from preclinical studies in rodent pain models, but virtually none in the neuropathic pain field has been verified in a Phase II clinical trial Citation[5]. Hence, the successful translation of findings from rodent models of peripheral neuropathic pain into a successful proof-of-concept clinical trial of EMA401 in patients with PHN affirms the strategy of antagonizing augmented angiotensin II signaling via the AT2R in the peripheral somatosensory system as a means to alleviate peripheral neuropathic pain states; these findings have considerable significance in the pain field. Additional clinical research is required to confirm the analgesic efficacy and safety profile of EMA401 for a range of doses administered over a longer treatment period for the relief of PHN, as well as for the relief of other types of peripheral neuropathic pain and chronic inflammatory pain.
6. Perspectives of AT2R-targeted analgesics
Many peptides and peptide families that were first isolated outside the nervous system and demonstrated to have a non-nociceptive physiological role have been shown subsequently to be present in sensory neurons and to be pro-hyperalgesic mediators Citation[28]. Examples include the endothelins, calcitonin gene-related peptide, cholecystokinin, substance P and now angiotensin II Citation[15,17,28,29,35]. Thus, inhibition of pro-hyperalgesicmediator signaling appears to hold promise as a strategy for novel analgesic drug discovery.
AT2R antagonists represent a novel class of analgesic agents for the relief of peripheral neuropathic pain conditions and potentially chronic inflammatory pain. By targeting only the peripheral components of the somatosensory system as with the peripherally restricted EMA401, CNS-related adverse effects are avoided. The analgesic effects of small-molecule AT2R antagonists in these pathological pain conditions appear to be underpinned by i) attenuation of augmented angiotensin II/AT2R signaling-induced hyperexcitability of DRG neurons as well as angiotensin II-induced potentiation of TRPV1 activity; ii) attenuation of augmented NGF/TrkA signaling; and iii) inhibition of p38 MAPK and p44/p42 MAPK activation in DRG neurons Citation[15,17,18]. These effects in turn would be expected to reduce MAPK-dependent phosphorylation of multiple receptors and ion channels implicated in DRG sensory neuronal hyperexcitability and chronic pain including TRPV1 Citation[43] and its NGF-dependent increase in neuronal membrane expression, as well as voltage-gated sodium Citation[44-46] and calcium channels Citation[47].
The clinical validity for targeting inhibition of MAPK activation as a strategy for discovery of new analgesics is supported by studies showing high expression levels of pp38 MAPK and pp42/pp44 MAPK in both painful neuromas in humans Citation[40] and experimental neuromas in rats Citation[46]. Although several clinical trials of p38 MAPK inhibitors have been terminated due to early toxicity, AT2R antagonists that indirectly regulate MAPK function appear to avoid these safety concerns Citation[9]. Hence, small-molecule, peripherally restricted, AT2R antagonists have promise as an attractive therapeutic approach for the improved relief of peripheral neuropathic and chronic inflammatory pain with good tolerability. As angiotensin II signaling via the AT2R induces neurite outgrowth in cultured cells of neuronal origin in vitro by sustained activation of p44/p42 MAPK, the analgesic effects of AT2R antagonists administered by chronic dosing regimens may be underpinned by inhibition of abnormal ectopic sprouting of primary afferent sensory nerve fibers Citation[37] in addition to attenuated hyperexcitability.
7. Expert opinion
To date, there are no clinically available medications that target the AT2R and the functional importance of angiotensin II signaling via the AT2R has remained enigmatic for decades Citation[14]. Herein we present in vivo data showing a pathobiological role for augmented angiotensin II/AT2R signaling in the peripheral components of the somatosensory system in rodent models of peripheral neuropathic and/or chronic inflammatory pain. We also present data showing that selective, peripherally restricted, small-molecule AT2R antagonists alleviate these pathological pain conditions.
Of major significance in the pain field, the AT2R is the first novel ’pain target’ in more than a decade where promising analgesic efficacy data in rodent pain models have translated into a successful proof-of-concept clinical trial in patients with neuropathic pain. Hence, the AT2R is receiving considerable attention as a novel therapeutic target with human validation for the relief of pathological pain states. The importance of this breakthrough in pain therapeutics discovery is underscored by the fact that numerous ‘pain targets’ have been identified in the past two decades, but the new molecules modulating these targets lacked efficacy in proof-of-concept clinical trials despite promising data from rodent pain models Citation[5]. This situation has led directly to the exit of several global pharmaceutical companies from the novel analgesics discovery field Citation[2] despite the large unmet medical need characterized by the fact that one in five adults in the general population suffer from poorly alleviated chronic pain Citation[1].
It is widely accepted that the pathobiology of peripheral neuropathic pain and chronic inflammatory pain conditions is underpinned by persistent ectopic firing and/or abnormal sprouting of primary afferent nerve fibers resulting in the development of so-called ‘central sensitization’ in the spinal cord and higher centers of the somatosensory system Citation[28]. Hence, many pain therapeutics discovery researchers have focused on generating compounds that can cross the BBB to attenuate CNS mechanisms underpinning central sensitization and/or augment CNS inhibitory mechanisms as a means to alleviate human peripheral neuropathic pain conditions Citation[5].
However, a less common strategy favored by us is to inhibit ectopic primary afferent input into the spinal cord as a means to enable central sensitization to be potentially reversed to alleviate peripheral neuropathic pain states without CNS side effects. This latter notion is supported by clinical trial data showing that EMA401 produced analgesia above placebo in patients with PHN by the third week of treatment but without CNS side effects Citation[9]. Our view that ongoing ectopic primary afferent input is key to the maintenance of peripheral neuropathic pain is supported by observations that ultrasound-guided peripheral nerve blocks with lidocaine in patients with distal polyneuropathy and peripheral nerve injury produced ipsilateral analgesia in the absence of clinically relevant systemic lidocaine exposure Citation[48].
Apart from their promise as novel analgesics for improved relief of peripheral neuropathic pain conditions Citation[9,17,29,35] and for relief of unremitting bone pain due to advanced skeletal metastases Citation[15], peripherally restricted small-molecule AT2R antagonists, such as EMA401, may also have potential for relief of osteoarthritis pain but without the safety concerns of anti-NGF therapy Citation[49]. This is because the pharmacokinetic profile of oral EMA401 Citation[29] will enable normal physiological levels of NGF to signal via TrkA to maintain normal bone health. Other types of chronic pain underpinned by inflammatory pain mechanisms that have potential for relief by peripherally restricted small-molecule AT2R antagonists include fibromyalgia and visceral pain such as chronic pancreatitis.
Although selective, peripherally restricted, small-molecule AT2R antagonists hold excellent promise as novel, well-tolerated analgesics for the relief of pathological pain states in humans Citation[9], the regulatory requirements for approval of new analgesic medications are rigorous. Hence, additional Phase II and new drug application-enabling Phase III clinical trials are required to define the analgesic dose range and to more fully assess the clinical utility, safety and tolerability profiles of the clinical candidate, EMA401.
The AT2R is a pain target that has human validation and so is attracting considerable research attention. Interestingly, although the AT2R was first cloned in the 1990s, its physiological significance remained enigmatic until recently. The key role of augmented angiotensin II signaling via the AT2R in the peripheral components of the somatosensory system in the pathobiology of peripheral neuropathic and/or chronic inflammatory pain conditions underpins the high promise of small-molecule AT2R antagonists as novel analgesic agents for relief of intractable peripheral pain conditions in the near term.
The functional importance of angiotensin II signaling via the angiotensin II type 2 receptor (AT2R) has been enigmatic, and there are no clinically available medications that target the AT2R.
Neuropathic and chronic inflammatory pain are areas of large unmet medical need.
Small-molecule AT2R antagonists produced dose-dependent analgesia in rodent models of neuropathic pain that was abolished by genetic deletion of the AT2R.
The analgesic mechanism involves inhibition of cross-talk with augmented nerve growth factor/neurotrophic tyrosine kinase A signaling in the lumbar dorsal root ganglia. The net effect is inhibition of the activation p38 MAPK and p44/p42 MAPK, key enzymes in the pathophysiology of pathological pain conditions.
EMA401 is a first-in-class small-molecule AT2R antagonist that successfully alleviated neuropathic pain in patients with postherpetic neuralgia.
The AT2R is receiving considerable attention as it is the first new pain target in more than a decade with human validation for the relief of pathological pain.
Declaration of interest
MT Smith is a named inventor of The University of Queensland (UQ) patents for the use of AT2R antagonists for the relief of neuropathic pain and inflammatory pain. This technology is being commercialized by the company Spinifex Pharmaceutical Pty Ltd. MT Smith is investigator on a grant-in-aid of AUD $50,406 to The University of Queensland from Spinifex Pharmaceuticals for investigations on the mode of action of AT2R antagonists in neuropathic pain. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
This box summarizes key points contained in the article.
Bibliography
- Brennan F, Carr DB, Cousins M. Pain management: a fundamental human right. Anesth Analg 2007;105:205-21
- Woolf CJ. Overcoming obstacles to developing new analgesics. Nat Med 2010;16:1241-7
- Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc 2010;85:S3-14
- Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain 2010;150:573-81
- Mao J. Current challenges in translational pain research. Trends Pharmacol Sci 2012;33:568-73
- Kalliomäki J, Jonzon B, Huizar K, et al. Evaluation of a novel chemokine receptor 2 (CCR2)-antagonist in painful diabetic polyneuropathy. Scand J Pain 2013;4:77-83
- Goldstein DJ, Wang O, Gitter BD, et al. Dose-response study of the analgesic effect of lanepitant in patients with painful diabetic neuropathy. Clin Neuropharmacol 2001;24:16-22
- Huggins JP, Smart TS, Langman S, et al. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain 2012;153:1837-46
- Rice AS, Dworkin RH, McCarthy TD, et al. EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet 2014;383:1637-47
- Hay M, Thomas DW, Craighead JL, et al. Clinical development success rates for investigational drugs. Nat Biotechnol 2014;32:40-51
- Tigerstedt R, Bergman PQ. Niere und Kreislauf1. Skand Arch Physiol 1898;8:223-71
- Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 2006;86:747-803
- Chakrabarty A, Blacklock A, Svojanovsky S, et al. Estrogen elicits dorsal root ganglion axon sprouting via a renin-angiotensin system. Endocrinology 2008;149:3452-60
- Verdonk K, Danser AH, van Esch JH. Angiotensin II type 2 receptor agonists: where should they be applied? Expert Opin Investig Drugs 2012;21:501-13
- Muralidharan A, Wyse BD, Smith MT. Analgesic efficacy and mode of action of a selective small molecule angiotensin II type 2 receptor antagonist in a rat model of prostate cancer-induced bone pain. Pain Med 2014;15:93-110
- Patil J, Schwab A, Nussberger J, et al. Intraneuronal angiotensinergic system in rat and human dorsal root ganglia. Regul Peptides 2010;162:90-8
- Smith MT, Woodruff TM, Wyse BD, et al. A small molecule angiotensin II Type 2 receptor (AT2R) antagonist produces analgesia in a rat model of neuropathic pain by inhibition of p38 mitogen activated protein kinase (MAPK) and p42/p44 MAPK activation in the dorsal root ganglia. Pain Med 2013;14:1557-68
- Anand U, Facer P, Yiangou Y, et al. Angiotensin II type 2 receptor (AT2 R) localization and antagonist-mediated inhibition of capsaicin responses and neurite outgrowth in human and rat sensory neurons. Eur J Pain 2013;17:1012-26
- Steckelings UM, Unger T. Angiotensin II type 2 receptor agonists–where should they be applied? Expert Opin Investig Drugs 2012;21:763-6
- Guimond MO, Roberge C, Gallo-Payet N. Fyn is involved in angiotensin II type 2 receptor-induced neurite outgrowth, but not in p42/p44mapk in NG108-15 cells. Mol Cell Neurosci 2010;45:201-12
- Laflamme L, Gasparo M, Gallo JM, et al. Angiotensin II induction of neurite outgrowth by AT2 receptors in NG108-15 cells. Effect counteracted by the AT1 receptors. J Biol Chem 1996;271:22729-35
- Gendron L, Laflamme L, Rivard N, et al. Signals from the AT2 (angiotensin type 2) receptor of angiotensin II inhibit p21ras and activate MAPK (mitogen-activated protein kinase) to induce morphological neuronal differentiation in NG108-15 cells. Mol Endocrinol 1999;13:1615-26
- Stroth U, Blume A, Mielke K, et al. Angiotensin AT(2) receptor stimulates ERK1 and ERK2 in quiescent but inhibits ERK in NGF-stimulated PC12W cells. Brain Res Mol Brain Res 2000;78:175-80
- Lucius R, Gallinat S, Rosenstiel P, et al. The angiotensin II type 2 (AT2) receptor promotes axonal regeneration in the optic nerve of adult rats. J Exp Med 1998;188:661-70
- Cote F, Do TH, Laflamme L, et al. Activation of the AT(2) receptor of angiotensin II induces neurite outgrowth and cell migration in microexplant cultures of the cerebellum. J Biol Chem 1999;274:31686-92
- Kang J, Sumners C, Posner P. Modulation of net outward current in cultured neurons by angiotensin II: involvement of AT1 and AT2 receptors. Brain Res 1992;580:317-24
- Reinecke K, Lucius R, Reinecke A, et al. Angiotensin II accelerates functional recovery in the rat sciatic nerve in vivo: role of the AT2 receptor and the transcription factor NF-kappaB. FASEB J 2003;17:2094-6
- Basbaum AI, Bautista DM, Scherrer G, et al. Cellular and molecular mechanisms of pain. Cell 2009;139:267-84
- Smith MT, Wyse BD, Edwards SR. Small molecule angiotensin II type 2 receptor (AT2R) antagonists as novel analgesics for neuropathic pain: comparative pharmacokinetics, radioligand binding and efficacy in rats. Pain Med 2013;14:692-705
- Plouffe B, Guimond MO, Beaudry H, et al. Role of tyrosine kinase receptors in angiotensin II AT2 receptor signaling: involvement in neurite outgrowth and in p42/p44mapk activation in NG108-15 cells. Endocrinology 2006;147:4646-54
- Zhang JM, Strong JA. Recent evidence for activity-dependent initiation of sympathetic sprouting and neuropathic pain. Sheng Li Xue Bao 2008;60:617-27
- Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res 2009;196:115-28
- Gold MS, Flake NM. Inflammation-mediated hyperexcitability of sensory neurons. Neurosignals 2005;14:147-57
- Zheng Q, Fang D, Cai J, et al. Enhanced excitability of small dorsal root ganglion neurons in rats with bone cancer pain. Mol Pain 2012;8:24
- Smith MT, Lau T, Wallace VC, et al. Analgesic efficacy of small-molecule angiotensin II type 2 receptor antagonists in a rat model of antiretroviral toxic polyneuropathy. Behav Pharmacol 2014;25:137-46
- Smith MT. Method of treatment or prophylaxis of inflammatory pain. US20140142116 A1; 2014
- Chakrabarty A, Liao Z, Smith PG. Angiotensin II receptor type 2 activation is required for cutaneous sensory hyperinnervation and hypersensitivity in a rat hind paw model of inflammatory pain. J Pain 2013;14:1053-65
- Muralidharan A, Wyse BD, Smith MT. Optimization and characterization of a rat model of prostate cancer-induced bone pain using behavioral, pharmacological, radiological, histological and immunohistochemical methods. Pharmacol Biochem Behav 2013;106:33-46
- Muralidharan A, Smith MT. Pathobiology and management of prostate cancer-induced bone pain: recent insights and future treatments. Inflammopharmacology 2013;21:339-63
- Black JA, Nikolajsen L, Kroner K, et al. Multiple sodium channel isoforms and mitogen-activated protein kinases are present in painful human neuromas. Ann Neurol 2008;64:644-53
- Raffa RB, Buschmann H, Christoph T, et al. Mechanistic and functional differentiation of tapentadol and tramadol. Expert Opin Pharmacother 2012;13:1437-49
- Smith MT, Moore BJ. Pregabalin for the treatment of fibromyalgia. Expert Opin Pharmacother 2012;13:1527-33
- Ji RR, Samad TA, Jin SX, et al. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002;36:57-68
- Hudmon A, Choi JS, Tyrrell L, et al. Phosphorylation of sodium channel Na(v)1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons. J Neurosci 2008;28:3190-201
- Persson AK, Gasser A, Black JA, et al. Nav1.7 accumulates and co-localizes with phosphorylated ERK1/2 within transected axons in early experimental neuromas. Exp Neurol 2011;230:273-9
- Stamboulian S, Choi JS, Ahn HS, et al. ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Na(v)1.7 and alters its gating properties. J Neurosci 2010;30:1637-47
- Martin SW, Butcher AJ, Berrow NS, et al. Phosphorylation sites on calcium channel alpha1 and beta subunits regulate ERK-dependent modulation of neuronal N-type calcium channels. Cell Calcium 2006;39:275-92
- Haroutounian S, Nikolajsen L, Bendtsen TF, et al. Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain 2014;155:1272-9
- Bannwarth B, Kostine M. Targeting nerve growth factor (NGF) for pain management: what does the future hold for ngf antagonists? Drugs 2014;74:619-26