Abstract
Alzheimer’s disease (AD) is the most common age-related neurodegenerative disorder. Despite this, there are no drugs for preventing the onset of AD. Preclinical studies suggest that the interaction between amyloid-β peptides (Aβ) and the α7 nicotinic acetylcholine receptor (α7 nAChR) plays a key role in AD pathology, and that α7 nAChR agonists could act as potential therapeutic drugs for AD. A recent study demonstrated that tropisetron, a potent α7 nAChR agonist and serotonin 5-hydroxytryptamine3 receptor antagonist, also bound to the ectodomain of amyloid precursor protein. Furthermore, tropisetron promoted greater improvements in memory than current AD therapeutic drugs, such as memantine and donepezil. Positron emission tomography studies detected Aβ deposition and inflammation in the brains of subjects with amnestic mild cognitive impairment (MCI) before the onset of AD. Given the role of α7 nAChR in Aβ deposition and inflammation, tropisetron represents an attractive potential therapeutic drug to delay or prevent MCI and AD. Additionally as this drug is used internationally to treat chemotherapy-induced emesis, its safety record is already known.
1. Introduction
The World Alzheimer Report 2013 Citation[1] estimated that between 2010 and 2050, the worldwide numbers of older people needing care will nearly treble, from 101 to 277 million. Nearly half of these older people are likely to be living with and experiencing the effects of dementia. Furthermore, costs will increase in line with the number of older people suffering dementia. The World Alzheimer Report predicted a near doubling in worldwide societal costs from US$604 billion in 2010 to US$1117 billion, by 2030 Citation[1].
Alzheimer’s disease (AD) is the most common age-related neurodegenerative disorder, and AD is the most common cause of dementia. It is a slowly progressing disease characterized by three stages – an early preclinical stage with no symptoms, a middle stage with mild cognitive impairment (MCI) and a final stage with dementia (). Currently, there are no therapeutic agents for preventing the onset of MCI or AD. A decade of disappointing clinical trials testing agents aimed at modifying AD disease in patients, suggest that effective treatment should be targeted at earlier stages of the disease, that is, even before overt symptoms arise Citation[2].
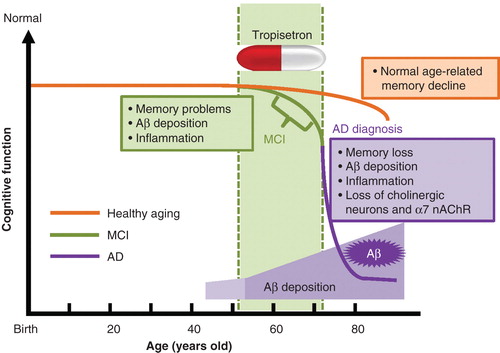
Figure 1. The life course in healthy aging, MCI subjects and AD patients. As people grow older, healthy subjects develop age-related memory loss. Subjects with MCI develop a greater degree of memory problems relative to age-matched healthy subjects, although they do not experience the personality changes or other problems characteristic of AD. Studies using PET showed that Aβ deposition and inflammation are present in the brains of MCI subjects. AD patients suffer severe memory loss, Aβ deposition, inflammation and loss of cholinergic neurons and α7 nAChRs. The deposition of Aβ in the brain starts before MCI and increases with age. Therefore, tropisetron could potentially prevent the onset of AD if administered during or before the onset of MCI.
2. Role of Aβ–α7 nicotinic acetylcholine receptor interaction in AD pathology
Although the precise mechanisms underlying AD pathology are currently unknown, the accumulation and aggregation of amyloid-β (Aβ) peptides in brain regions, such as the hippocampus and cerebral cortex, are believed to be an early event in the pathogenesis of this disease. Another prominent feature of AD pathology is the loss of cholinergic neurons and nicotinic acetylcholine receptors (nAChRs) throughout the brain () Citation[3,4]. However, the mechanisms linking Aβ to the loss of cholinergic neurons and nAChRs remain to be fully elucidated. The α7 subtype of nAChRs, a major subtype of nAChRs in the brain, is a functional homopentameric receptor as ACh-gated ion channels open when five identical α7-subunits assemble together Citation[4]. The α7 nAChRs are located presynaptically, including on cholinergic projection pathways from the basal forebrain, where they are active in the Ca2+-dependent release of neurotransmitters. Additionally, α7 nAChRs are localized postsynaptically on γ-aminobutyric acid-ergic inhibitory interneurons, particularly in the hippocampus and cerebral cortex Citation[4]. Accumulating evidence suggests that the α7 nAChR is integral to the pathogenesis of AD, and therefore that α7 nAChR agonists could be potential therapeutic drugs for MCI and AD Citation[3,4]. Despite the presence of high amounts of amyloid precursor protein (APP) and Aβ deposits in the brain, deleting α7 nAChR subunits in the mouse model of AD protects against dysfunction of synaptic integrity (pathology and plasticity) and cognitive function Citation[5]. Furthermore, chronic administration of nicotine prevented Aβ-induced reductions of α7 nAChR in an animal model of AD Citation[6]. Although the mechanism underlying the neuroprotective effect of nicotine is unclear, it is likely that nicotine protects cells against Aβ toxicity by upregulation of α7 nAChR Citation[6]. Subsequently, S 24795, a partial agonist at α7 nAChR, was found to reduce the Aβ42–α7 nAChR interaction and Aβ42-induced τ phosphorylation Citation[7]. Taken together, disrupting the Aβ–α7 nAChR interaction may represent a novel approach to reducing Aβ-mediated functional deficits, neurodegeneration, and possibly the neuropathological features of AD.
3. Tropisetron
Tropisetron (Navoban®), a 5-hydroxytryptamine (5-HT)3 receptor antagonist, is widely used to treat chemotherapy-induced emesis outside of the United States (US). In addition to its 5-HT3 receptor antagonism properties, tropisetron is also a partial agonist at the α7 nAChR. Tropisetron (5 – 20 mg/day) is reportedly effective in the treatment of auditory sensory gating P50 deficits, cognitive impairment, and negative symptoms in patients with schizophrenia Citation[8-11], indicating that tropisetron would be a potential therapeutic drug for schizophrenia Citation[3,12].
Using a clinical compound library, Spilman et al. Citation[13] recently identified tropisetron as an agent that consistently increased soluble APP α (sAPPα), which acts as a trophic factor (). A subsequent assay showed that tropisetron consistently increased the sAPPα/Aβ1 – 42 ratio, suggestive of a beneficial effect in ameliorating the AD phenotype. In vivo studies using J20 mice (an animal model of AD) showed that tropisetron (0.5 mg/kg/day) improved the sAPPα/Aβ ratio, along with spatial and working memory in mice, and that tropisetron was effective both during the symptomatic, pre-plaque phase (5 – 6 months) and in the late plaque phase (14 months). As well as possessing 5-HT3 receptor antagonism and α7 nAChR partial agonism properties, tropisetron also binds to the ectodomain of APP, with a sub-micromolar affinity Citation[13]. Interestingly, direct comparisons of tropisetron with current AD therapeutic drugs, such as memantine and donepezil, revealed that tropisetron induced greater improvements in memory and the sAPPα/Aβ1 – 42 ratio Citation[13]. Furthermore, it is reported that tropisetron protects against Aβ-induced neurotoxicity in vivo, through both 5-HT3 receptor-dependent and independent pathways Citation[14].
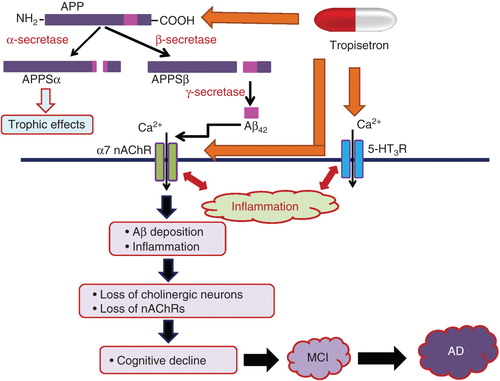
Figure 2. Schematic diagram of the proteolytic events and cleave products associated with APP and possible mechanistic action of tropisetron in the prevention of AD. APP is metabolized by a membrane-associated protease, α-secretase, and this cleavage releases the extracellular amino-terminal ectodomain of APP (APPSα), which displays trophic properties. The alternative cleavage pathway involves two sequential cleavages by β- and γ-secretase and gives rise to a series of Aβ. Accumulating evidence suggests that the interaction between Aβ peptides and α7 nAChRs is integral to the pathology of AD. The interaction between Aβ peptides and α7 nAChRs might induce Aβ deposition and inflammation in the brain, resulting in the loss of cholinergic neurons and α7 nAChR. These neurotoxic events may promote cognitive decline, followed by MCI, and ultimately lead to AD. In combination with an α7 nAChR agonist and serotonin 5-HT3 receptor antagonist, tropisetron, binds to APP, with a sub-micromolar affinity. The interaction of tropisetron with 5-HT3 receptors also plays a role in the anti-inflammatory and neuroprotective effect against Aβ-induced neurotoxicity. Taken together, tropisetron could act as a potential therapeutic drug for AD, if be administered during the period of MCI or early stages of AD.
4. Role of α7 nAChR in inflammation
Positron emission tomography (PET) studies using [11C]PIB (Pittsburgh Compound-B) and [11C](R)-PK11195 (an antagonist at the mitochondrial 18 kDa translocator protein) demonstrated that Aβ deposition and microglial activation could be detected in the brain of patients with amnestic MCI Citation[15,16]. Longitudinal studies suggested that MCI subjects with high PIB retention are much more likely to convert to AD than subjects with low PIB retention Citation[16], indicating that Aβ–PET may play a prognostic role in the clinical evaluation of MCI. Most significantly, longitudinal studies showed that cognitively normal subjects with elevated PIB were at much higher risk for longitudinal cognitive decline and the emergence of clinically significant cognitive impairment, relative to PIB negative, age- and education-matched subjects Citation[16]. In addition, the AD Neuroimaging Initiative research suggests that AD begins with Aβ accumulation and inflammation in the brain, which ultimately leads to synaptic dysfunction, neurogeneration, and cognitive or functional decline, although inflammation is not always detrimental in the AD pathological process ( and ) Citation[16].
In 2003, Wang et al. Citation[17] reported on the key role of α7 nAChR in the inflammatory process. They found that the anti-inflammatory action of vagal stimulation worked in wild-type, but not α7 nAChR knock-out mice Citation[17]. This led to the proposal of a ‘cholinergic anti-inflammatory pathway’ Citation[18]. Preclinical models have provided a plethora of evidence supporting a beneficial effect for activation or mimicry of the ‘cholinergic anti-inflammatory pathway’ in a number of disorders (e.g., inflammatory bowel disease, postoperative and endotoxin-induced ileus) Citation[18]. Interestingly, a report showed that tropisetron can attenuate serum levels of the pro-inflammatory cytokine, IL-6, in rats after cecal ligation and puncture Citation[19], indicating a potent anti-inflammatory effect for tropisetron. It is also known that 5-HT3 receptor antagonists, including tropisetron, conferred anti-inflammatory properties. Considering the crucial role of α7 nAChR on Aβ deposition and inflammation in the brain, tropisetron may act to prevent or delay the onset of AD from the state of MCI ( and ).
5. Encenicline (EVP-6124)
EnVivo Pharmaceuticals, Inc. (now Forum Pharmaceuticals, Inc.) reported results for a Phase IIb trial of encenicline hydrochloride (EVP-6124; 0.3, 1 or 2 mg, 23-week), another α7 nAChR partial agonist, in patients (n = 409) with mild to moderate AD Citation[20]. Encenicline (2 mg) showed statistically significant effects on a number of cognitive and clinical endpoints, including the primary endpoint of AD Assessment Scale-Cognitive Subscale status. The drug was safe and well tolerated, showing predominantly mild treatment-emergent adverse effects (1 mg = 48.5%, 2 mg = 53%). Next, the company initiated COGNITIV AD, a Phase III clinical trial program, using encenicline in mild to moderate AD. The program consists of two randomized, double-blind, placebo-controlled trials, enrolling ∼ 1600 patients at sites in the US and other countries worldwide Citation[20].
6. Conclusion
As mentioned above, tropisetron interacts with both the 5-HT3 receptor and α7 nAChR, as well as interfacing directly with APP, targets that are all associated with early AD pathology (). This makes tropisetron an attractive potential therapeutic drug to delay or prevent MCI and AD, particularly as current medications do not affect the underlying disease process. Further clinical studies are needed to fully evaluate the efficacy of tropisetron in AD prevention.
7. Expert opinion
AD is the most common cause of dementia in the elderly, with a worldwide prevalence estimated to quadruple over the next 50 years. It is a slowly progressing disease, characterized by three stages: an early preclinical stage with no symptoms, a middle stage with MCI and a final stage with dementia. However, no effective treatment is available to slow down or stop the onset of MCI or AD.
A prominent feature of AD pathology is the loss of cholinergic neurons and nAChRs throughout the brain (). Given the role of the Aβ–α7 nAChR interaction in AD pathology, both Aβ and α7 nAChR are valid therapeutic targets for AD. A number of PET studies demonstrated the presence of Aβ depositions in the brain, some 10 – 20 years before dementia or even MCI is diagnosed (). A recent preclinical study showed that in addition to interaction with both the 5-HT3 receptor and α7 nAChR, tropisetron interacts directly with APP, targets that are all associated with early AD pathology Citation[13]. Interestingly, direct comparisons of tropisetron with current AD therapeutic drugs (e.g., memantine and donepezil) revealed greater improvement in memory and sAPPα/Aβ1–42 ratios with tropisetron Citation[13]. In addition, tropisetron showed excellent oral bioavailability, brain penetration, cognitive effects and biomarker effects at currently used human equivalent doses Citation[13].
Taken together, current evidence highlights tropisetron as a promising therapeutic drug to delay or prevent MCI and mild to moderate AD, especially as it already has worldwide approval for clinical use in a different disorder ( and ). Future studies using a combination of tropisetron and other drugs, such as β-site APP cleaving enzyme (BACE1) inhibitors will be interesting.
Declaration of interest
The study was supported by a Grant-in-Aid from the Minister of Education, Culture, Sports, Science, and Technology of Japan. K Hashimoto holds a patent for the use of tropisetron in neuropsychiatric diseases, including schizophrenia and Alzheimer’s disease. In addition, K Hashimoto has served as a scientific consultant to Astellas, Dainippon-Sumitomo and Taisho, and he has also received research support from Abbvie, Dainippon-Sumitomo, Otsuka, and Taisho. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
Bibliography
- World Alzheimer Report 2013. Journey of Caring. An analysis of long-term care for dementia. Alzheimer’s Disease International; London, UK; 2013
- Friedrich MJ. Researchers test strategies to prevent Alzheimer’s disease. JAMA 2014;311:1596-8
- Toyohara J, Hashimoto K. Alpha7 Nicotinic receptor agonists: potential therapeutic drugs for treatment of cognitive impairments in schizophrenia and Alzheimer’s disease. Open Med Chem J 2010;4:37-46
- Deutsch SI, Burket JA, Benson AD. Targeting the alpha7 nicotinic acetylcholine receptor to prevent progressive dementia and improve cognition in adults with Down’s syndrome. Prog Neuropsychopharmacol Biol Psychiatry 2014;54:131-9
- Dziewczapolski G, Glogowski CM, Masliah E, et al. Deletion of the alpha7 nicotinic acetylcholine receptor gene improves cognitive deficits and synaptic pathology in a mouse model of Alzheimer’s disease. J Neurosci 2009;29:8805-15
- Srivareerat M, Tran TT, Salim S, et al. Chronic nicotine restores normal Abeta levels and prevents short-term memory and E-LTP impairment in Abeta rat model of Alzheimer’s disease. Neurobiol Aging 2011;32:834-44
- Wang HY, Bakshi K, Shen C, et al. S 24795 limits beta-amyloid - alpha7 nicotinic receptor interaction and reduces Alzheimer’s disease-like pathologies. Biol Psychiatry 2010;67:522-30
- Koike K, Hashimoto K, Takai N, et al. Tropisetron improves deficits in auditory P50 suppression in schizophrenia. Schizophr Res 2005;76:67-72
- Shiina A, Shirayama Y, Niitsu T, et al. A randomized, double-blind, placebo-controlled trial of tropisetron in patients with schizophrenia. Ann Gen Psychiatry 2010;9:27
- Zhang XY, Liu L, Liu S, et al. Short-term tropisetron treatment and cognitive and P50 auditory gating deficits in schizophrenia. Am J Psychiatry 2012;169:974-81
- Noroozian M, Ghasemi S, Hosseini SM, et al. A placebo-controlled study of tropisetron added to risperidone for the treatment of negative symptoms in chronic and stable schizophrenia. Psychopharmacology (Berl) 2013;228:595-602
- Ishikawa M, Hashimoto K. Alpha7 nicotinic acetylcholine receptor as a potential therapeutic target for schizophrenia. Curr Pharm Des 2011;17:121-9
- Spilman P, Descampus O, Gorostiza O, et al. The multi-functional drug tropisetron binds APP and normalizes cognition in a murine Alzheimer’s model. Brain Res 2014;1551:25-44
- Rahimian R, Fakhfouri G, Ejtemaei Mehr S, et al. Tropisetron attenuates amyloid-beta -induced inflammatory and apoptotic responses in rats. Eur J Clin Invest 2014;43:1039-51
- Okello A, Edison P, Archer HA, et al. Microglial activation and amyloid deposition in mild cognitive impairment: a PET study. Neurology 2009;72:56-62
- Cohen AD, Klunk WE. Early detection of Alzheimer’s disease using PIB and FDG PET. Neurobiol Dis 2014;72:117-122
- Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003;421:384-8
- Matteoli G, Boeckxstaens GE. The vagal innervation of the gut and immune homeostasis. Gut 2013;62:1214-22
- Setoguchi D, Nakamura M, Yatsuki H, et al. Experimental examination of anti-inflammatory effects of a 5-HT3 receptor antagonist, tropisetron, and concomitant effects on autonomic nervous function in a rat sepsis model. Int Immunopharmacol 2011;11:2073-8
- Forum Pharmaceuticals, Inc. EnEivo Pharmaceuticals initiates COGNITIV AD, a phase 3 clinical trial program of encenicline (EVP-6124) in Alzheimer’s disease. January 22 2014. Available from: http://www.forumpharma.com/content/news-events/cognitiv-ad-program
